First developed over 80 years ago, electroconvulsive therapy (ECT) is an evidence-based, essential medical procedure. This brain stimulation treatment involves passing an electrical charge through the brain to induce a generalised seizure lasting about 30 s, under controlled conditions, using brief general anaesthesia along with a muscle relaxant and continuous oxygenation. Usually, 8–12 treatments are administered in a course, two or three times per week. ECT is an acutely effective treatment for resistant, severe and sometimes life-threatening depressive episodes, in both unipolar and bipolar disorders, as well as catatonia and treatment-resistant mania. These specific indications for ECT have been approved by the National Institute for Health and Care Excellence in the UK. ECT can also be used as a maintenance therapy to prevent relapse after successful treatmentReference Elias, Phutane, Clarke and Prudic1 and, beyond the scope of this analysis, there is meta-analytical evidence to support its use for treatment-resistant schizophrenia.Reference Sinclair, Zhao, Qi, Nyakyoma, Kwong and Adams2
The USA Food and Drug Administration (FDA) completed a comprehensive review of ECT as part of its reclassification of ECT devices in 2018 (https://www.federalregister.gov/documents/2018/12/26/2018-27809/neurological-devices-reclassification-of-electroconvulsive-therapy-devices-effective-date-of). They considered all of the scientific data and more than 3400 submissions, and concluded that ECT is ‘safe and effective’ and that further trials are not needed to confirm this for patients with severe depression and catatonia. The FDA consequently reclassified ECT devices from class III (highest risk) to class II (moderate risk, requiring ‘special controls’) for these indications, but not for use in schizophrenia and mania, where the FDA required additional studies despite the fact that, globally, schizophrenia and related disorders are probably the main indications for ECT.Reference Leiknes, Jarosh-von Schweder and Hoie3 Additionally, because of its robust evidence base and global clinical experience, ECT is routinely incorporated into, and endorsed by, international professional treatment guidelines for mood disorders.
About 1.4 million people worldwide are treated annually with ECT, with treatment-resistant depression being the most common indication in Western industrialised nations.Reference Leiknes, Jarosh-von Schweder and Hoie3 In England the annual treated person rate is 0.43 per 10 000 population.Reference Buley, Copland, Hodge and Chaplin4 ECT is the most reliably effective procedure for severe depression, and exerts its effect within a few weeks. Most people who receive ECT see an improvement in their condition. Clinical Global Impression scores collected by the Royal College of Psychiatrists’ ECT Accreditation Service on 1527 persons treated with a course of ECT in England in 2015, showed that about 31% were ‘very much improved’ and another 43% were ‘much improved’.Reference Buley, Copland, Hodge and Chaplin4 Remission rates reported in modern ECT randomised clinical trials are 52%,Reference Kolshus, Jelovac and McLoughlin5 and up to 75% in large, open-label studies.Reference Husain, Rush, Fink, Knapp, Petrides and Rummans6
These are remarkable results, considering that the majority of patients with depression referred for ECT are treatment-resistant and have failed to benefit from two or more antidepressant drugs with or without psychotherapies. Unfortunately, ECT is often used after prolonged and repeated treatment failures and is mistakenly viewed as a ‘treatment of last resort’. We rarely see this phenomenon in other branches of medicine, where the most rapidly acting and most effective treatment is consigned to the very end of the list of treatment options. In contrast, a recent economic analysis from the USA found that ECT is cost-effective and probably should be considered sooner in treatment algorithms, i.e. after failure of only two or more trials of antidepressants or psychotherapy.Reference Ross, Zivin and Maixner7
Optimising ECT for depression
Nearly 20 years ago, in 2003, the UK ECT Review Group performed a rigorous systematic review and meta-analysis of real versus sham ECT trials. They identified six relevant trials from the 1960s to the 1980s that used now outmoded forms of ECT. Analysis of these historical trials demonstrated real ECT was more effective than sham ECT, with a large standardised effect size of –0.91 (95% CI –1.27 to –0.54).8 It is worth noting that no major sham-controlled trials of ECT for depression have taken place since this meta-analysis and, for obvious ethical reasons, it is unlikely that there will be or that this could be justified. Also of note, the Review Group found that ECT was considerably more effective than antidepressant drugs in treating depression (effect size –0.80, 95% CI –1.29 to –0.29).8
Not surprisingly, ECT practice has evolved considerably since the 1980s. Contemporary randomised controlled trials have focused on reducing side-effects while maintaining effectiveness. Originally, ECT was delivered via a sine-wave electrical stimulus with a long pulse width (8.3 ms; Fig. 1a). This produced substantial cognitive side-effects and was replaced in the 1980s by a more efficient square-wave brief pulse (0.5–1.5 ms) stimulus. This reduced side-effects but maintained efficacy. A more recent ECT refinement is using an ultrabrief pulse (0.25–0.3 ms) stimulus, which is closer to the neuronal chronaxie (a measure of the stimulus duration required for neuronal discharge, i.e. 0.1–0.2 ms). Ultrabrief pulse ECT has advantages in terms of cognitive side-effects, but may not be as effective in treating depression as brief pulse ECT.Reference Tor, Bautovich, Wang, Martin, Harvey and Loo9 Most recently, there has been renewed interest in reducing the electrical stimulus pulse amplitude (usually fixed at 800–900 mA) to 500–700 mA, to reduce the size of the electrical field induced by ECT. Such forms of low-amplitude ECT may result in fewer cognitive side-effects, although clinical effectiveness needs to be evaluated and optimised.Reference Abbott, Quinn, Miller, Ye, Iqbal and Lloyd10
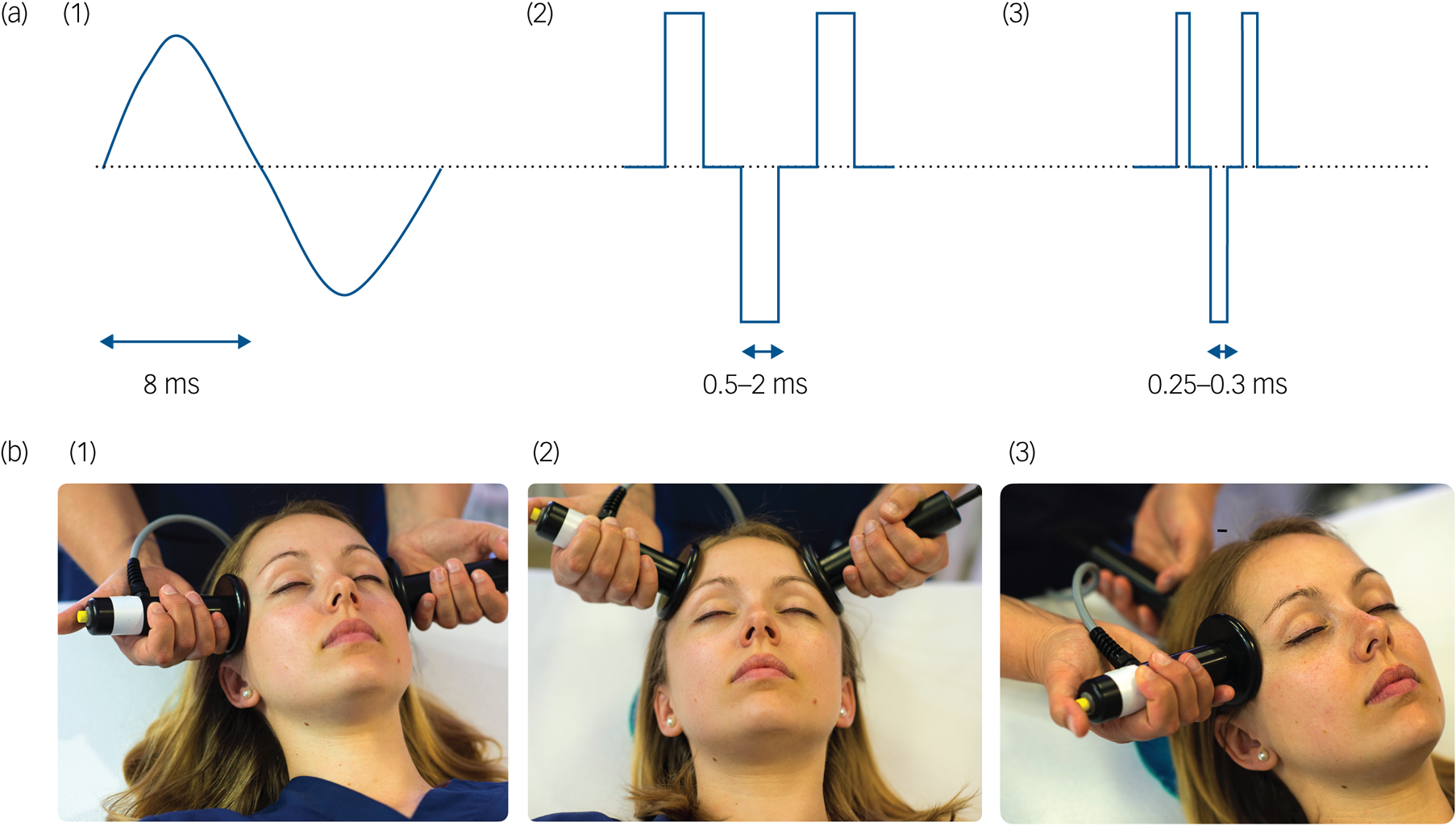
Fig. 1 Technical parameters in electroconvulsive therapy: stimulus waveforms and electrode placement. (a) (1) Sine-wave, (2) brief pulse and (3) ultrabrief pulse stimulus wave forms. (b) (1) Bitemporal, (2) bifrontal and (3) unilateral electrode placements.
Electrode placement also affects the efficacy and side-effect profile of ECT. Bitemporal electrode placement is the most commonly used worldwide (Fig. 1b).Reference Leiknes, Jarosh-von Schweder and Hoie3 Right unilateral (i.e. non-dominant hemisphere) placement was developed in an attempt to minimise cognitive side-effects. It used to be regarded as less effective than bitemporal ECT.8 However, randomised trials have since shown that right unilateral ECT given at a high dose (i.e. six times the seizure threshold, the minimum electrical stimulus charge required to induce a generalised seizure) is as effective as moderate-dose (i.e. 1.5 times seizure threshold) bitemporal ECT, and has cognitive advantages regarding recovery of orientation and autobiographical memory.Reference Kolshus, Jelovac and McLoughlin5 Another form of bilateral ECT uses a bifrontal electrode placement, but this does not appear to be associated with major clinical advantages.Reference Dunne and McLoughlin11 Some recent small, open-label trials indicate that further cognitive advantages may be derived from an experimental technique called focal electrically administered seizure therapy (FEAST). This uses a novel asymmetric electrode configuration, coupled with a unidirectional (posterior to anterior) current flow, that targets seizure initiation within the right prefrontal cortex.Reference Sahlem, McCall, Short, Rosenquist, Fox and Youssef12 As with any new development, large-scale randomised and adequately powered studies are required to establish a role for FEAST in clinical practice.
A wide range of non-surgical brain stimulation treatments for depression has been developed in an attempt to find treatments with fewer side-effects. These have recently been evaluated by a network meta-analysis that included ECT, various forms of repetitive transcranial magnetic stimulation (rTMS), theta burst stimulation, magnetic seizure therapy and transcranial direct current stimulation.Reference Mutz, Vipulananthan, Carter, Hurlemann, Fu and Young13 Bitemporal and high-dose right unilateral ECT showed the strongest effect and achieved the highest rankings. Compared with sham therapy, the odds ratio for response rates was 8.91 (95% CI 2.57–30.91) for bitemporal ECT and 7.27 (95% CI 1.9–27.78) for unilateral ECT.Reference Mutz, Vipulananthan, Carter, Hurlemann, Fu and Young13 These findings are underlined by a previous meta-analysis of trials comparing various forms of rTMS and ECT for depression, in which ECT was found to have a cumulative probability of 65% for being the most efficacious therapy, compared with only 25% for bilateral rTMS, the most efficacious of the studied rTMS modalities.Reference Chen, Zhao, Liu, Fan and Xie14
To summarise and place the trial and meta-analytical evidence in a routine clinical context, three different forms of ECT are now commonly used but differ in efficacy and cognitive side-effects, depending upon stimulus pulse width, electrical dose and electrode placement. In terms of average efficacy of ECT, ultrabrief pulse high-dose unilateral ECT is less efficacious than brief pulse high-dose unilateral ECT, which is similar to brief pulse bitemporal ECT, although the latter has a possibly faster onset of action.Reference Kellner, Knapp, Husain, Rasmussen, Sampson and Cullum15 Ultrabrief pulse high-dose unilateral ECT has fewer cognitive side-effects than brief pulse high-dose unilateral ECT, which in turn affects cognition less than brief pulse bitemporal ECT. In terms of antidepressant efficacy, ECT is superior to other brain stimulation therapies and antidepressant drugs. It is therefore possible to inform patient choice and tailor ECT to the individual patient, regarding effectiveness and side-effect profile.
Safety and side-effects of ECT
ECT is a serious treatment for serious illness. As with all effective medical procedures, ECT has adverse effects. These vary in severity, need to be weighed against benefits of treatment and should be fully discussed with patients. Adverse effects also have to be considered in the background of side-effects caused by medications and the devastating effects of unresolved severe mental illness.
For a treatment that involves general anaesthesia and seizure induction, ECT is remarkably safe and well tolerated. Common physical side-effects experienced by patients include nausea (12–16%), muscle pain (9–12%) and headache (26–28%).Reference Semkovska, Landau, Dunne, Kolshus, Kavanagh and Jelovac16 These are usually temporary and mild and, if required, can be symptomatically managed with antiemetics and simple analgesics. Cardiovascular changes that occur during ECT (e.g. vagally mediated bradycardia and asystole, tachycardia and hypertension) are typically self-limiting and uneventful, and are managed as part of routine anaesthetic care. Mortality is extremely low, with a recent pooled analysis estimating 2.1 deaths per 100 000 treatments.Reference Torring, Sanghani, Petrides, Kellner and Ostergaard17 In fact, ECT is associated with reduced all-cause mortality, possibly because of patient selection and the medical attention provided to patients referred for ECT.Reference Jørgensen, Rozing, Kellner and Osler18 Despite occasional sensationalist reports in the general media, there is no credible evidence that ECT results in brain damage at the cellular or macroscopic level. For example, recent large registry studies have shown that ECT does not increase the risk for dementia or stroke.Reference Osler, Rozing, Christensen, Andersen and Jorgensen19,Reference Rozing, Jorgensen and Osler20
The side-effects of most concern relate to the effects of ECT on cognition. Comparing standardised assessments before and after a course of ECT, the cognitive domains most affected include short-term memory (e.g. delayed verbal recall effect size −1.12, 95% CI −1.29 to −0.95) and executive function (e.g. Trail Making Test B effect size −1.10, 95% CI −1.53 to −0.67; letter fluency effect size −0.79, 95% CI −0.96 to −0.63).Reference Semkovska and McLoughlin21 Of course, interindividual differences occur, with some patients performing worse, others improving and some remaining unchanged. Importantly, at the overall group level, these objectively measured deficits resolve within weeks, by which time most performance measures improve when compared with before ECT.
However, the impact of ECT on retrospective autobiographical memory is less clear and more challenging to quantify.Reference Semkovska and McLoughlin22 For example, the normal loss of consistency in recall of autobiographical memories is 25–40% over 1.5–3.0 months. Loss of recall consistency in modern ECT trials is within this range, although it is clear that bitemporal ECT has more pronounced effects than other forms of ECT.Reference Kolshus, Jelovac and McLoughlin5,Reference Tor, Bautovich, Wang, Martin, Harvey and Loo9 To further complicate the issue, depression itself is well-known to cause ‘overgeneralisation’ in autobiographical memory, especially episodic memory. Additionally, objective measures of cognition may not correlate with subjective reports in patients with depression. Nonetheless, in a recent Swedish national register-based study (n = 1212 patients), 26% reported subjective memory worsening after a course of ECT.Reference Brus, Nordanskog, Båve, Cao, Hammar and Landén23 Risk seemed to be associated with female gender, younger age, fewer subjective memory problems before ECT, non-remission status and use of a brief pulse rather than ultrabrief pulse electrical stimulus. At the current time, it is impossible to predict who will experience cognitive impairment, and it is therefore important to monitor both objective and subjective cognitive function before and throughout the course of ECT, to allow the treatment to be adjusted to minimise any cognitive side-effects.
Getting better and staying well
Although ECT can be considered a first-line and relatively rapid treatment for psychotic depression, the utility of other clinical features in predicting outcomes with ECT is less clear. The most recent systematic review and meta-analysis of predictive clinical factors found that the presence of psychosis (odds ratio 1.47, 95% CI 1.16–1.85) and, to a much lesser extent, older age (standard mean difference 0.26, 95% CI 0.13–0.38), predicted remission with ECT.Reference van Diermen, van den Ameele, Kamperman, Sabbe, Vermeulen and Schrijvers24 The mean difference in age between ECT remitters and non-remitters was only 4.3 years. Of note, the presence of melancholia did not significantly predict remission or response following ECT; however, greater overall depression severity modestly predicted response (standard mean difference 0.19, 95% CI 0.07–0.31), but not remission. Psychomotor disturbances that are core features of both melancholia and catatonia, such as agitation and retardation, may mediate the predictive value of illness severity. Treatment resistance and longer duration of illness appear to be associated with somewhat poorer outcome with ECT, although outcomes are still better than with pharmacotherapy.
Although the main aims of treating depression are achieving and maintaining remission, ECT is typically used as an acute, limited course of treatment. Perhaps not surprisingly, ECT is often criticised for having a high relapse rate. However, the meta-analytical evidence is that relapse rates after a successful course of ECT, and continuing afterward on antidepressants, are similar to those after successful treatment of resistant depression with only antidepressants (i.e. 27.1% after 3 months, 37.7% after 6 months and 51.1% after 12 months).Reference Jelovac, Kolshus and McLoughlin25 Thus relapse following ECT reflects the nature of the underlying depressive illness. Relapse rates are probably better for patients who do not discontinue antidepressant drug treatment during the ECT course. Importantly, the same meta-analysis also showed that not being on continuation antidepressant drug therapy doubles the relapse rate at 6 months to 78%.Reference Jelovac, Kolshus and McLoughlin25 It is therefore essential for patients to be taking some form of continuation therapy after ECT, although the optimal form of this is not yet known.
Unlike antidepressant pharmacotherapy or psychotherapy, ECT is often abruptly discontinued once the patient's symptoms have fully improved or, worse, is stopped prematurely when signs of improvement are only beginning to appear. There is an emerging evidence base to support a role for tapered (rather than abruptly stopped) acute ECT courses, continuation ECT to maintain recovery and longer-term maintenance ECT to prevent relapse.Reference Elias, Phutane, Clarke and Prudic1 This might well be expected when one considers that for some people with resistant depression, ECT has been the only treatment that has appreciably worked for them. This is illustrated in the randomised phase of the Prolonging Remission in Depressed Elderly trial, involving older (≥60 years) adults who had achieved remission with an acute course of ultrabrief pulse high-dose unilateral ECT (n = 120).Reference Kellner, Husain, Knapp, McCall, Petrides and Rudorfer26 Patients allocated to continuation ECT (initially once weekly for 4 weeks, followed by an adaptive flexible schedule) plus pharmacotherapy (venlafaxine and lithium) had a lower relapse rate (13.1%) over 24 weeks than those in the pharmacotherapy alone arm (20.3%; odds ratio 1.7, 95% CI 0.6–4.5). ECT practice need not be rigid, and courses of ECT should be sufficiently flexible to meet the needs of the individual patient, regarding effectiveness, side-effects and maintaining well-being.
The future of ECT
It is astonishing that, after more than 80 years, no other treatment for depression has been developed that is the equivalent of ECT. Despite this, ECT is difficult to access because of a combination of factors, including limited availability, lack of professional training in modern ECT techniques, misinformation campaigns, stigma and genuine concerns about effects on cognition. Another issue is that, like many effective medical treatments, the precise mechanism of ECT is not yet fully unravelled. Preclinical (including sham-controlled rodent and primate studies) and translational research increasingly support a central role for neuroplastic mechanisms in ECT.Reference Bouckaert, Sienaert, Obbels, Dols, Vandenbulcke and Stek27 In a reverse technology fashion, understanding the molecular and cellular mechanisms of action of ECT will also contribute to our knowledge of the biology of depression itself, and aid the development of novel therapies. The formation of new international consortia to perform large-scale neuroimaging and genomics studies of ECT (e.g. GEMRIC,Reference Ousdal, Argyelan, Narr, Abbott, Wade and Vandenbulcke28 Gen-ECT-icReference Soda, McLoughlin, Clark, Oltedal, Kessler and Haavik29) are welcome developments in this endeavour, reflecting the resurgence of interest in the neurobiology of ECT and an ever-evolving future for what continues to be one of the most effective treatments we have for some of our most seriously ill patients.
Author contributions
G.K. and D.M.M. contributed to the conception of this article. D.M.M. wrote the first draft. P.S. produced the figure. All authors revised the paper and approved the final submission.
Declaration of interest
G.K. has no interests to declare. S.J. is funded by a John, Margaret, Albert and Steward Sim Fellowship Sim Scholarship from the Royal College of Physicians (Edinburgh) and National Institute for Health Research Biomedical Research Centre at South London and Maudsley National Health Service Foundation Trust and King's College London. S.J. has received honoraria for educational talks given for Sunovian and King's College London, and has received funding for educational talks given for Lundbeck. P.S. has received research funding from the Fonds Wetenschappelijk Onderzoek (Fund for Scientific Research, Flanders, Belgium) for research on ECT. C.H.K. has received ECT research funding support from the National Institute of Mental Health (USA), fees from UpToDate and Psychiatric Times for ECT articles, and royalties from Cambridge University Press for an ECT textbook. D.M.M. has received brain stimulation research grants from the NHS Health Technology Assessment Programme (UK), National Association for Research on Schizophrenia and Affective disorders (USA) and the Health Research Board (Ireland); and received speaker's honoraria from MECTA and Otsuka, and an honorarium from Janssen for participating in an esketamine advisory board meeting.





eLetters
No eLetters have been published for this article.