INTRODUCTION
Campylobacter spp. are ubiquitous foodborne pathogens [Reference Silva1], and among the most common cause of bacterial gastroenteritis in low-, middle- and high-income countries [2, Reference Coker3]. The global distribution of Campylobacter is attributed to its universal ability to colonize and form part of the commensal flora of a wide range of species including poultry [Reference Hermans4], pigs [Reference Fosse, Seegers and Magras5] and cattle [Reference Chatre6]. The epidemiology of symptomatic Campylobacter infections in humans reflects the complexity and variability of sources of Campylobacter. Risk factors for infection include the consumption of undercooked meat, unpasteurized dairy products or contaminated drinking water, and direct contact with farm animals [Reference Humphrey, O'Brien and Madsen7–Reference Domingues9]. Of all Campylobacter spp., Campylobacter jejuni and Campylobacter coli are the two most common causes of human infection [Reference Sheppard10]. Globally, C. jejuni is more prevalent in poultry, whereas C. coli is more common in pigs [Reference Fosse, Seegers and Magras5, Reference Horrocks11, 12]. Poultry meat is considered to be the primary source of human Campylobacter infection; this is particularly well established for C. jejuni [Reference Humphrey, O'Brien and Madsen7, 12–Reference Adzitey, Huda and Ali14].
Diarrhoeal disease is common in Vietnam; community-based studies indicate a moderately high burden in children aged <5 years (about 1–3 cases/child per year), which is higher than typical levels from developed countries [Reference Isenbarger15, Reference Hien16]. Campylobacter is likely to play a significant role, with some aetiological investigations indicating that Campylobacter may be responsible for 2–7% of cases of gastroenteritis in children aged <5 years [Reference Isenbarger15, Reference Bodhidatta17]. Notably, and in common with other developing countries, unpublished data suggests that Campylobacter-induced diarrhoea is relatively rare in adult populations [Reference Coker3]. Limited data on species identification on 88 isolates from cases of clinical diarrhoea from 1996 to 1999 indicate that 85% were C. jejuni, and the remainder C. coli [Reference Isenbarger15]. Previous surveys of fresh chicken samples collected from retail markets and canteens in Vietnam have indicated a Campylobacter prevalence of 28–31% [Reference Ha and Pham18, Reference Luu19]. However, none of 75 pork and beef samples collected from the same sites tested positive for Campylobacter [Reference Ha and Pham18].
To date there are no published studies regarding the prevalence or distribution of Campylobacter spp. in animal reservoirs in Vietnam, where mixed species farming occurs with low levels of biosecurity. We aimed to determine the prevalence of Campylobacter in the main species farmed in the Mekong delta of Vietnam by surveying 343 pigs and poultry farms in Dong Thap province. Risk factors that may explain the presence of Campylobacter at the individual-animal level were investigated, and the isolated organisms were genotyped and evaluated for antimicrobial susceptibility to determine their circulation and potential threat to human health.
MATERIALS AND METHODS
Study location
The survey was conducted between February and May 2012 in Dong Thap province, Vietnam, a rural province located in the Mekong delta between latitude 10° 58′ 01·70″ and 10° 07′ 36·56″ N and longitude 105° 56′ 47·22″ and 105° 11′ 16·30″. Covering an area of 3238 km2, the province is home to about 1·6 million people, 3·1 million ducks, 1·3 million chickens, 274 000pigs, and 22 000 cattle and buffalo. The study included 4/12 districts (Cao Lanh, Chau Thanh, Hong Ngu, Thanh Binh) (Fig. 1).

Fig. 1. The geographical distribution of chicken, duck and pig farms and human population density in Dong Thap province, Mekong delta, Vietnam. Maps showing Dong Thap province (total area), highlighting the four study districts (Hong Ngu, Thanh Binh, Cao Lanh, Chau Thanh) and communes (smallest shaded areas). (a) The density of chicken farms (per km2, as shown by shading in key) and the number of farms sampled per commune (numeral within shaded area). (b) The density of duck farms (per km2, as shown by shading in key) and the number of farms sampled per commune (numeral within shaded area). (c) The density of pig farms (per km2, as shown by shading in key) and the number of farms sampled per commune (numeral within shaded area). (d) Human population density per km2, as shown by shading in key).
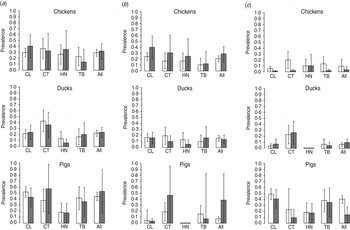
Fig. 2. The prevalence of Campylobacter by district in Dong Thap province, Mekong delta, Vietnam. Bar charts showing the unadjusted (white bars) and adjusted (grey bars) prevalence of (a) Campylobacter spp., (b) C. jejuni and (c) C. coli in chickens, ducks and pigs in Cao Lanh (CL), Chau Thanh (CT), Hong Nhu (HN) and Thanh Binh (TB) districts in Dong Thap province.
Survey design
We aimed to collect samples from 612 animals from 306 clusters (farms) (i.e. two animals per farm). Such sample size would theoretically allow the detection of an overall 35% prevalence with an estimated relative error of 10% and 80% confidence level, assuming a design effect of 2 (i.e. low-level clustering). Based on a farm census provided by the Dong Thap Sub-Department of Animal Health (sDAH), farms were stratified by district (X = 4), animal species (Y = 3; chickens, ducks, pigs) and size (Z = 3; small, medium, large). The cut-offs for animal numbers that determined farm size were as follows: for chickens 20–50 (small), 51–100 (medium) and >100 (large); for ducks 50–200 (small); 201–1000 (medium) and >1000 (large); and for pigs 5–20 (small), 21–50 (medium) and >50 (large). Each farm was defined by the species of animal that represented the highest source of income to the farmer, and was included in only one species category. The total number of strata was, therefore, X*Y*Z = 36. We aimed to sample ten randomly selected farms from each stratum and each farm was sampled on a single occasion. From each farm faecal samples from 1–3 individual animals were collected, except from a subset of 20 farms in Cao Lanh district, where more intensive sampling was performed (10–14 animals per farm). About 5 g freshly voided faeces were aseptically collected from each animal and placed immediately into sterile 25 ml universal tubes. Samples were kept at 4°C and were transported to the laboratory within 24 h, where culture was immediately initiated.
Laboratory methods
Fresh faecal samples were incubated on Campylobacter selective agar (Blood Free with CCDA supplement) (Oxoid Ltd, UK) for 48 h at 42 °C within a gas jar with microaerophilic conditions using CampyGen gas packs (Oxoid Ltd, UK). Colonies from these plates were screened by Gram stain for Gram-negative Vibrio-like bacteria. Differentiation between C. jejuni and C. coli/C. lari was done by hypurate test [Reference Hwang and Ederer20] and by PCR (see below). All isolates were antimicrobial susceptibility tested against ampicillin, chloramphenicol, gentamycin, sulfamethoxazole–trimethoprim, nalidixic acid, ofloxacin, ciprofloxacin, clindamycin and erythromycin using the Kirby–Bauer (disk diffusion) method on Muller–Hinton Agar (Oxoid, UK) and interpreted using CLSI guidelines for Enterobacteriaceae (www.clsi.org).
Molecular speciation and multi locus sequence typing (MLST)
C. coli and C. jejuni were distinguished using PCR primers targeting the hipuricase gene (hipO) for C. jejeni and the aspartokinase gene (asp) for C. coli as described previously [Reference Linton21, Reference Persson and Olsen22]. MLST was performed on DNA extracted using the wizard genomic DNA extraction kit (Promega, USA) from one full loop of pure Campylobacter colonies of overnight culture in blood agar media. PCR amplification was performed on the seven MLST loci [aspA (aspartase), glnA (glutamine synthetase), gltA (citrate synthase), glyA (serine hydroxy methyl transferase), tkt (transketolase), pgm (phospho glucomutase) and uncA (ATP synthase alpha subunit)], as described previously [Reference Dingle23]. PCR products were purified using QIAquick column-based PCR purification system (Qiagen) and sequenced in forward and reverse directions using the Big Dye cycle sequencing kit (Applied Biosystems, USA) on an ABI 3770 automatic sequencer according to the manufacturer's instructions. Minimum spanning trees were created from the resulting MLST data using bionumerics (Applied Mathematics, Belgium).
Statistical analyses
Separate analyses were performed for chickens, ducks and pigs. District- and species-specific estimates of prevalence were calculated before and after adjusting for the sampling frame that implied a known (but non-random) probability of an animal being selected. This was performed by assigning a sample weight (1/W) to each observation [Reference Dohoo, Martyn and Stryhn24], where W = P(S) × P(F) × P(A), and P(S) = probability of a stratum being selected (since the size strata were determined arbitrarily); P(F) = probability of a farm being selected from each stratum (since strata contained different numbers of farms); P(A) = probability of an animal being selected from a selected farm (since a variable number of animals were sampled from each farm).
Clustering of infection within farms was investigated using the subset of samples (N = 244) from intensively sampled farms (N = 20) in Cao Lanh district. The intra-class correlation coefficient (ICC) was calculated separately for each host species (chickens, ducks, pigs) for Campylobacter spp., C. jejuni and C. coli using a one-way random-effects model [Reference Golstein, Browne and Rasbash25].
Survey data on animal and farm characteristics were collected using validated questionnaires and entered into an Access database (Microsoft, USA). To explore potential risk factors for Campylobacter infection, two-level random-effects logistic regression models (animals nested within farms) were built to account for the variable numbers of animals sampled from each farm [Reference Dohoo, Martyn and Stryhn24]. The outcomes modelled were: all Campylobacter spp., C. jejuni, and C. coli. Models were built separately for each animal species and outcome (total nine models). Variables investigated as potential risk factors included farm-level descriptive management variables (number of flocks, number of animals, type of water, use of antibiotics), density of farms and humans at the commune level, district and other individual animal-level variables (age, presence of diarrhoea). Variables were considered as candidates for multivariable analysis based on their biological plausibility and a P value <0·15 in the univariable analysis. Candidate variables were ranked by their degree of significance and were included in the model starting with the most significant ones using a step-wise forward approach [Reference Hosmer and Lemeshow26]. In the final multivariable model for each species, variables were retained if their P value was <0·05 for any of the three outcome variables. The suitability of each new variable included in the model was assessed using Akaike's Information Criterion [Reference Burnham and Anderson27]. All statistical analyses were performed using R (packages Epicalc and EpiR; http://www.r-project.org). Random-effects logistic regression modelling was performed using the lme4 package. Maps were produced using Quantum GIS v. 1·7·4 (http://qgis.osgeo.org).
RESULTS
Baseline farm data
A total of 343 farms distributed across Dong Thap province were sampled (Fig. 1), and 634 faecal specimens were collected and tested for the presence of Campylobacter. The number of samples collected and analysed from each host species (pig, chicken, duck) and the number of farms sampled are summarized in Supplementary Table S1 (available online). The distribution of the farms sampled by district within Dong Thap province, and the underlying farm density and human population density, are shown in Figure 1. The district of Chau Thanh contained over 70% of the pig production in the study area. In two of the four surveyed districts, there were few pig farms in the large size stratum, and all farms were sampled. Notably, the sampling fraction was considerably higher in the larger farms. Across the study area chicken farms had the greatest density (37·9 farms/km2) followed by duck farms (12·5 farms/km2) and pig farms (1·3 farms/km2). The density of pig farms was greatest in Chau Thanh district (3·6 farms/km2) and lowest in Thanh Binh district (0·5 farms/km2) (Table 1).
Table 1. Human population and farm statistics (per km2) for four districts of Dong Thap province, Mekong delta, Vietnam

IQR, Interquartile range.
Adjusted estimates of Campylobacter prevalence
A total of 207 isolates were recovered from 202 Campylobacter-positive animals (five pigs tested positive for both C. jejuni and C. coli). Two isolates (1%) could not be identified as either C. jejuni or C. coli. The overall unadjusted prevalence of Campylobacter from faecal specimens of chickens, ducks and pigs was 29·4%, 22·5% and 44·9%, respectively. The adjusted prevalence was 31·9% [95% confidence interval (CI) 19·0–44·8] in chickens, 23·9% (95% CI 15·1–32·7) in ducks, and 53·7% (95% CI 17·6–89·7) in pigs. The adjusted prevalence of C. jejuni in chickens, ducks and pigs was 28·4%, 13·8% and 38·6%, respectively, whereas the adjusted prevalence of C. coli in chickens, ducks and pigs was 3·5%, 9·7% and 14·1%, respectively (Fig. 2). In chickens, the highest crude prevalence (45%) was reported in the 10–24 weeks age group. The high adjusted prevalence of C. jejuni in pigs was a consequence of the large sampling weight applied to pigs from large herds in Chau Thanh district, which had a notably higher prevalence of C. jejuni (47%) compared to other districts (6%). Clustering of Campylobacter infection by farm was investigated with 244 samples from 20 farms in Cao Lanh district. Results indicated a low level of clustering of Campylobacter infection in farms, ranging from ICC = 0·196 (the highest) in pig farms to ICC = 0·053 (the lowest) in chicken farms (Table 2).
Table 2. The animal- and farm-level prevalence of Campylobacter on 20 farms in Cao Lanh district, Dong Thap province, Mekong delta (Vietnam)

ICC, Intra-class correlation coefficient.
Table 3. Variables investigated for their potential association with Campylobacter infection, and summary of their descriptive values in animals sampled in Dong Thap province, Mekong delta, Vietnam

IQR, Interquartile range.
Risk factors for Campylobacter infection in chickens, ducks and pigs
A number of variables with plausible biological associations with Campylobacter colonization were investigated using multivariable hierarchical modelling (Table 3). A combination of animal species on farms was common; with 35% of pig farms also having chickens, and 18% of poultry farms having pigs. We found important differences in management between poultry species, with the majority of ducks (99·5%) raised in synchronized-age flocks, compared to 53·6% of chickens, the remainder being raised in continuous mixed-age flocks. Age was investigated both as a (log-transformed) continuous variable and as a categorical variable (quintiles) in both chicken and duck models. For chickens, the age group corresponding to the third quartile (10–24 weeks) had the greatest risk and the best fit in the final models [odds ratio (OR) 3·6] (Table 4). There was an association between C. coli and the occurrence of diarrhoea in both chickens and ducks (OR 13·6 and 4·4, respectively), but not in pigs. Risk factors for Campylobacter in ducks included the presence of more than one flock per farm and the use of municipal water. The use of municipal water for ducks was specifically associated with increased risk of C. jejuni, but not C. coli. The model also showed that older pigs had reduced risk of C. coli, with estimates indicating that a pig aged 5·5 months (third quartile) has an OR of 0·19 compared to a pig aged 2·5 months (first quartile) for Campylobacter infection. Notably, pigs in Chau Thanh had increased risk of C. jejuni (OR 6·5) compared to other districts.
Table 4. Results of models for risk factors of Campylobacter infection in chickens, ducks and pigs in Dong Thap province, Mekong delta, Vietnam

OR, Odds ratio; CI, confidence interval.
Antimicrobial susceptibility testing
A summary of the antimicrobial susceptibility patterns and resistance prevalence for all 202 Campylobacter isolates is shown in Figure 3. Of the eight antimicrobials in the panel to which Campylobacter normally exhibits susceptibility, strains demonstrated resistance against a median of four antimicrobials [interquartile range (IQR) 4–5]. Notably, the proportion of strains demonstrating resistance against erythromycin was 100%, while 99% of isolates exhibited resistance against sulfamethoxazole–trimethoprim, 92% against nalidixic acid and ofloxacin, and 20·8% against ciprofloxacin. The susceptibility patterns were almost identical for C. jejuni and C. coli and there was no statistically significant difference in prevalence of resistance between host species. While antimicrobial resistance was common, there was no significant difference in the number of antimicrobials to which isolates were resistant based on whether farms had (n = 185) or had not (n = 17) used antimicrobials within the 2 months prior to sampling (4·59 vs. 4·41, respectively) (Kruskal–Wallis P = 0·450)

Fig. 3. The antimicrobial susceptibility profiles of Campylobacter isolated from animals in Dong Thap province, Mekong delta, Vietnam. Bar charts showing the antimicrobial susceptibility patterns of C. jejuni (white bars) and C. coli (grey bars) isolated from (a) chickens (68 organisms), (b) pigs (85 organisms), (c) ducks (49 organisms) and (d) all species combined (202 organisms) against 12 antimicrobials: AMP, ampicillin; C, chloramphenicol; CN, gentamycin; SXT, sulfamethoxazole-trimethoprim; NA, nalidixic acid; OFL, oxfloxacin; CIP, ciprofloxacin; CL, clindamycin; and ERY, erythromycin.
MLST
One hundred and forty-three available Campylobacter isolates (73 C. jejuni, 70 C. coli) were subjected to seven-loci MLST (Fig. 4). The Campylobacter strains (76 from chickens, 43 from pigs, and 24 from ducks) could be divided into 112 individual sequence types (53 C. jejuni, 59 C. coli), including 75 novel sequence types (Supplementary Table S2), and one major (ST828) (>3 isolates), and 10 minor (⩽3 isolates) single allele variants (clonal) complexes. Overall, C. jejuni (Fig. 4 a) demonstrated a higher level of genetic diversity than C. coli (Fig. 4 b), with all but 16 C. coli isolates lying outside the major ST828 clonal complex. There was a strong association between the animal species of isolation and the clonal complex/Campylobacter spp., as the majority of strains isolated from pigs formed a single C. coli complex. However, this association was not absolute, as a number of C. coli isolated from ducks and chickens lay within the same clonal complex as isolates from pigs. Furthermore, four C. coli strains isolated from chickens belonging to sequence types 828, 966, 4939 and 5935 were identical to strains identified from pigs (Fig. 4 b).

Fig. 4. Multi-locus sequence typing (MLST) of Campylobacter isolated from animals in Dong Thap province, Mekong delta, Vietnam. (a) Minimum spanning tree of 73 C. jejuni isolates from animals in Dong Thap province, calculated by sequencing of the 7-target MLST genes. Each individual sequence type is distinguished by separate circles and linked by lines indicating allelic variation. The colour of each sequence type signifies from which animal species each bacterial strain was isolated: blue, ducks; yellow, chickens; pink, pigs. Background shading highlights clonal complexes. (b) Minimum spanning tree of 70 C. coli isolated from animals in Dong Thap province, calculated by sequencing of the 7-target MLST genes. Each individual sequence type is distinguished by separate circles and linked by lines indicating allelic variation.
DISCUSSION
This is first animal survey of its type investigating the prevalence of Campylobacter in farms in Vietnam. The key findings from this study are: (1) the predominance of C. jejuni as the main Campylobacter species colonizing pigs and poultry; (2) an age-related difference in Campylobacter colonization in pigs and chickens; (3) a high prevalence of resistance (>90%) against erythromycin, nalidixic acid and ofloxacin, and moderate levels of resistance (21%) against ciprofloxacin; (4) evidence for cross-species recombination and the transmission of C. coli between pigs and poultry; and (5) an association between C. coli infection and diarrhoea in poultry.
In spite of a low-level of biosecurity and mixing of age groups, the calculated prevalence of Campylobacter in chickens (31·9%) was not higher than that observed in other studies in Southeast Asia (Malaysia and Thailand), where bird-level prevalence in broilers sampled in farms and wet markets has been reported to range between 64% and 76% [Reference Saleha28–Reference Padungtod and Kaneene30]. However, since our study is cross-sectional, it is difficult to draw direct comparisons. With the exception of the study by Padungtod et al. [Reference Padungtod and Kaneene30], the other investigations were performed on chickens at slaughter age, the age at which chickens are predicted to have their highest prevalence of Campylobacter. Campylobacter infections in chickens are highly age-dependent, with a prevalence approaching 100% in broilers slaughtered after 35–42 days [Reference Bouwknegt31, Reference Gregory32]. Studies on older chickens kept for laying production have shown a decreasing prevalence after 10–20 weeks, potentially reflecting acquired immunity [Reference Lindblom, Sjörgren and Kaijser33, Reference Kalupahana34]. The observed highest prevalence in the 10–24 weeks age group (45%) in our investigation is consistent with these findings, and suggests substantial differences between production systems. In Vietnam most consumed chickens are produced in backyard and semi-intensive systems, using hybrid and local breeds, and are normally slaughtered at around 10–12 weeks (compared to 5–6 weeks in typical broiler production) [35]. In addition, consumption of older laying hens is also common in backyard production. The lower stocking densities of low-intensity production systems may also explain these differences. In contrast, the observed Campylobacter prevalence in pigs (53·7%) was only marginally lower than the overall prevalence compiled from 20 studies on Campylobacter in pigs worldwide (69·7%). We can also report an important age effect associated with the prevalence of Campylobacter in pigs. Our data also demonstrated low-level of clustering of Campylobacter on farms, predicting that most production units would test positive for Campylobacter if sufficient numbers of animals were tested. This is probably a reflection of limited biosecurity, a mix of species and variable animal ages on farms.
Ninety-nine per cent of the culture isolates were C. jejuni or C. coli, the two main Campylobacter species responsible for clinical cases of diarrhoea in Vietnam [Reference Isenbarger15]. C. jejuni was the most common species in pigs and poultry. An unexpectedly high prevalence of C. jejuni was observed in pigs in the district (Chau Thanh) with the highest pig production. Studies have shown that colonization of pigs with C. jejuni is common in animals reared outdoors [Reference Jensen36]. This suggests that pigs may be an important source of C. jejuni in Vietnam, and merits further investigation.
The observed Campylobacter species distribution in chickens differs from a 2005–2006 study in Ho Chi Minh City (HCMC), where 73·9% of chicken meat samples were identified as C. lari [Reference Garin37]. C. lari was also identified in chicken carcasses from markets sampled in neighbouring Cambodia (21% of all isolates) [Reference Lay38]. This discrepancy may reflect differences in the sources of chickens, since it is likely that a larger fraction of birds sold in HCMC are representative of chickens produced under industrial conditions. Despite the high potential for inter-species transmission on the farms, we found no statistical difference in the prevalence of C. jejuni between pigs kept on farms that raised and did not raise poultry; similarly, there was no difference in risk of C. coli infection in poultry that were and were not exposed to pigs. In contrast, we found an association between presence of C. coli in ducks and high density of pigs at the commune level, and genotypic evidence of strain transfer between pigs and chickens. Duck farming in the Mekong delta is characterized by low levels of biosecurity and itinerant grazing practices [Reference Minh39], which are likely to expose ducks at risk of infection when they graze in areas where pig effluent is used. Furthermore, the presence of multiple flocks of ducks on a single farm was associated with an increased prevalence of Campylobacter, similar to an observation from broiler operations [Reference Henry40].
Our results indicate a high level of antimicrobial resistance in Campylobacter isolates. This is a particular a concern for erythromycin (100% resistance) and ciprofloxacin (21% resistance), two antimicrobials commonly used to treat serious Campylobacter infections. We found high rates of antimicrobial usage on farms, with a wide range of antimicrobial compounds, suggesting a sustained selective pressure inducing the potential for the development of antimicrobial resistance. In a previous study by Garin et al. a higher prevalence of resistance to ciprofloxacin (95%) but a lower prevalence to erythromycin (25%) was reported in isolates from chickens [Reference Garin37]. Antimicrobial resistance in human Campylobacter isolates from Vietnam from children with diarrhoea presenting to healthcare settings in HCMC has been increasing over recent years and current isolates show almost identical antimicrobial resistance patterns to those presented in this study (data not shown). Data from 88 human isolates from 1996 to 1999 indicate that 7% of isolates were resistance to nalidixic acid and ciprofloxacin and all strains were sensitive to azithromycin [Reference Isenbarger41]. A likely explanation for this is the increased use of antimicrobials in human and veterinary medicine over the same period [Reference Hoa42, 43].
The last few years have seen the establishment of a large number of integrated broiler, commercial chicken layer, and pig companies in Vietnam which supply meat to the increasing population. As a consequence of increasing demand, these production systems are likely to become common in future years. Except for the large pig units in Chau Thanh district, industrial size operations are still uncommon in Dong Thap province, but this is changing. It is likely that industrialized production systems will contribute to a higher burden of Campylobacter organisms in the food chain, due to increased stocking density and stress, homogenization of diet, and reduced levels of immunity [Reference Graham44]. In addition, changes associated with urbanization, improved sanitation and water quality are likely to reduce the exposure to bacterial pathogens during childhood, reducing levels of population immunity. Something similar has been observed in China, where an increase in Campylobacter cases in older age groups has been observed [Reference Hou, Sun and Wang45]. This combination of increased exposure through food and reduced population immunity is likely to contribute in an increased burden of human Campylobacter infections throughout the next decade.
We conclude that Campylobacter, an important cause of diarrhoea in children, is highly prevalent in animal production systems in Vietnam. We predict that intensification of animal production systems and increased urbanization will result in a further increase in the incidence of this infection and a change in the epidemiology in animals and humans. Given the high levels of resistance to antimicrobials in these organisms it is imperative to improve the surveillance and control of Campylobacter throughout the length of the food chain. Future interventions targeting a reduction in the prevalence of Campylobacter in farmed animal populations in Vietnam will become increasingly important.
SUPPLEMENTARY MATERIAL
For supplementary material accompanying this paper visit http://dx.doi.org/10.1017/S0950268813002410.
ACKNOWLEDGEMENTS
We thank the Sub-Department of Animal Health of Dong Thap for their support. We are indebted to Dr Marcel Wolbers (OUCRU) for statistical advice. This work has been co-funded by ZoNMW/WOTRO (The Netherlands), VIBRE Project (No. 205100012) and the Vietnam Initiative on Zoonotic Infections (VIZIONS), part of the Wellcome Trust Major Overseas Programme (UK).
DECLARATION OF INTEREST
None.










