The need for innovative approaches for the care of serious mental illnesses such as schizophrenia is clear. Schizophrenia affects approximately 1% of the global population and is a leading cause of disability, according to the World Health Organization (WHO). It is associated with a pooled 2.5 times relative risk of mortality and reduced life expectancy of upward of 30 years compared with the general population. The WHO has declared schizophrenia a priority condition for its mental health gap action programme because of the lack of available services, especially in low- and middle-income countries. Predicting and preventing relapse presents a crucial opportunity and first step to improve outcomes and reduce the care gap for persons living with schizophrenia. Relapse occurs within 5 years for the overwhelming majority of patients.Reference Robinson, Woerner, Alvir, Bilder, Goldman and Geisler1,Reference Brown, Kim, Mitchell and Inskip2 Each subsequent episode is associated with poorer lifetime outcomes, worse chronicity of the illness, reduced functioning and declining cognition. These arise as a combined consequence of neurotoxic, as well as psychological and social ‘toxic’ effects of relapse.Reference Muliyala and Thirthalli3 Innovative approaches to predict and prevent relapse while reducing the care gap are thus the focus of this global mental health protocol, the Smartphone Health Assessment for Relapse Prevention (SHARP) study, supported by the Wellcome Trust.
Predicting relapse and offering preventive interventions in schizophrenia requires an accurate and holistic measurement of patients’ lived experiences. Although self-reporting by patients remains the foundation, there is now the ability to augment self-report with objective insights into the effect of environment, psychosocial stressors and sleep on symptoms and functioning. Using commercially available smartphones and smartwatches, technology now affords opportunities to capture real-time and longitudinal profiles of patients’ symptoms, behaviours, cognition, physiology and social patterns. This novel data makes it possible to explore relationships between behaviours, physiology and symptoms, which may yield personalised relapse signals. Preliminary efforts have demonstrated that digital biomarkers related to mobility, sociability and self-reported symptoms may help predict relapse in schizophrenia.Reference Barnett, Torous, Staples, Sandoval, Keshavan and Onnela4 Other studies have also shown how smartphone digital biomarkers can predict stress, hallucinations and mood changes in patients with schizophrenia.Reference Ben-Zeev, Brian, Wang, Wang, Campbell and Aung5 The SHARP study will build on this preliminary work to inform the development of a scalable and sharable digital health solution that will monitor personal risk of relapse in individuals living with schizophrenia, and support timely response to potential risks by using real-time and evidence-based adjunctive digital resources.
The partnership between the Beth Israel Deaconess Medical Center (BIDMC) in Boston, Massachusetts; the All India Institute of Medical Sciences (AIIMS) Bhopal and Sangath in Bhopal, central India; and the National Institute of Mental Health and Neuroscience (NIMHANS) in Bengaluru, south India, represents a vision for global mental health solutions that are broadly applicable. Spanning rural and urban settings and low-to middle-income patient populations, these institutions are a microcosm for the global population living with schizophrenia. Each site, distinct from the others in geography and culture, will engage patients, family members and clinicians with diverse perspectives and markedly different lived experiences. The results of this global research will inform technology that can be utilised by populations around the world, to understand and improve mental health services for individuals living with schizophrenia.
Objectives
The overarching goal of this project is to guide the systematic development and adaptation of an open-source smartphone application and digital dashboard – Learn, Assess, Manage, Prevent (LAMP) – across diverse cultures and contexts in a patient-centred and transparent manner, to promote personalised care. The resulting app will be studied toward predicting and preventing relapse among individuals diagnosed with schizophrenia spectrum disorders. The BIDMC has used LAMP to collect preliminary evidence for relapse prediction in schizophrenia, and prior studies have demonstrated that the use of the app is safe, and offers potentially valuable clinical insights to patients and clinicians.Reference Wisniewski, Henson and Torous6 With new means to measure cognition, LAMP technology is increasingly better equipped to predict relapse. In this study, we seek to expand LAMP by co-designing a more engaging digital toolkit with patients, support systems and caregivers. Although our published work supports the feasibility of this new study, and the potential of digital phenotyping for global mental health has been increasingly recognised, this study will explore how app-based tools can realise such potential across diverse settings. The international collaboration supporting this study can break new ground for informing novel approaches to addressing pressing challenges in global mental health with digital tools.
The objectives and methods laid out in this protocol were jointly determined by the research teams at Sangath and AIIMS Bhopal, BIDMC, Harvard Medical School and NIMHANS. Collaborators from each institution met together in Bhopal, India to discuss logistics and ethics; ensure cultural relevance across sites in both the USA and India; and finalise the study details. The meeting in February 2020, familiarised researchers with LAMP and aligned institutions to shared study procedures, timelines and milestones, and overarching goals.
Technology: mindLAMP
The LAMP platform used throughout this study represents a patient-centred, transparent and collaborative approach to digital health.Reference Wisniewski, Henson and Torous6 Open-source and customisable, LAMP is a mobile application, dashboard and digital health platform, built with the knowledge that mental illness is as universal as it is personal. Researchers, patients and care providers can build on LAMP's software to create, customise and share digital health tools that meet their needs. LAMP has already been used in five countries and translated into six languages (Figure 1).
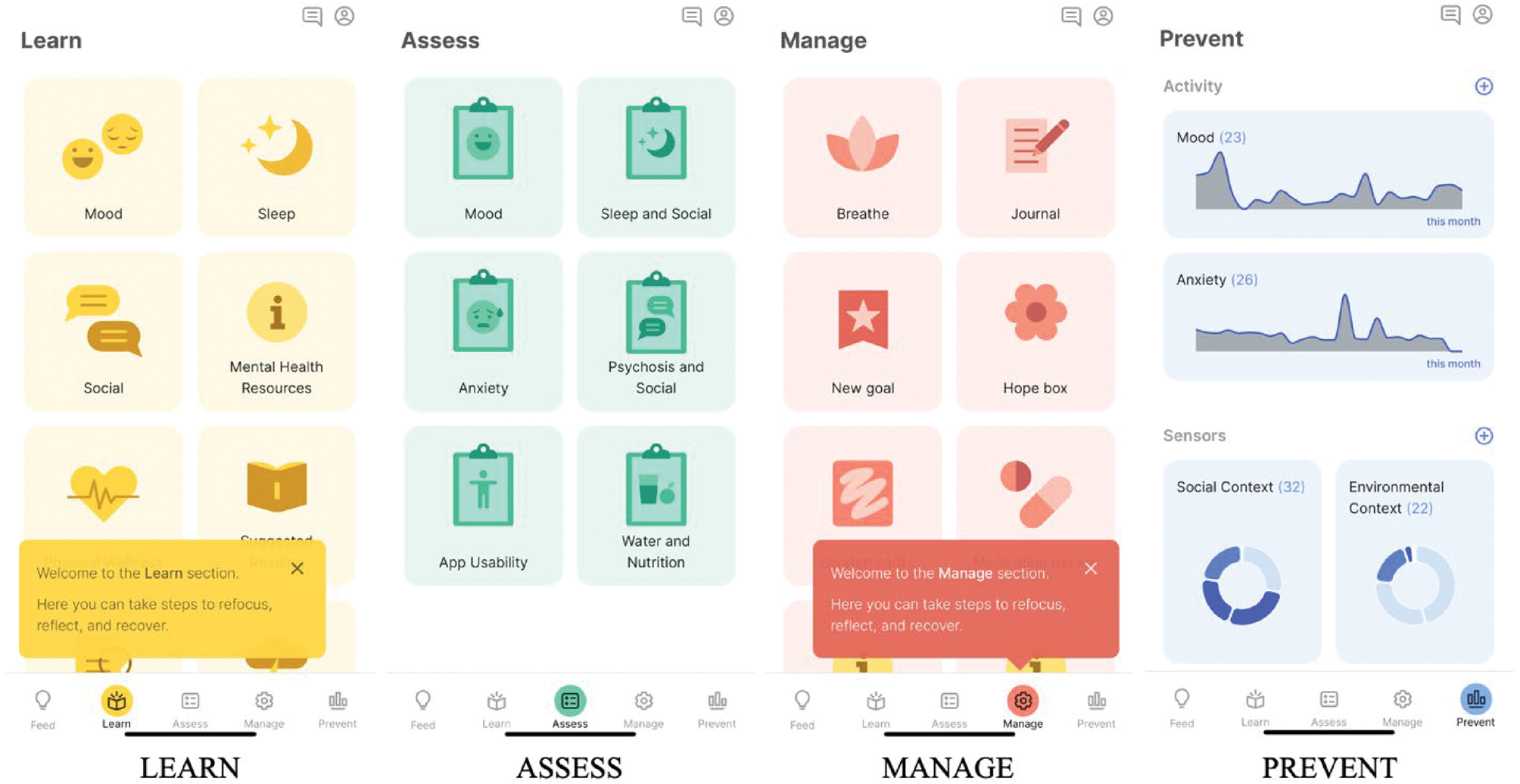
Fig. 1 LAMP (Learn, Assess, Manage, Prevent) is a customisable digital platform that offers researchers, patients and clinicians tools designed to help inform and supplement clinical care.
With an estimated 65% of people worldwide having access to mobile technology, LAMP offers accessible and scalable support for understanding and treating mental illness in countries such as India, where smartphone ownership for young adults grew 21% in 5 years.7
The Division of Digital Psychiatry at BIDMC designed the LAMP platform through a partnership with the Zco Corporation, a software development company. LAMP comprises three major components:
(a) mindLAMP, a smartphone app that captures surveys, physiological data via Apple HealthKit and Google Fit, passive data and games that assess cognition. The app is able to offer tips and mental health on demand or as triggered by patterns of captured data.
(b) A dashboard that aggregates and displays patient data that can be shared with clinicians, family members and trusted peers.
(c) A back-end secure database, server, application programming interface and middleware layer to support flexible use cases (Figure 2).
With smartphones increasingly owned and used by patients today, mindLAMP is well-positioned to collect temporally dense and longitudinal data. By analysing movement via GPS, exercise and sleep via accelerometer, cognition via on-screen neuropsychological assessments and symptoms via surveys, mindLAMP captures an integrated record of patients’ experiences, cognitive abilities and activities. The ability to capture diverse forms of digital data, an approach defined and referred to as digital phenotyping by the Digital Psychiatry Team at BIDMC, allows patients and clinicians to identify when behaviour patterns change.Reference Torous, Kiang, Lorme and Onnela8 An aberration from a patient's baseline data might indicate an early warning for relapse and an opportunity to intervene (Figure 3).
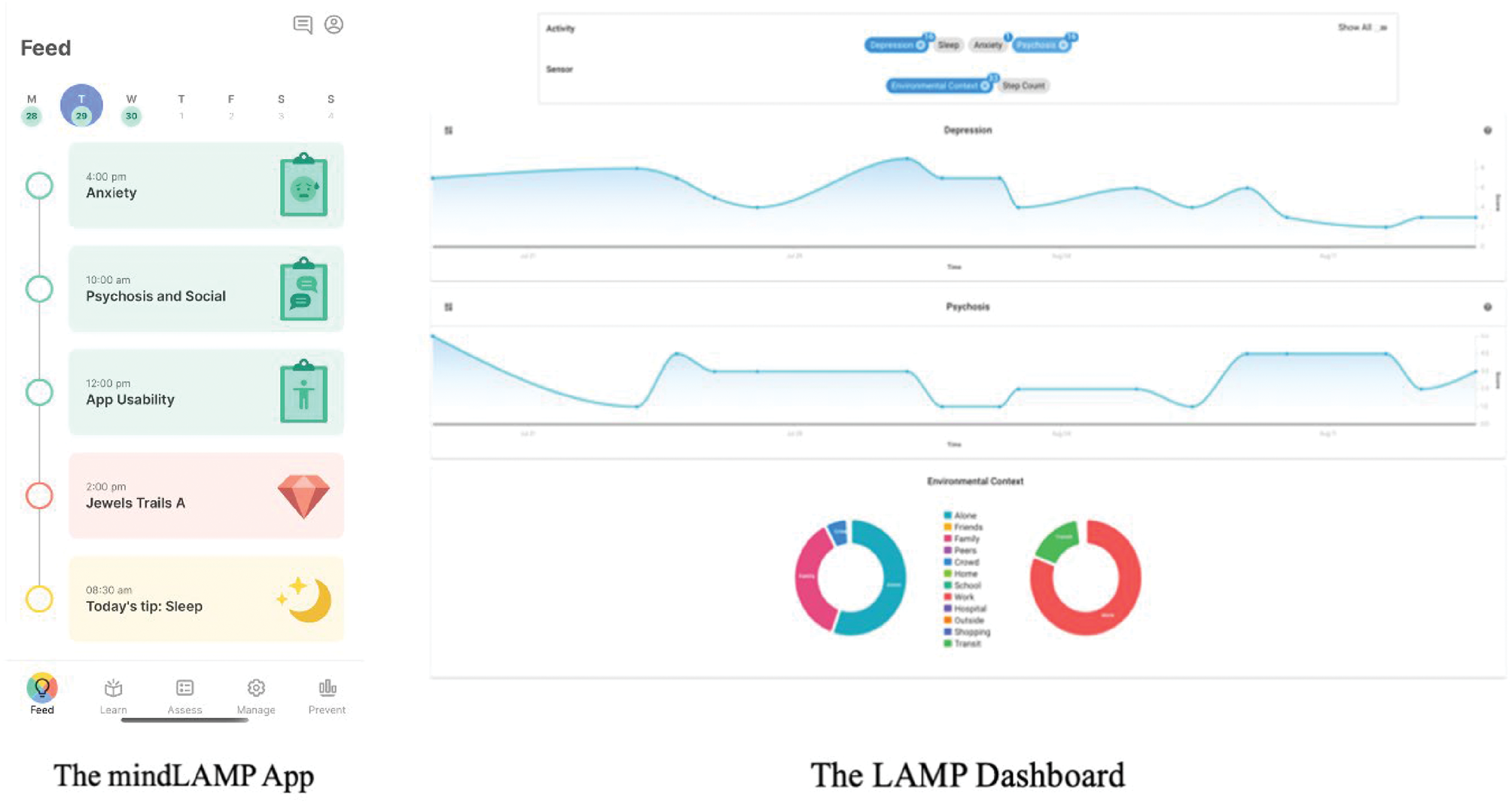
Fig. 2 Core components combine to make LAMP a data collection and visualisation tool to inform and augment mental health treatment.
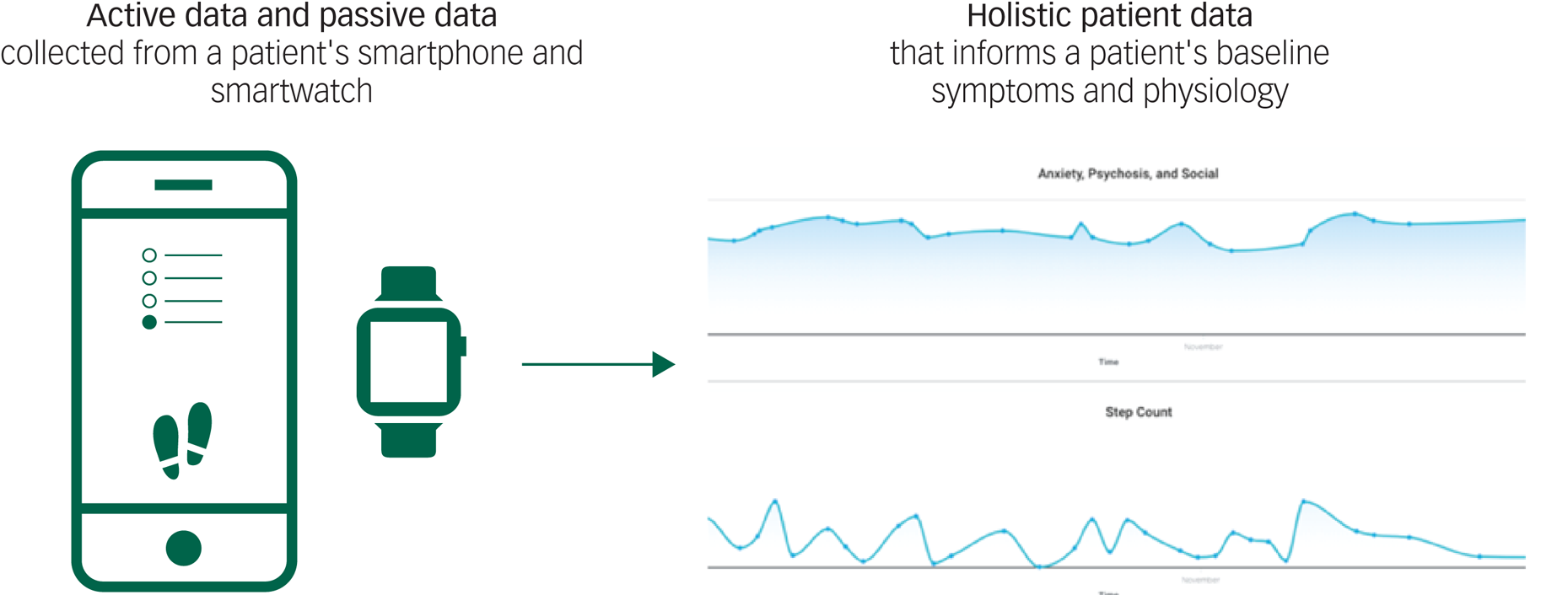
Fig. 3 Active and passive data collection makes it possible for LAMP to detect abnormal patient activity or mood fluctuations.
This study will further develop and explore simple activities designed to moderate sudden shifts or drastic changes in a patient's baseline symptoms or physiology. Although these app activities are not designed to replace treatment, they are designed to offer in the moment and relevant support and education. Activities will include resources ranging from psychoeducation tips on sleep to brief breathing exercises (Figure 4).
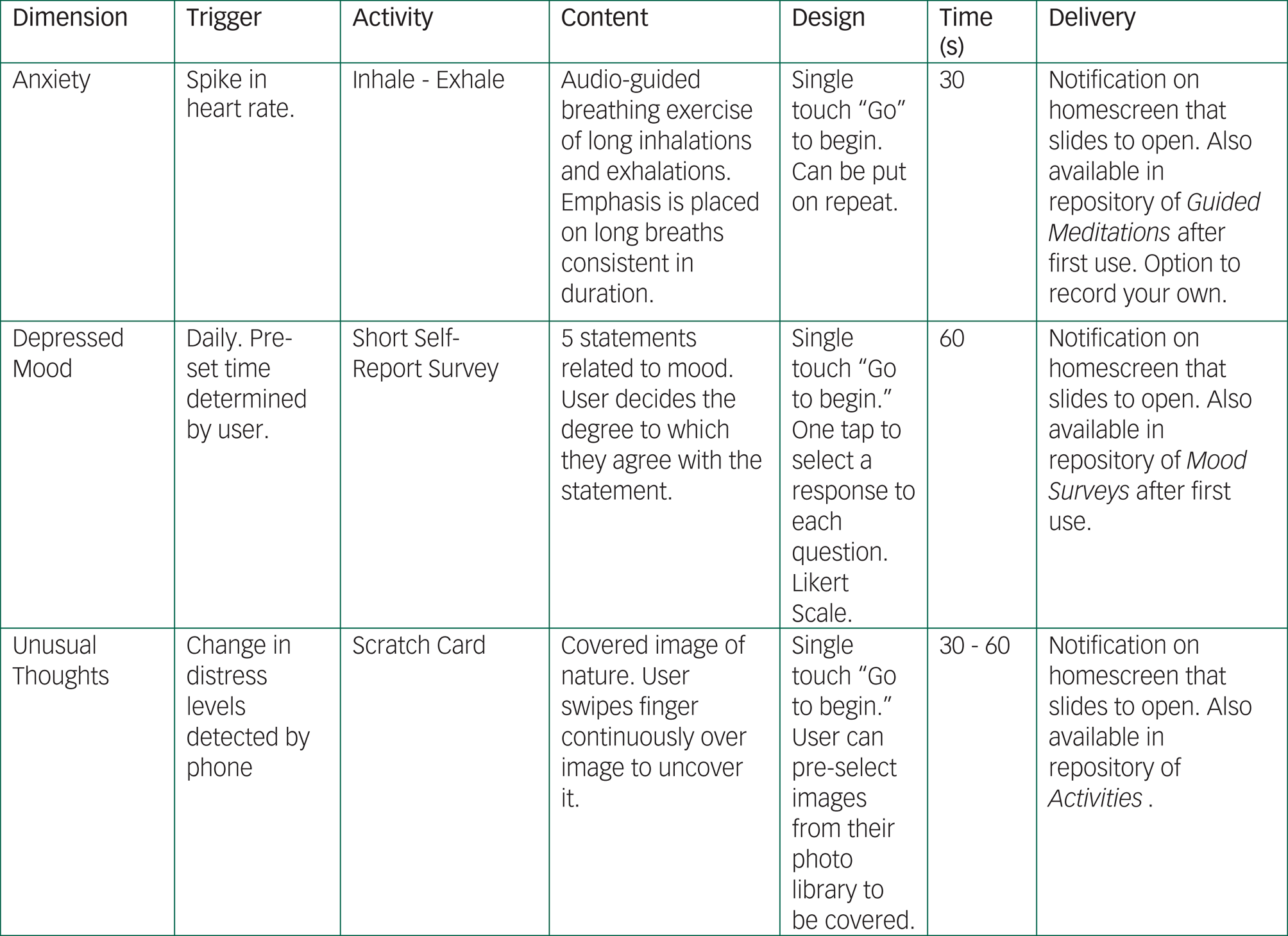
Fig. 4 mindLAMP offers patients psychoeducation and activities when abnormal behaviour or symptom reporting is detected. These featured are also available on command through the app.
Method
Setting
The study collaboration between BIDMC, NIMHANS, Sangath and AIIMS Bhopal brings together a diversity of clinical experiences spanning multiple sites in the USA and India. The international partnership ensures that study design, research methods and updates to LAMP are globally relevant; tailored to local culture, language and context; and useful across healthcare systems and regional settings.
The BIDMC, a Harvard Medical School affiliate, is a teaching hospital located in Boston, Massachusetts. The psychiatry department includes an in-patient unit with 25 beds, and offers out-patient services and consultation to approximately 5200 patients annually.
Sangath and the AIIMS Bhopal will be implementing this project in Bhopal, India. Sangath was founded in 1996, and is a leading health research, non-governmental organisation in India. Sangath's primary goal is to continuously innovate solutions to improve access to interventions, so that the treatment gap for mental disorders is significantly reduced. Sangath has led some of the largest population-based cohort studies and randomised controlled trials for mental disorders in the world, spanning adult mental health, severe mental disorders, child development, adolescent and youth mental health and addictions research. Since 2011, Sangath has been working closely with the health system in Madhya Pradesh, to advance research efforts aimed at implementing evidence-based mental health services in primary care settings.
AIIMS Bhopal is a premier institute of India, established through an Act of Parliament by the Ministry of Health & Family Welfare, Government of India, under the Pradhan Mantri Swasthya Suraksha Yojna. AIIMS Bhopal imparts both graduate and postgraduate medical education in all branches of healthcare activity, in addition to nursing and paramedical training. AIIMS Bhopal is a tertiary medical centre and a national centre of excellence in medical education, biomedical research and services delivery. In Madhya Pradesh, AIIMS Bhopal represents a leading regional institution for provision of psychiatric services and, specifically, treatment and management of schizophrenia spectrum disorders.
NIMHANS is located in Bengaluru, India, and is a tertiary care teaching facility that caters to about 400 out-patients per day, with a 600-bed psychiatric in-patient facility. The facility's schizophrenia population comprises a mix of individuals with first episode and chronic disease, coming from urban and surrounding rural localities, and individuals with difficult-to-treat symptoms, referred from across the country.
Study design
Phase 1: qualitative feedback and mindLAMP adaptation
The specific aim of phase 1 of the study is to involve patients, family members and clinicians in co-designing and adapting LAMP to meet their needs, and to account for contextual factors specific to each research site. Too often, technology solutions are created for patients and clinicians without their input, resulting in pervasive challenges in digital mental health, including low uptake and poor engagement.Reference Baumel, Muench, Edan and Kane9 Thus, in line with human-centred design philosophy, we are interested in understanding how clinicians and patients and their families may want to use the app, as well as identifying the features, visualisations and functionality that can make the app most appealing and relevant for use in their daily lives. For LAMP to be effective in predicting relapse and responding to patients who may be at risk, user engagement is essential because the collection of both active and passive data is necessary to inform risk prediction. A personalised approach, centred around the unique needs of users across distinct contexts and diverse settings, will guide the design of LAMP.Reference Yardley10 Phase 1 will help us build a global digital mental health platform that attracts and retains users to support regular engagement and participant activity. It will also inform what type of learning materials and psychoeducation resources could be created and offered via the app.
In this phase, each site (Boston, Bengaluru and Bhopal) will lead focus groups for three subsets of participants (i.e. individuals living with schizophrenia, their family members and clinicians). Insights from these focus groups will inform modifications to LAMP and creation of new content tailored to each study setting. Resulting adaptations to the app will be presented back to participants for them to evaluate and test, in light of their initial impressions and suggestions. The goal of this phase is to improve usability and functionality, as well as to ensure cultural relevance across sites by engaging users in discussion and developments.
Focus groups
Focus groups will be separated into two distinct sections: patients and their family members, and clinicians, with the potential for successive conversations that include all participants from both sections. Focus groups for both sections will include a demonstration of the mindLAMP app, discussion on preferences related to features and functions and a survey on smartphone access and use for participants. Each site will compile comments, feedback and observations from the focus group results and surveys into an initial report. The insights collected will be analysed by each site's research team and converted into a technical specification for app improvements. Participants will have the opportunity to test any improvements made to the app and offer additional comments in a follow-up focus group. Focus groups will follow an iterative design process that engages participants, research staff and developers in feedback, software building and testing (Figure 5).
Fig. 5 Focus group discussions collect insights that inform adaptions and updates to mindLAMP. Participants test the app's new features and share their feedback in subsequent focus groups.
There will be approximately a total of 135 participants recruited for the focus groups in the study. For the patient and family members focus groups, we will recruit a target of n = 25 at each site. For clinician focus groups, we will recruit a target of n = 20 at each site. All focus groups will be subdivided further into two sections no larger than n = 10, to facilitate engagement and encourage active participation. All focus group participants will give informed verbal consent to participate in phase 1. Research staff moderating the sections will be educated around mental illness and will also be made familiar with the LAMP technology. Focus group facilitators will undergo an additional training to equip them with interviewing skills and applicable design-thinking techniques.
Sample prompts for discussion include the questions, what kind of apps on your phone do you regularly use? Tell me about a recent time you deleted an app because you found it not usable? Why wasn't it usable? What do you think makes an app more or less usable? What information do you like having about yourself?
mindLAMP adaptations
Based on results of phase 1, improvements in functionality, content and design will be made by Zco Corporation over the course of 3 months. Changes to LAMP will be guided and overseen by the research team at BIDMC to ensure they reflect the insights collected from focus group participants at each study site. In keeping with the study's aim to build a scalable and shareable digital solution, the final code base of LAMP will be publicly posted online. This will ensure the reproducibility of the research and enable others to build and expand upon LAMP in the future.
Phase 2: observational LAMP study
The specific aim of phase 2 of the study is to assess the real-world use and effect size of the new and updated LAMP app in both predicting and preventing relapse. No prior research has measured the effectiveness, usability or feasibility of a mobile mental health app being used across different contexts, cultures and countries. This study will help answer critical questions around whether digital phenotyping captures universal signals of mental illness or more regional and cultural signals specific to the context at each site.
The 12-month observational study will be conducted independently at each of the research sites. At each site, we will recruit a target of 25 patients with schizophrenia (total of 75 participants). Patients’ diagnoses will be confirmed through clinician referrals. Patients with schizophrenia who consent to participate in-person – and meet eligibility criteria for the study including a basic understanding of smartphone functioning and language requirements – will be offered use of the mindLAMP app and a data-enabled smartwatch device for the duration of the study. Each site will also recruit 25 healthy participants with no current or past history of mental illness, to serve as a control group for the study. The healthy control participants will also be offered a generic version of the mindLAMP app as well as a data-enabled smartwatch to allow active and passive data collection. A total of 150 participants across all three sites will partake in five clinical assessments over the course of the study. Before beginning the first clinical assessment, participants will provide written informed consent. Study visits will help engage participants and capture any significant changes in symptoms and behaviour. Clinical measurements collected at each visit will include scales validated and commonly used internationally: the Positive and Negative Syndrome Scale for schizophrenia, to capture symptoms; the Patient Health Questionnaire-9, to capture depression; and the Generalized Anxiety Disorder-7, to capture anxiety.
Digital data collection and analysis
Over the course of months 0–12, the smartwatch device and the mindLAMP app will collect a combination of active and passive data. Active and passive data collection are described below.
Active data collection with surveys and cognitive tests
For the first month of the study, participants will be prompted twice a day to complete a basic survey on mindLAMP that asks them to report on their mood, medication, anxiety, sleep, social behaviours and psychosis. After the first month, participants will be prompted twice a week to complete these surveys.
mindLAMP will also offer cognitive tests that involve users tapping the screen to assess motor response, memory, executive functioning or attention. These cognitive tests appear like games and are designed to be engaging and simple to use (e.g. Trail Making Test). Participants will be prompted to play a game after they complete a survey. Games are also accessible on-demand to participants through the app.
Passive data collection based on participant mobility, activity and screen use metrics
Without participants actively engaging with the app, mindLAMP will capture metrics related to mobility by recording how far and how many unique locations a participant travels each day. This data will be derived from the smartphone GPS, but actual locations will not be stored. Instead, they will be converted into summary metrics that do not contain specific geolocation data, as shown in Figure 6 (blue boxes). mindLAMP can also capture metadata around phone use, as shown in Figure 6 (yellow boxes). Although both GPS and phone data are sensitive and private, the way they are captured, stored and used in this study ensures that nothing identifiable is used.
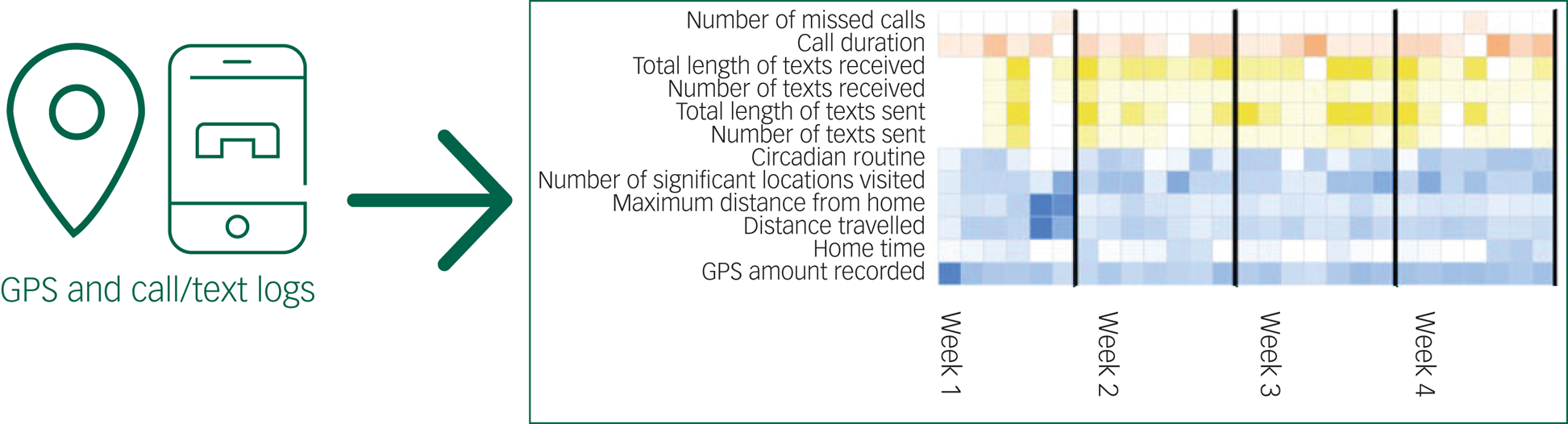
Fig. 6 mindLAMP protects privacy by ensuring that sensitive data is de-identified and summarised in aggregate, as shown with how GPS and call/text data is handled.
From both the smartwatch and smartphone accelerometer, LAMP will also measure step count, sleep data and heart rate variability. This data is not identifiable. Lastly, mindLAMP will track phone use, including metrics such as number of times a patient's smartphone is turned off or on per day.
At any time, participants can immediately opt out from passive data collection by turning the GPS feature off in the app, turning off the GPS feature on their phone or not wearing the smartwatch. Participants’ decision to opt out of passive data collection will not affect their participation in the overall study.
The primary analysis of digital data will focus on using anomaly detection methods to predict relapse at various antecedent time points to the actual event. Given the personalised nature of relapse prediction, model accuracy for each participant will be compared with a naïve predictor model that only takes the mean of that participant's digital phenotyping data, a static risk factor model for early-course schizophrenia that does not use any digital phenotyping data and a combined static risk factor and naïve model to represent digital phenotyping superimposed on baseline risk.
It is our hypothesis that metrics that deviate from a participant's baseline reports in active and passive data signal onset of relapse. Anomalies in symptoms, behaviours and physical activity will precede a deterioration in cognition and/or a psychotic episode. Aggregate findings related to relapse prediction will be emanated through publication after the study's completion.
Psychoeducation
The mindLAMP app will offer participants basic prompts containing psychoeducation, information, and helpful tips related to mental health in the local language of participants. Psychoeducation topics will be informed by focus group insights collected by research teams at each site during phase 1. This feedback will likely vary across sites, reflecting the unique needs of a site's context and their patient and clinician populations. The design of resources, therefore, will suit the local environment and cater to participants’ preferences at each study site location. However, the teams will ensure that participants have access to comparable resources by matching the type of resources in quantity, duration and frequency across sites.
At any time, participants can access on-demand, static information about medications, sleep, social skills and mindfulness. If passive or active data collected from a participant shows unusual patterns or differs from their baseline activity, then the mindLAMP app will provide a prompt containing relevant psychoeducation. For example, if a participant is showing lower mobility metrics than their baseline, the mindLAMP app might offer a brief article on how higher step count can improve mood or provide a prompt to encourage activity and other healthy lifestyle behaviours. Although this is an observational study to assess if the smartphone data outlined above can help predict relapse, offering participants information and helpful tips that are relevant to their behaviour and activity will likely increase engagement with the app and encourage participants’ interest and agency in their own recovery. There is also robust evidence showing that encouraging positive lifestyle behaviours related to activity, sleep and healthy eating can contribute to improved well-being and better mood among patients living with schizophrenia.
Ethical approval
Ethical approval for SHARP will be obtained independently from each site's institutional review boards. BIDMC and partners Sangath, AIIMS Bhopal and NIMHANS have well-constituted ethics review boards whose operations are guided by standard operating procedures and national applicable guidelines, and have overseen many study proposals involving use of novel digital technologies or treatment of complex mental disorders.
A joint application from both Indian sites has been submitted to India's Health Ministry Screening Committee with secretariat at the Indian Council of Medical Research for clearance. The final clearance will be given after institutional ethics committee approvals are obtained from all sites.
This study includes participants who suffer from serious mental illness. Our research team is cognisant that this population is considered a vulnerable patient group and is more likely to experience prejudices, social disadvantages and poverty relative to the general population in both the USA and India. To ensure the comfort and safety of study participants, focus groups and clinical assessments will be conducted in private rooms by study staff with clinical training and exposure, with supportive referral protocols in place where required. Insights and data collected from participants during phases 1 and 2 will be anonymous to maintain patient confidentiality and protect privacy. LAMP does not record or store any private identity information. Instead, it assigns participants a randomly generated user identification, and the data collected by mindLAMP remain on the smartphone only until it can be automatically transferred to a secure local server. All data transmission is encrypted and LAMP accounts are password protected to safeguard participant activity. LAMP features technical safeguards to protect against unauthorised access, alteration, disclosure or destruction of user data.
Conclusions
Schizophrenia spectrum disorders have devastating impact on individuals, their families and society. In both the USA and India, few individuals living with schizophrenia have access to quality mental healthcare. Emerging digital technologies, such as the mindLAMP smartphone app, hold promise for ensuring that more timely, personalised and high-quality treatment is available. This international effort, spanning sites in the USA and India, is a blueprint for conducting rigorous mental health research across multiple countries, cultures and contexts. The collaborative effort is poised to break new ground in advancing the early detection, prediction and prevention of relapse in patients living with schizophrenia. The findings from this study will highlight the potential for digital mental health tools to inform and contribute to meaningful clinical care in both low-income and higher-income settings. By engaging diverse stakeholder groups in the design and testing of a digital mental health solution in real-world clinical settings, this research offers an important contribution to the field of digital psychiatry.
Funding
This work was supported by the Wellcome Trust UK (grant no. 215843/Z/19/Z).
Acknowledgements
The authors would like to thank the many people who have helped test and provide input into the LAMP app. Without their efforts and feedback, this work would not have been possible. The authors would also like to thank Kate Steward and Amanda DeJesus of BIDMC, for their support in advancing this project.
Author contributions
J. Torous conceived the study and all authors helped in the creation of the study at an in-person meeting in Bhopal, India. E.R.-V., J. Torous and J.N. wrote the initial draft based on input from all authors. All authors edited this draft and reviewed the final version of the paper.
Declaration of interest
None. J. Torous reports unrelated research support from Otsuka.










eLetters
No eLetters have been published for this article.