Introduction
The disposal of radioactive waste from nuclear power plants (NPP) containing 14C implies considerations that are different from those encountered with other usual isotopes. First of all, its long half-life (5730 yr) excludes any significant loss by decay during mid-term storage periods, and secondly, carbon is one of the most commonly encountered elements in living matter, occurring in nature in a wide number of chemical forms.
The understanding of the mechanisms by which the 14C can be released from different types of radioactive waste under its final storage conditions (organic, inorganic, dissolved or gaseous species) can be a crucial aspect in the design of this kind of facility (Toulhoat et al. Reference Toulhoat, Narkunas, Zlobenko, Diaconu, Petit, Schumacher, Catherin, Capone, Von Lensa, Piña, Williams, Fachinger and Norris2015).
The management of radioactive waste in Spain, carried out within the framework of other international organizations as IAEA, involves its previous classification in two major groups (Ministry of Industry of Spain 2008; IAEA 2007):
∙ The so-called low and intermediate level waste, which, given their characteristics, may be conditioned and indefinitely stored at the near surface repository of El Cabril (Córdoba, Spain).
∙ The high level waste, consisting basically of spent nuclear fuel and other irradiated materials. This group also includes other intermediate level waste which, in view of their characteristics, cannot be disposed of under the conditions established for El Cabril.
At the El Cabril facility, there is a limitation of 20 TBq on the total amount of 14C that can be disposed of (Official State Gazette of Spain 2001). Based on that, and the room available in the disposal cells, limits for acceptance of individual waste packages can be derived.
Nowadays, a large number of full commercial reactor dismantling projects are now in the execution phase in Spain. These dismantling and decommissioning activities may be seriously hampered depending on the management capacity of, not only the spent fuel, but materials of moderate (but appreciable) quantities of 14C (Ministry of Industry of Spain 2006).
Because of the reasons mentioned above, one of the R&D activities planned in the Spanish context for the next years should provide support for the drawing up and/or revision of the management strategies for radioactive waste, based on the better understanding of its physical, chemical, environmental and radiological properties. With this purpose, several works at international (Banford et al. Reference Banford, Eccles, Graves, Von Lensa and Norris2008; Von Lensa et al. Reference Von Lensa, Vulpius, Steimetz, Girke, Bosbach, Thomauske, Banford, Bradbury, Grambow, Graves, Jones, Petit and Piña2011) and national level (Esteban-Duque et al. Reference Esteban-Duque, Romero, Fabrellas, Rodríguez, Molina, Caturla and Molina1996; Rodríguez Reference Rodríguez1997) were performed aiming to provide data on the content and distribution of 14C in radioactive waste mainly coming from nuclear reactors.
Within this framework, the understanding of the 14C behavior in waste packages could lead to a revaluation of the near surface repository for the disposal of waste containing this radionuclide in high concentrations. To achieve these objectives, leaching experiments were planned considering two different scenarios. One, in which the leaching solution (granite-bentonite water, GBW) simulates some of the expected conditions of a repository in which a granite-bentonite mixture has been used as backfill material, and the other, using deionized water as a high efficiency chemical removal agent and for reference purposes.
The particular aerobic conditions in which the tests were made can facilitate, by comparison with the anaerobic conditions relevant to most of the other national disposal concepts considered in the CAST project, the understanding of the leaching and speciation processes happening in these experiments.
MATHERIALS AND MethodS
Activation History of the Material
Vandellós I was a Spanish nuclear power plant of 460 MW operated from 1972 to 1989 which used natural uranium as fuel, graphite as moderator and CO2 as coolant. In 1989 a fire in the turbine building led to the closure of the plant with the consequent need of the removal of nuclear waste and the decontamination of the site.
The graphite in the nucleus acted as moderator and reflector in the protector shield of the steam generator, and it was composed of pile graphite (2.5×103 tonnes), which remains there for the whole life of the reactor, and graphite fuel sleeve (1.1×103 tonnes) which was replaced together with the fuel.
During operation, the sleeves were placed inside the reactor pool while removing the fuel, therefore non-activation products like 137Cs, transuranics, etc., were incorporated from those present in the pool. At the latest stages of the dismantling, the sleeve graphite was extracted, crushed and stored at the Vandellós I site in 220 cubic containers of 6 m3 each.
Reagents and Standard Solutions
Leaching solutions
∙ Ultrapure water of type 1: 18.2 MΩ· cm at 25 °C / Total Organic Carbon=2 ppb (Millipore)
∙ Synthetic air, 21% O2 and 79% N2 (Praxair)
Gas Chromatography/Mass Spectrometry (GC-MS) calibration standards
∙ CO standard: 5000 mg/L±2% (Air Liquide)
∙ Methanol ≥99.9% (Fluka)
∙ Ethanol, Chromasolv Gradient Grade, ≥99.9% (Sigma Aldrich)
∙ Formaldehyde solution, ACS reagent, 37wt% in H2O (10–15% methanol) (Sigma Aldrich)
∙ Acetaldehyde, ACS reagent, ≥99.5% (Sigma Aldrich)
∙ Propionaldehyde, reagent grade, 97% (Sigma Aldrich)
Ionic Chromatography System (ICS) calibration standards
∙ KOH Dionex EGCIII eluent generator (Thermo Fisher Scientific)
∙ Sodium acetate trihydrate puriss. p.a., >99.5 % (Sigma Aldrich)
∙ Sodium formate puriss. p.a., >98% (Sigma Aldrich)
∙ Sodium oxalate puriss. p.a., ACS reagent, >99.5% (Sigma Aldrich)
Catalytic combustion and Liquid Scintillation Counter (LSC) systems
∙ Copper Oxide (II), catalysts (Sigma Aldrich)
∙ Silver Vanadate (Ag3VO4), catalysts (Sigma Aldrich)
∙ Platinum, catalysts (Sigma Aldrich)
∙ CO2 absorber: Oxysolve C-400 (Zynsser Analytic)
∙ Instagel Plus and Hionic Fluor cocktails (Perkin Elmer)
∙ 14C standard solution (DL-Tartaric acid-1,4-14C) (Amersham)
Equipment
∙ Gas-tight reactor vessel from Berghof for leaching of irradiated graphite. The inner part of the reactor is made of radiation stable cross-linked PTFE, preventing material sorption on the walls. The outer vessel is made of stainless steel. Construction of the reactor allows pressure measurement and gas sampling (see Berghof website).
∙ GC-MS system: Agilent GC/MS 7890B/5977A series. Turbo inert Electron-ionization source, auto sampler CTC Combi-PAL and Headspace.
∙ Dionex ICS-900 Ion Chromatography System with Ionic Reagent Free Controller (RFC-30), AS11-HC column, AERS 500 Carbonate Electrolytically Regenerated Suppressor and AS40 auto sampler (all by Thermo Scientific).
∙ OX-500 Biological Material Oxidizer (combustion oven).
∙ Packard Tricarb 3110 TR/LL and 1220 QUANTULUS Ultra Low Level LSC.
∙ Canberra Gamma Spectrometer System with BEGe 3830 HPGe detector. The acquired spectra were evaluated using the software Genie 2000.
Sample Preparation
The samples of irradiated graphite received at CIEMAT were used for manufacturing fuel sleeves and ranged in size from powder to 30 mm irregular fragments. The average 14C activity of the material declared in the transport document was 1.35×104 Bq/g.
In order to obtain test samples compatible with the leaching containers and with well-known dimensions, core samples of about 11 mm×12 mm (Ø×H) were extracted by drilling from the raw material received at the laboratory (see Figure 1).

Figure 1 Leaching containers and graphite samples.
Radiological Characterization of Initial Sample
To perform the characterization of the graphite, an aliquot (ca 20 mg) of sample was introduced in the combustion oven where it was burned to 900ºC in an atmosphere of oxygen. The resulting gaseous products were passed through a catalyst bed (CuO, Pt, and Ag3VO4) where the carbon compounds were converted to CO2, trapped in a vial containing the carbon absorber Oxysolve C-400, added Instagel Plus and finally analyzed by LSC.
To be sure that no activity remains in the combustion chamber, each sample was burned three times for 3 min, in this way, the 14C activity collected was about 99.9% and all the interferences coming from beta-gamma emitters different from 14C were removed.
Additionally, to determine the activity of the high energy beta-gamma emitters present in the initial sample, another aliquot of sample (ca. 1g) was placed into a glass vial and measured by gamma spectrometry.
Leaching Process
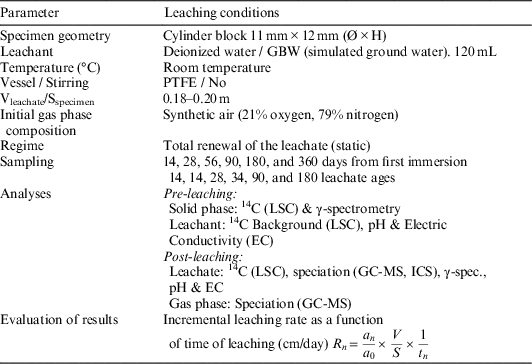
Once graphite cylindrical samples were obtained, the leaching process, based on the standard (ISO 6961, 1982) and in the harmonized leaching parameters recommended by Petrova et al. (Reference Petrova, Shcherbina, Williams and Piña2015), was carried out as follows:
∙ As leachants, ultra-pure water type 1 (pH=5.8, EC=0.05 µS/cm), and granite-bentonite water (pH=7.2, EC=16.8 mS/cm), were used (see Table 1 for GBW composition).
∙ The leachant container was made of PTFE (material recommended for its chemical inertness) and the value of the ratio provided by dividing the volume of leachant by the exposed geometric surface area of specimen was 0.2 m. Although the leaching tests were carried out at room temperature, the losses of leachant by vaporization were negligible.
∙ The specimens were suspended in 120 mL of leachant by means of a PTFE thread and surrounded by at least 1cm of liquid in all directions (Figure 1). The total volume of the vessel is 150 mL, and prior to its first use, it was cleaned up with deionized water.
∙ The initial gas phase composition consisted of synthetic air (21% oxygen, 79% nitrogen) supplied from a 5-L compressed air cylinder. The pressure vessel was set up at 1 bar.
∙ The frequency of leachant renewal was stablished at specific intervals (15, 28, 56, 90, 182, and 359 days from the start of the test). After each leaching step, the gas sample is collected through the gas sample extraction valve by means of a gas-tight syringe, and immediately injected into the GC-MS.
∙ Once the gas sample has been analyzed, the container is opened, the specimen is withdrawn from the leachant and the leachate is filtered and finally transferred to a polyethylene bottle.
∙ Finally, the PFTE vessel is cleaned up with deionized water, refilled with fresh leachant, the specimen immersed in the liquid, and the reactor closed.
∙ The headspace of the reactor was purged with synthetic air to remove interfering elements that could disturb or invalidate the later measurements. The leaching process and general conditions of the experiments are depicted and summarized in Figure 2 and Table 2.
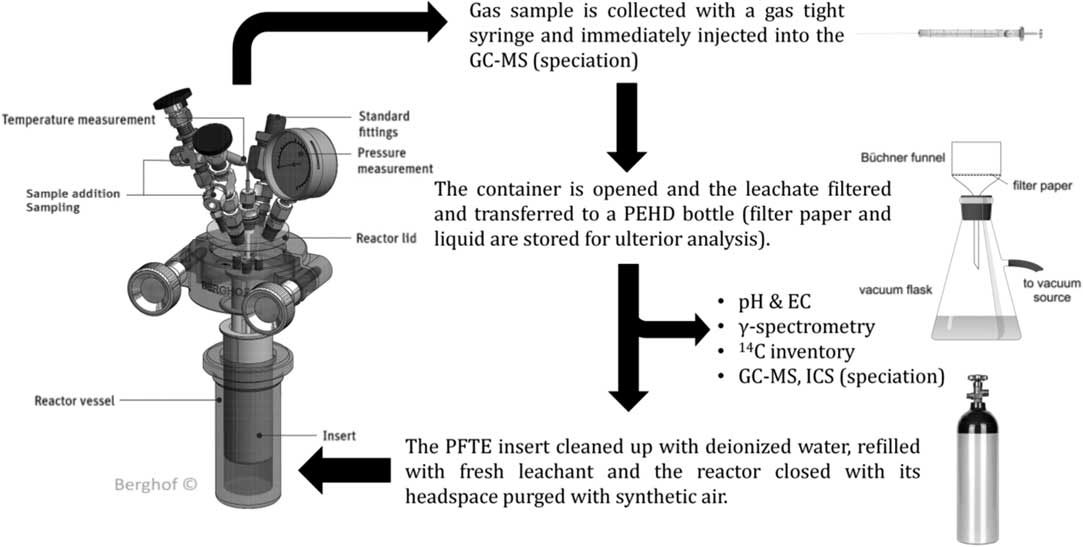
Figure 2 Leaching process sampling methodology.
Table 1 Granite-bentonite water (GBW) composition.
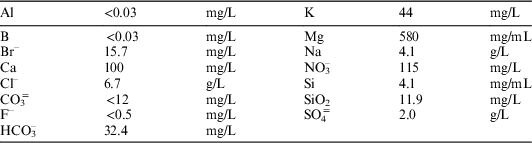
Table 2 General conditions of graphite leaching experiments.
Analytical Determinations
14C Inventory
The 14C content of the leachates was analyzed twice, first by means of a conventional LSC system (Packard Tricarb 3110 TR/LL) and, after a preliminary evaluation of the results (where no 14C activity was detected above the detection limits), with an ultra-low level LSC equipment (Quantulus). For these analyses, 0.5 mL of sample was introduced in the combustion oven, following the same methodology as was used for the characterization of the initial graphite.
Gamma Spectrometry
For the gamma characterization of the leachates, a polyethylene bottle containing 100 mL of sample was placed in contact with the BEGe 3830 HPGe detector (calibrated counting geometry).
GC-MS Analyses
For the speciation analyses, the following range of temperatures were set: GC-MS injector 150°C, GC transfer line 250°C, MS ion source 230°C and MS Quadrupole 150ºC. All the injections were made in the split/splitless mode and the mass spectra were collected in Scan and SIM mode. MassHunter software was used for data acquisition and processing.
Two different Agilent chromatographic columns were used: the “Select Permanent Gases/CO2 HR” column, to analyze CO, CO2 and hydrocarbons C1 to C5, and the DB-624UI column for alcohols and aldehydes.
The assays to analyze CO in gaseous samples were carried out by manual injection of 1 mL of sample under isothermal conditions (70ºC) and with a split flow of 50 mL/min. To analyze alcohols and aldehydes in liquid samples the following temperature-time profile was stablished: 40ºC (2 min); 1ºC/min to 45ºC (5 min); 1ºC/min to 50ºC (5 min) and 50ºC (2 min) with a split flow of 100:1. In this last case, the technique of head space sampling was used, with 30 sec of incubation time at 40ºC.
ICS Analyses
Short chain carboxylic acids were determined by means of a Dionex ICS-900 chromatography system. Separation was accomplished with a Dionex Ion Pac AS-11-HC anion separator column (4 mm×250 mm) and IonPac AG-11-HC guard column (4 mm×50 mm). The detection was performed with a conductivity detector (digital range: 0–1000 μS/cm). Chromeleon SE Software was used for data acquisition and processing.
The eluent used was KOH at a flow rate of 1 mL/min. The eluent concentration was prepared in situ by an eluent generator (Dionex Reagent Free Controller). In order to obtain higher sensitivity and lower noise for carbonate separations, suppression was accomplished with a Dionex AERS-500 operated in the auto suppression recycle mode. The sample size was 50 μL and the separation was carried out at room temperature.
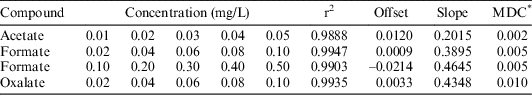
A set of standard solutions of acetate, formate and oxalate were prepared, ranging from 0.01 ppm to 0.5 ppm. The calibration was carried out by using the three mixed standards with different concentrations: 1.5 mM KOH (0–8 min), 25 mM KOH (8–30 min), and 1.5 mM KOH (30–40 min) and with a flow rate of 1 mL/min. The calibration result for each analyte is shown in Table 3.
Table 3 ICS calibration results in deionized water.
* Minimum detectable concentration.
Results
Radiological Characterization of the Initial Sample
The results of 14C and the most representative high energy beta-gamma emitters of the irradiated graphite samples are indicated in Table 4.
Table 4 Radiological characterization of graphite samples.

* All the uncertainties were below 10% at the 95% confidence.
Leaching Process and Speciation
14C and Main β-γ Emitters Results
Regarding the LSC analyses, it was tried to lower the 14C minimum detectable activity (MDA), first, by increasing the volume of leachate that is introduced in the combustion oven, and second, by measuring the trapped CO2 solution in low background equipment. Although, this procedure reduces the detection limits up to 0.005 Bq/g, in none of the media tested has been found 14C activity above MDA.
The results of the activity, leaching rates (Rn), and the accumulated activity (ΣAn/Ao) of 14C, 137Cs and 60Co, found in deionized water and GBW leachates after each stage, are indicated in Tables 5 and 6. Where A0 and An are respectively the activity of the radionuclide initially present in the specimen and the activity leached during each leaching interval.
Table 5 Activity, leaching rate and accumulated activity of 14C, 137Cs, and 60Co in deionized water leachates. Sample V-I-1. Dimensions: S=6.49 cm2; V=1.21 cm3; M=2.02 g.
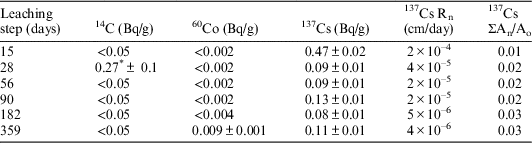
* Although this value is higher than the minimum detectable activity (MDA), it is more likely that this could be because of failure in the filtering process of the sample, or cross-contamination in the laboratory.
Table 6 Activity, leaching rate and accumulated activity of 14C, 137Cs. and 60Co in GBW leachates. Sample V-I-2. Dimensions: S=6.29 cm2; V=1.21 cm3; M=2.02 g.
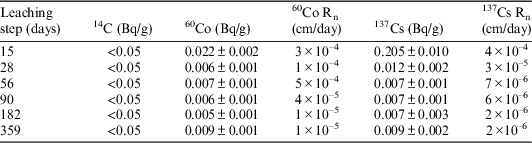
As can be observed in Table 6, 137Cs has been detected in every leaching step. After 359 days of testing in deionized water (177 continuous days of leaching), the leaching rate of 137Cs has decreased to 4×10–6 cm/day, this value corresponds to a leached accumulated activity for this isotope of 3%. On the contrary, only one value of 60Co, which represents the 0.02% of the total 60Co of the initial sample, has been found in the leachates analyses of the 359 days stage. Therefore, it has not been possible to establish a leaching trend for this radionuclide.
Regarding the main beta-gamma emitters detected in GBW (see Table 6), in the sample taken after 359 days (177 continuous days of leaching) a leaching rate value of 2×10–6 cm/day for 137Cs and 1×10–5 cm/day for 60Co has been obtained. This represents a leached accumulated activity of 4% in the case of 137Cs and 6% for 60Co.
Organic Carbon Compounds Results
Regarding the gas and leachate volatile species analyses, using the GC-MS technique with the parameters and methods previously detailed, neither alcohols nor aldehydes were detected in leachates in any step of the leaching process. Regarding gas samples, CO was not detected above 3.5 ppm (MDC).
With reference to the ICS analyses, due to the high concentration of anions and cations in GBW, which saturates the ICS signal, it has not been possible to use this technique to analyze the leachates obtained using this methodology.
Table 7 represents the results of the ICS analyses on leachates in deionized water. In this case, it is worth pointing out that acetate was detected in the fourth leaching step (90 days); formate in the 15, 56, and 90 days steps and oxalate in the 56 and 90 days steps. In Figure 3 is represented the acetate, formate and oxalate peaks detected in the 90 days step.
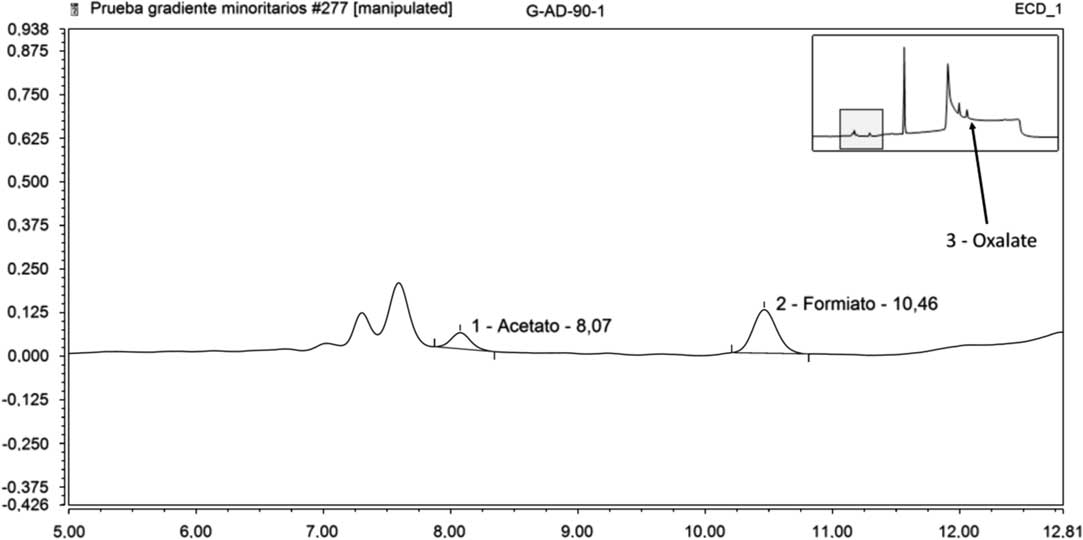
Figure 3 Acetate, formate, and oxalate peaks in pure water (90 days step).
Table 7 ICS results of deionized water leachates. Compound concentration in mg/L±2u.

Discussion and Conclusion
Although significant heterogeneity has been observed both in the detection and in the activity of the high energy beta-gamma emitters, the 14C content of the core samples tested, shows a quite constant mean value, with an average specific activity of 11,700±1023 Bq/g.
Among all the analyses of leachate samples carried out, only one sample (corresponding to the 28 days leaching step in deionized water) presented a value of 14C higher than the detection limit. It is more likely that this could be because of a failure during the filtration of the sample (clogged or broken filter paper) or cross contamination in the equipment than because of the leaching process itself.
Using pure water as leachant, in the ICS analyses, acetate was detected, although close to the MDA, after 90 days of leaching time; formate after 15, 56, and 90 days and oxalate in the 56 and 90 periods. However, this technique cannot be used to analyze GBW solutions because of the high concentration of anions and cations present in this media.
Both alcohols and aldehydes in leachates have not been detected in any step of the leaching process, and regarding the gas samples, nor was CO, whose values were again below MDC (3.5 ppm).
After 359 days of leaching, only leaching rates regarding beta-gamma emitters were observed: 2×10–6 cm/day for 137Cs and 1×10–5 cm/day for 60Co in GBW, and 4×10–6 cm/day for 137Cs in ultra-pure water.
Acknowledgments
These results were obtained from the work carried out by CIEMAT in CAST project that has received funding from the European Union’s European Atomic Energy Community’s (EURATOM) Seventh Framework Program FP7/2007-2013 under grant agreement no. 604779, the CAST project.












