INTRODUCTION
The knowledge of species composition of a given ecosystem is one the most basic aspects of biodiversity research, being essential for natural resource management and to assure quality in biological and ecosystem sciences (Costello et al., Reference Costello, Wilson and Houlding2013). Many areas lack comprehensive systematic inventories and this is particularly true for marine ecosystems that in general are much less studied than terrestrial ones (Bouchet, Reference Bouchet and Duarte2006). Additionally, biodiversity is often considered to be under major threat mostly due to anthropogenic pressure (Carlton, Reference Carlton1996, Reference Carlton, Mooney and Hobbs2000; Dulvy et al., Reference Dulvy, Sadovy and Reynolds2003; Costello et al., Reference Costello, Wilson and Houlding2013). However, the lack of historical data hampers an accurate detection of assemblage changes such as invasions and/or local extinctions. This may be particularly true for planktonic hydrozoans which are typically small, delicate and historically under-studied worldwide (e.g. Bouillon et al., Reference Bouillon, Medel, Pagès, Gili, Boero and Gravili2004), and particularly in Brazil (Haddad & Marques, Reference Haddad, Marques, Ribeiro-Costa and Rocha2009), underscoring the need for comprehensive efforts to develop taxonomic inventories such as the present study.
Estuarine ecosystems are particularly vulnerable since they harbour an abundant and diversified biota (Dolan & Gallegos, Reference Dolan and Gallegos2001; Bouchereau et al., Reference Bouchereau, Chaves and Monti2008; Paiva et al., Reference Paiva, Chaves and Araújo2013; Seguro et al., Reference Seguro, García, Papaspyrou, Gálvez, García-Robledo, Navarro, Soria-Píriz, Aguilar, Lizano, Morales-Ramírez and Corzo2015) and are often pressured by human occupation and activities nearby with associated impacts (e.g. Cremer et al., Reference Cremer, Morales and Oliveira2006). Among the many human pressures, such as pollution and habitat fragmentation, growing attention has been given to non-indigenous species. There is an increased awareness that invasive species can change abundance of indigenous species and play an important role in species extinctions (Gallardo et al., Reference Gallardo, Clavero, Sánchez and Vilà2016), being considered a great threat to marine ecosystems.
The Paranaguá Estuarine System (PES) is one of the largest estuarine systems of South America, with ~550 km2 of total water body (Noernberg et al., Reference Noernberg, Lautert, Araújo, Marone, Angellotti, Netto and Krug2006). PES harbours a large biodiversity and has been adopted a Natural World Heritage site by UNESCO (2016). Besides its ecological importance, PES is also important for harbouring two ports; the port of Paranaguá, the main South American grain-shipping port (Marone et al., Reference Marone, Machado, Lopes, Silva, Smith, Dupra, Marshall Crossland and Crossland2000), and the port of Antonina. No comprehensive surveys on planktonic hydrozoans from PES have been performed, despite their ecological importance (e.g. Matsakis & Conover, Reference Matsakis and Conover1991; Mills, Reference Mills1995) and high diversity. The few existing studies recorded 18 species, three of them regarded as probably exotic (Montú & Cordeiro, Reference Montú and Cordeiro1988; Nogueira Júnior & Oliveira, Reference Nogueira Júnior and Oliveira2006; Bardi, Reference Bardi2011; Haddad et al., Reference Haddad, Bettim and Miglietta2014). In the present study we aim to provide a comprehensive overview of planktonic hydrozoan diversity from PES, along with evidence of regional assemblage change compared with the past decades.
MATERIALS AND METHODS
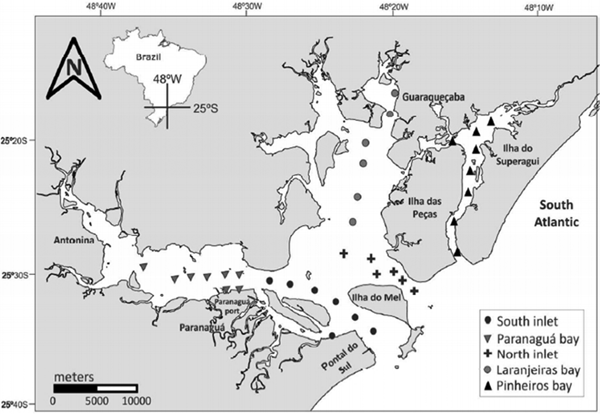
Planktonic hydrozoans were collected in five seasonal sampling campaigns: summer (13–16 March) and winter (23 and 24 August) 2012, summer (21 and 22 February) and winter (18 and 19 June) 2013, and summer (28 and 29 March) 2014. For each campaign, 37 stations were sampled (Figure 1), totalling 185 samples. PES can be divided in five sectors (Paranaguá, Antonina, Laranjeiras and Pinheiros Bays and a mixing zone) based on Noernberg et al. (Reference Noernberg, Lautert, Araújo, Marone, Angellotti, Netto and Krug2006). In the present study, with exception of Antonina Bay, all the sectors, along with two main tidal channels were sampled.

Fig. 1. Map of Paranaguá Estuarine System, southern Brazil, showing the 37 stations sampled on March and August 2012, February and June 2013, and March 2014, in each sector: inlets, mixing zone, Paranaguá, Laranjeiras and Pinheiros Bays. Map was made in the QGis 2.18 software.
Oblique tows (speed = 2 knots for 2 min) from near the bottom to the surface were made, with a WP-2 plankton net (2 m long, 0.5 m mouth diameter, 200 µm mesh) equipped with calibrated mechanical flowmeters Hydrobios in 2012 samplings campaigns and General Oceanics in 2013. Samples (N = 185) were preserved with a 4% formaldehyde (borax-buffered) solution for later analysis in the laboratory. In addition, surface temperature and salinity were measured at each station with a mercury thermometer and calibrated portable refractometer Atago, respectively.
Whole samples were analysed in the laboratory under a stereomicroscope and all the planktonic hydrozoans were sorted and identified (mostly following Bouillon, Reference Bouillon and Boltovsky1999 and Pugh, Reference Pugh and Boltovsky1999). A list with all species found, along with the literature records was compiled and is presented (Table 1). Classification follows Schuchert (Reference Schuchert2016). The number of analysed individuals (colonies for siphonophores) and the frequency of capture of a given species are also tabulated.
Table 1. Taxonomic classification, frequency of capture (FC) and number of individuals (colonies for siphonophores) of planktonic hydrozoans recorded in the present study, in Paranaguá Estuarine System, southern Brazil, from 185 plankton hauls.
Damaged and unidentifiable organisms (N = 173) were not included. Salinity (S) and temperature (T, in °C) range and PES sector occurrence of each species in the present study and in previous studies are also shown.
a Species whose polyps have also been recorded in PES.
b Specimen only sighted. Data source: 1=present study; 2=Nogueira Júnior & Oliveira (Reference Nogueira Júnior and Oliveira2006); 3=Bardi (Reference Bardi2011); 4=Montú & Cordeiro (Reference Montú and Cordeiro1988); 5=Lopes et al. (Reference Lopes, Vale and Brandini1998); 6=Haddad et al. (Reference Haddad, Bettim and Miglietta2014); Bettim & Haddad (Reference Bettim and Haddad2017).
In, inlets; MZ, mixing zone; Pguá, Paranaguá Bay; Lar, Laranjeiras Bay; Pin, Pinheiros Bay.
Species accumulation curves and diversity estimators such as Chao 1 and 2, and Jackknife 1 and 2 were constructed for all sampling campaigns, using the program Primer v6 (Clarke & Gorley, Reference Clarke and Gorley2006).
RESULTS AND DISCUSSION
In the present study we have sampled and analysed about 49,000 hydrozoan specimens belonging to 34 species, these being three siphonophores and 31 hydromedusae, apart from actinula larvae (Table 1). Some representative examples are shown in Figures 2–14. In many cases the quality of the material was not good, as is usual with these delicate organisms and therefore for detailed morphological analyses, examination of live organisms is recommended whenever possible. In the present collection, the diagnostic characters could be seen in most cases, allowing a reliable identification aided by the strong taxonomic background of some of the authors (LSN and MNJ). In fact, damaged unidentifiable organisms did not represent a significant proportion of the collection and accounted for less than 1% of the total organisms recorded here. Four taxa, namely Clytia spp. (Figure 9), Obelia spp., Solmaris sp. and Helgicirrha sp. (Figure 8), could not be identified to species level since the latter is probably an undescribed species, and all the other genera have taxonomic problems (Nagata et al., Reference Nagata, Nogueira Júnior and Haddad2014a). All the planktonic hydrozoan species found here have already been recorded for the Brazilian coast (Oliveira et al., Reference Oliveira, Miranda, Araujo, Ayón, Cedeño-Posso, Cepeda-Mercado, Córdova, Cunha, Genzano, Haddad, Mianzan, Migotto, Miranda, Morandini, Nagata, Nascimento, Nogueira Júnior, Palma, Quiñones, Rodriguez, Scarabino, Schiariti, Stampar, Tronolone and Marques2016), but 19 species are new records for PES and nine for Paraná State (Table 1; Nagata et al., Reference Nagata, Nogueira Júnior and Haddad2014a). Although these new records fill gaps in their distribution their occurrence was expected, since polyps and/or medusae of them have been found to the south and/or north of the PES (Oliveira et al., Reference Oliveira, Miranda, Araujo, Ayón, Cedeño-Posso, Cepeda-Mercado, Córdova, Cunha, Genzano, Haddad, Mianzan, Migotto, Miranda, Morandini, Nagata, Nascimento, Nogueira Júnior, Palma, Quiñones, Rodriguez, Scarabino, Schiariti, Stampar, Tronolone and Marques2016).
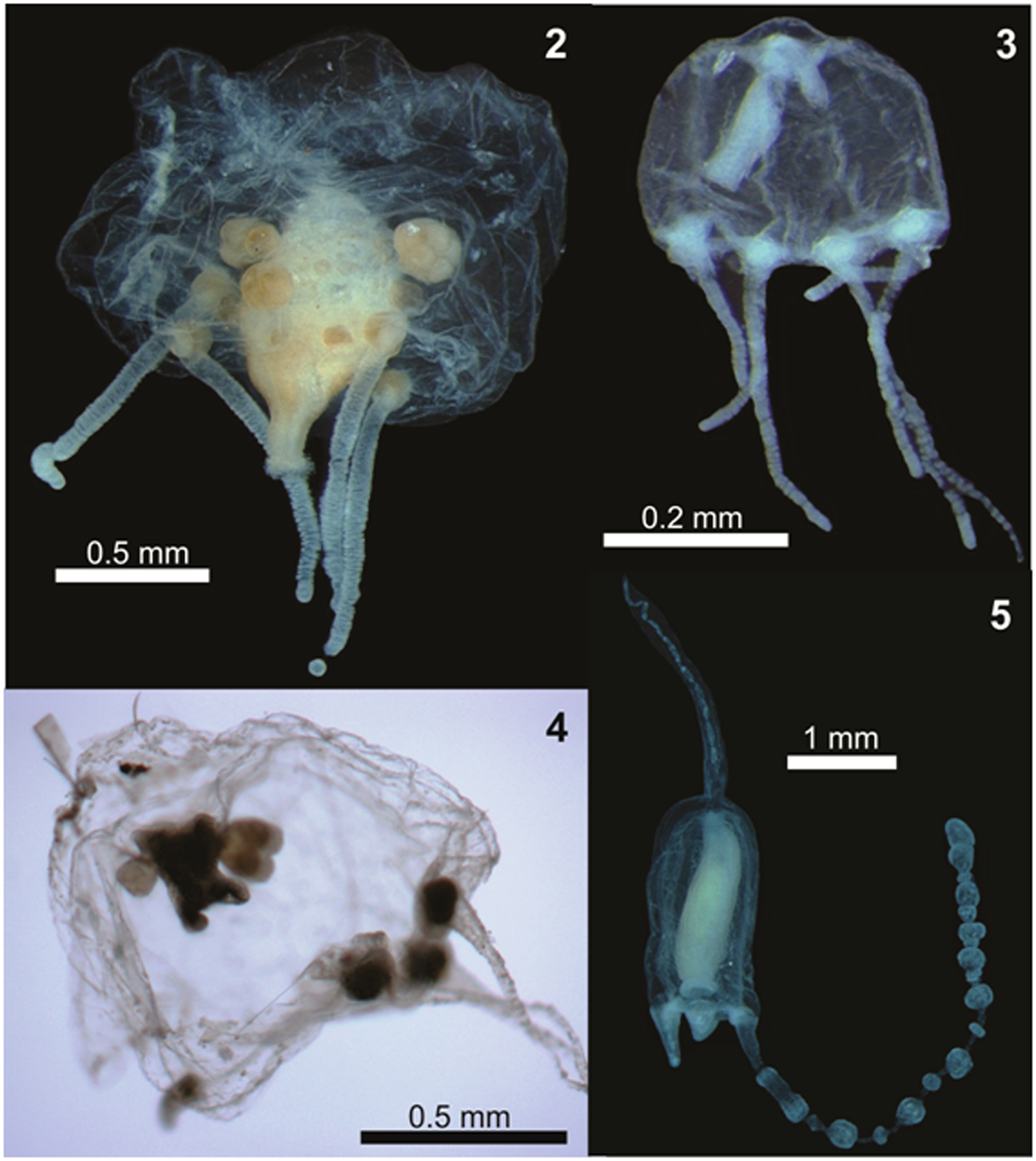
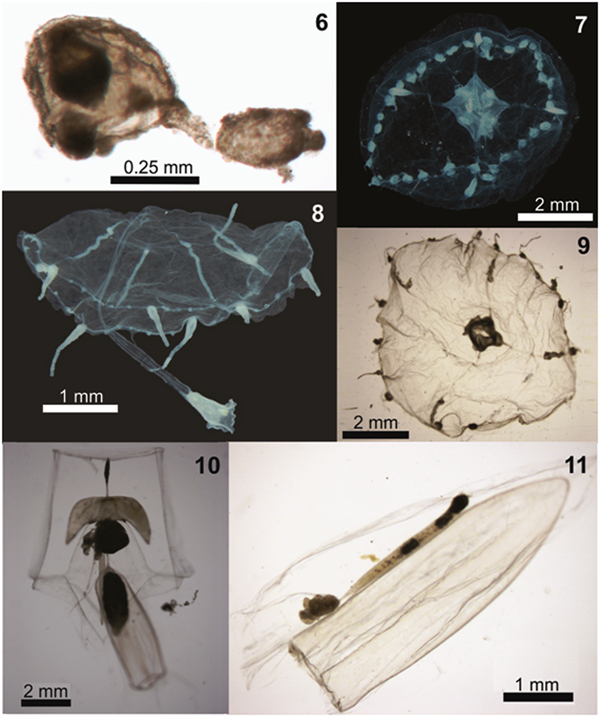
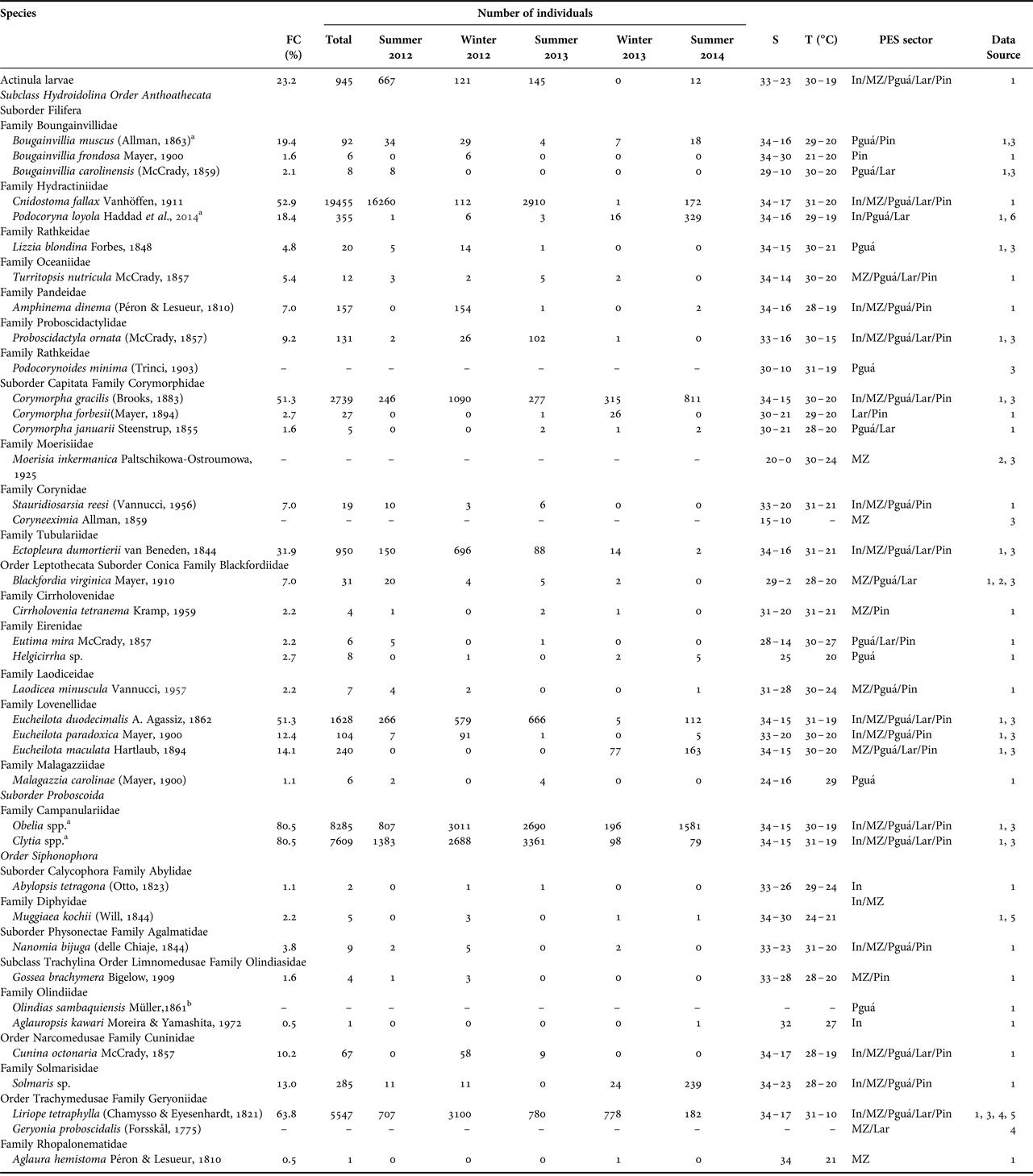
Fig. 2–5. Examples of planktonic hydrozoans from Paranaguá Estuarine System, southern Brazil. Cnidostoma fallax, lateral view (2); Podocoryna loyola, lateral view (3); Proboscidactyla ornata, lateral view (4) and Corymorpha gracilis, lateral view (5).

Fig. 6–11. Examples of planktonic hydrozoans from Paranaguá Estuarine System, southern Brazil. Corymorpha forbesii, lateral view (6); Malagazzia carolinae, oral view (7); Helgicirrha sp., lateral view (8); Clytia spp., oral view (9); Abylopsis tetragona eudoxid (10) and Muggiaea kochii, lateral view (11).
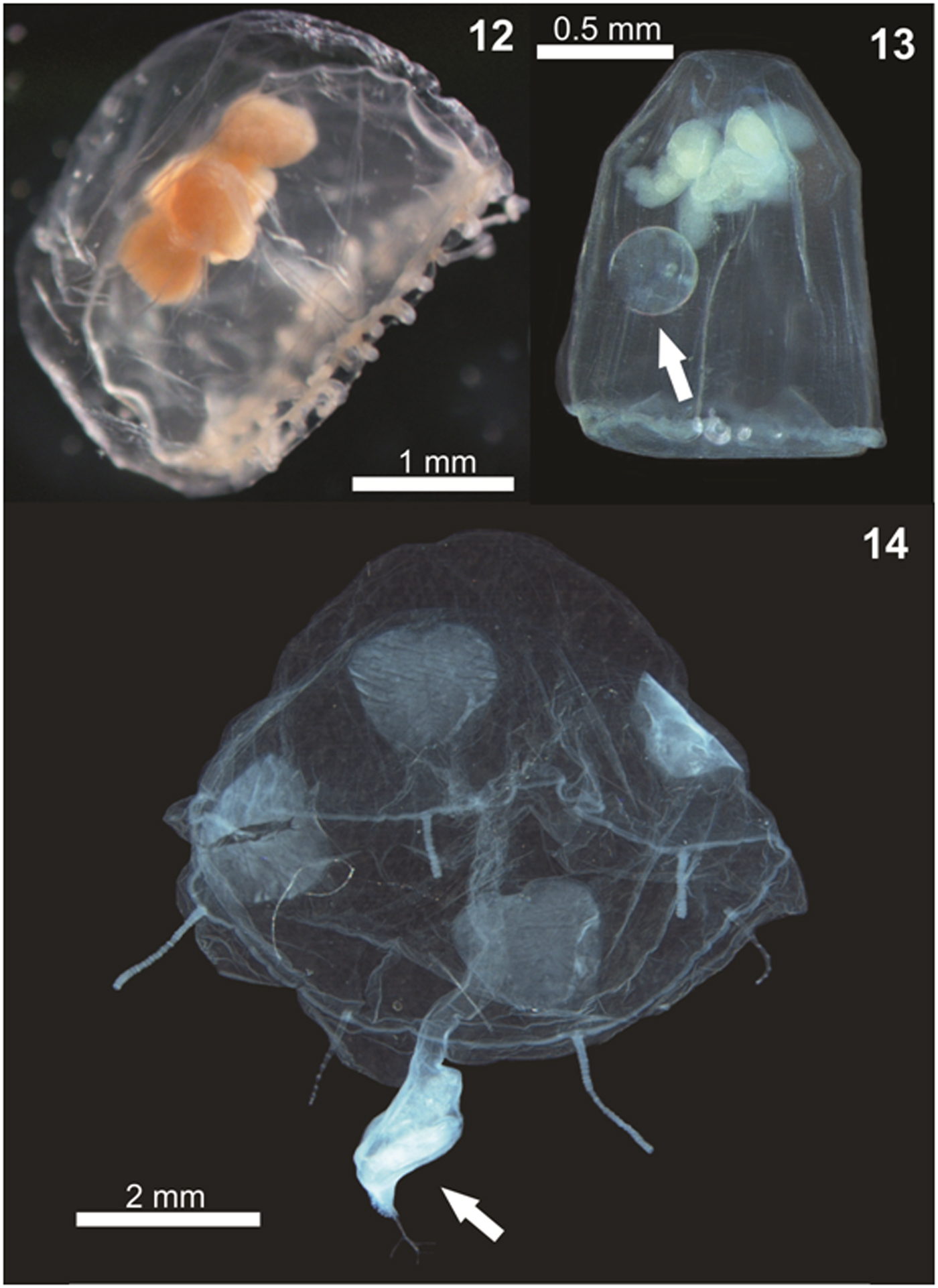
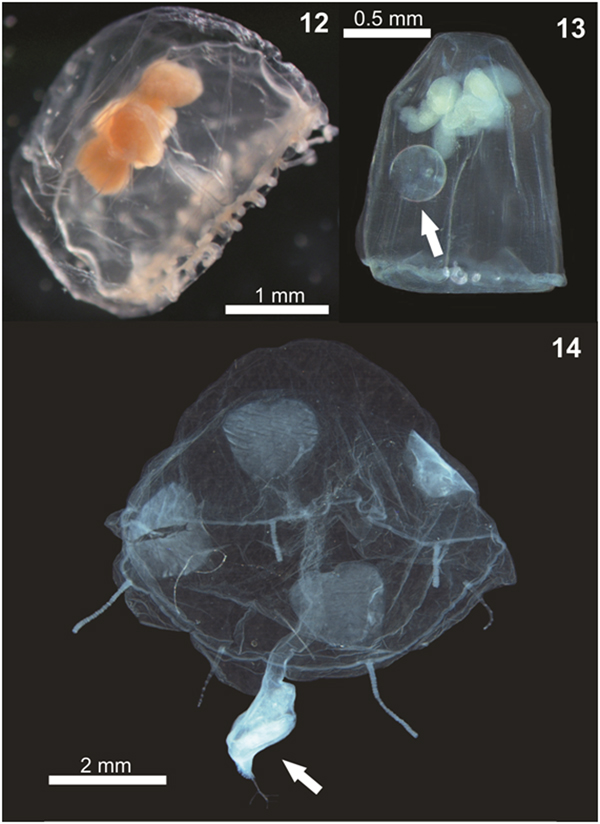
Fig. 12–14. Examples of planktonic hydrozoans from Paranaguá Estuarine System, southern Brazil. Aglauropsis kawari, lateral view (12); Aglaura hemistoma, lateral view, white arrow indicates a diatom inside the umbrella (13) and Liriope tetraphylla, lateral view, white arrow indicates copepods as possible prey in digestion process (14).
The accumulated number of sampled species nearly stabilized (Figure 15), and the diversity recorded here (34 spp.) is quite similar to the estimators that reached up to 36 in the Jackknife 2, and is considerably greater than that estimated by the Chao 2 (Figure 15). Therefore, the diversity of planktonic hydrozoans estimated here can be considered robust, and only a few additional species are likely to be found in future studies. Yet, the total species richness reported probably still is slightly underestimated due to the difficulty of identifying the different species of Clytia and Obelia based on medusa morphology, the notorious fragility of planktonic hydrozoans which result in damaged and unidentifiable individuals (even when relatively few) that could represent additional species. Moreover, the use of different gear types also may help to find additional species such as Rhacostoma atlanticum and Olindias sambaquiensis, not sampled here but known to commonly occur in coastal waters nearby and occasionally entering estuaries (Nogueira Júnior et al., Reference Nogueira Júnior, Nagata and Haddad2010; Nogueira Júnior, Reference Nogueira Júnior2012). These species are usually large-sized (reaching up to >40 mm) and thus rarely sampled in standard zooplankton nets as used here (Nagata et al., Reference Nagata, Nogueira Júnior and Haddad2014a). In fact, O. sambaquiensis has been observed a few times in the PES tidal channels in samples with larger nets not included here (LSN, personal observations). Sampling in other seasons also could result in additional species. For instance, Octophialucium haeckeli was not found here at PES perhaps due to absence of sampling in autumn and/or spring seasons when this medusa was found in more southerly estuarine waters (Nogueira Júnior et al., Reference Nogueira Júnior, Pukanski and Souza-Conceição2015). Nevertheless, the other three species exclusively found in these seasons (see Nogueira Júnior et al., Reference Nogueira Júnior, Pukanski and Souza-Conceição2015: their supplementary online material table 1), namely Amphinema dinema, Gossea brachymera and Abylopsis tetragona, were sampled in the present study (Table 1).
Fig. 15. Accumulation curves of richness estimators from planktonic hydrozoan species in Paranaguá Estuarine System, southern Brazil.
The limited literature data available indicate that four additional species can be included in the PES planktonic hydrozoan checklist: Moerisia inkermanica Paltschikowa-Ostroumowa, 1925, Coryne eximia Allman, 1859, Podocorynoides minima (Trinci, 1903) and Geryonia proboscidalis (Forsskål, 1775) (Montú & Cordeiro, Reference Montú and Cordeiro1988; Nogueira Júnior & Oliveira, Reference Nogueira Júnior and Oliveira2006; Bardi, Reference Bardi2011; Table 1), that along with O. sambaquiensis (see above) totals 39 species. The general species composition of planktonic hydrozoans from PES is a highly diverse estuarine fauna, with many brackish-water tolerant species. Typical examples are Cnidostoma fallax, Moerisia inkermanica and Malagazzia carolinae which are more abundant or exclusive inside estuaries (Teixeira-Amaral et al., Reference Teixeira-Amaral, Amaral, de Ortiz, Agostini and Muxagata2017; Nogueira Júnior et al., Reference Nogueira Júnior, Nascimento, Maciel, Tilbert, Oliveira, Hoffmeyer, Sabatini, Brandini, Calliari and Santinelli2018; Nogueira Júnior & Silva Nascimento, Reference Nogueira Júnior and Nascimento2018) rather than on the adjacent shelf (Vannucci, Reference Vannucci1957, Reference Vannucci1963; Nagata et al., Reference Nagata, Nogueira Júnior and Haddad2014a).
The PES fauna is quite similar to other tropical/subtropical estuaries from the south-western Atlantic (Nogueira Júnior et al., Reference Nogueira Júnior, Nascimento, Maciel, Tilbert, Oliveira, Hoffmeyer, Sabatini, Brandini, Calliari and Santinelli2018) and elsewhere (e.g. Vannucci et al., Reference Vannucci, Santhakumari and dos Santos1970; Santhakumari et al., Reference Santhakumari, Ramaiah and Nair1997, Reference Santhakumari, Tiwari and Nair1999). This high diversity is similar to the few other comprehensively sampled nearby estuaries (Nogueira Júnior et al., Reference Nogueira Júnior, Nascimento, Maciel, Tilbert, Oliveira, Hoffmeyer, Sabatini, Brandini, Calliari and Santinelli2018), but contrasts with the general view that estuarine hydrozoan fauna is impoverished (Calder, Reference Calder1971; Santhakumari et al., Reference Santhakumari, Ramaiah and Nair1997, Reference Santhakumari, Tiwari and Nair1999). In fact, tropical/subtropical estuarine systems in the south-western Atlantic seem to harbour more hydromedusae species (e.g. Nogueira Júnior , Reference Nogueira Júnior2012 – 36 spp.; present study – 36 spp.; Aguilar et al., Reference Aguilar, Costa, Miyashita, Brandini and Nogueira Júnior2015 – 35 spp.) than adjacent shelf regions (e.g. Vannucci, Reference Vannucci1957 – 27 spp.; Vannucci, Reference Vannucci1963 – 17 spp.; Nagata et al., Reference Nagata, Nogueira Júnior and Haddad2014a, Reference Nagata, Nogueira Júnior, Brandini and Haddadb – 22 spp.). These observations emphasize the necessity of comprehensive sampling of other tropical/subtropical estuaries from the south-western Atlantic and worldwide. These could potentially harbour high diversity and are commonly poorly studied, since most previous studies focus on temperate estuaries commonly with considerably lower diversity (e.g. Calder, Reference Calder1971; Petrova et al., Reference Petrova, Dautova and Shkoldina2011; Pestorić et al., Reference Pestorić, Krpo-Ćetković, Gangai and Lučić2012; Vansteenbrugge et al., Reference Vansteenbrugge, Regenmortel, Troch, Vincx and Hostens2015; Zuo et al., Reference Zuo, Wu, Wang and Li2016; Dutto et al., Reference Dutto, Genzano, Schiariti, Lecanda, Hoffmeyer and Pratolongo2017).
Meroplanktonic species represented 79% of total PES planktonic hydrozoans (30 spp.), as is typical from tropical and subtropical estuaries (Navas Pereira, Reference Navas-Pereira1980; Santhakumari et al., Reference Santhakumari, Ramaiah and Nair1997, Reference Santhakumari, Tiwari and Nair1999; Nogueira Júnior, Reference Nogueira Júnior2012). Meroplanktonic Anthoathecata from PES represent 24.6% of those recorded from Brazil and 15.7% from the South Atlantic, while meroplanktonic Leptothecata represent 22% and 18.6%, and Limnomedusae represent 40 and 22.2%, respectively (Bouillon, Reference Bouillon and Boltovsky1999; Oliveira et al., Reference Oliveira, Miranda, Araujo, Ayón, Cedeño-Posso, Cepeda-Mercado, Córdova, Cunha, Genzano, Haddad, Mianzan, Migotto, Miranda, Morandini, Nagata, Nascimento, Nogueira Júnior, Palma, Quiñones, Rodriguez, Scarabino, Schiariti, Stampar, Tronolone and Marques2016). These proportions are quite high considering that PES (~550 km2), harbours nearly a quarter of all meroplanktonic hydrozoans so far recorded from the >8000 km of Brazilian coastline.
On the other hand, holoplanktonic hydrozoans from PES (i.e. Siphonophorae, Narcomedusae and Trachymedusae) represent only ~7 and 5% of all species from Brazil and the South Atlantic, respectively (Bouillon, Reference Bouillon and Boltovsky1999; Pugh, Reference Pugh and Boltovsky1999; Oliveira et al., Reference Oliveira, Miranda, Araujo, Ayón, Cedeño-Posso, Cepeda-Mercado, Córdova, Cunha, Genzano, Haddad, Mianzan, Migotto, Miranda, Morandini, Nagata, Nascimento, Nogueira Júnior, Palma, Quiñones, Rodriguez, Scarabino, Schiariti, Stampar, Tronolone and Marques2016). With the exception of Liriope tetraphylla, present in 64% of the samples and representing 11% of all hydrozoans sampled, all other holoplanktonic species presented relatively low frequencies of occurrence and abundance. They were mostly found in the outer sectors of the estuary, in the inlets, mixing zone and outer stations of Pinheiros Bay (Table 1). These observations suggest their populations are not resident and were probably advected from adjacent shelf waters where they are common and abundant (Vannucci, Reference Vannucci1957, Reference Vannucci1963; Nagata et al., Reference Nagata, Nogueira Júnior, Brandini and Haddad2014b; Nogueira Júnior et al., Reference Nogueira Júnior, Brandini and Codina2014).
The additional records of Dipurena sp. (Bardi, Reference Bardi2011) and Aglantha sp. (Montú & Cordeiro, Reference Montú and Cordeiro1988) from PES were not considered, pending confirmation due to taxonomic uncertainties. The genus Dipurena is no longer accepted, and the species were moved either to the genus Slabberia or Stauridiosarsia (Schuchert, Reference Schuchert2016). Moreover, Corynidae medusae are very similar to each other, particularly when juveniles, and Bardi (Reference Bardi2011) does not state the reasons for identifying the three individuals only at the generic level (i.e. juveniles, damaged, not exactly fitting any known description). Since voucher, illustrations or descriptions of the studied individuals were not provided (Bardi, Reference Bardi2011) a critical re-analysis of the identification is not possible. Yet, we suspect that the Dipurena sp. record from PES may be juveniles and/or damaged Staurodiosarsia reesii, which is commonly found on South Brazilian Bight estuaries (Vannucci, Reference Vannucci1957; Nogueira Júnior et al., Reference Nogueira Júnior, Nascimento, Maciel, Tilbert, Oliveira, Hoffmeyer, Sabatini, Brandini, Calliari and Santinelli2018) including PES (present study; Table 1) and not recorded by Bardi (Reference Bardi2011). The record of Aglantha sp. (Montú & Cordeiro, Reference Montú and Cordeiro1988) is also doubtful (Nagata et al., Reference Nagata, Nogueira Júnior and Haddad2014a) because it is typically an oceanic genus (Bouillon, Reference Bouillon and Boltovsky1999) and is probably a misidentification of Aglaura hemistoma (Figure 13), which is roughly similar and commonly found in the adjacent shallow shelf (Nagata et al., Reference Nagata, Nogueira Júnior and Haddad2014a, Reference Nagata, Nogueira Júnior, Brandini and Haddadb), occasionally with a few individuals entering the estuary, as found here (Table 1).
Cnidostoma fallax (Figure 2) was the most numerous species, with 19,455 individuals sampled, followed by Obelia spp. (8285 individuals), Clytia spp. (7609), Liriope tetraphylla (Figure 14; 5547), Corymorpha gracilis (2739) and Eucheilota duodecimalis (1628), all of them sampled both in summer and winter campaigns and in all PES sectors (Table 1). Some other species were frequently captured (>15% of samples), but were not so abundant (<1000 individuals), such as Ectopleura dumortierii, actinula larvae, Bougainvillia muscus and Podocoryna loyola (Figure 3).
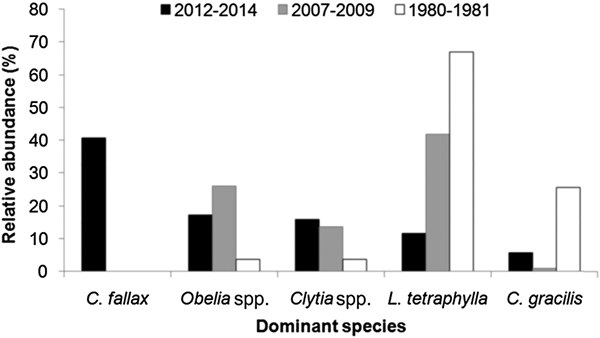
The high dominance of C. fallax in the present study is noteworthy. This is in contrast to results from the PES in the 1980s (Montú & Cordeiro, Reference Montú and Cordeiro1988), 1990s (Lopes, Reference Lopes1997; Lopes et al., Reference Lopes, Vale and Brandini1998) and 2000s (Bardi, Reference Bardi2011) when L. tetraphylla was the dominant hydromedusa, followed by Obelia spp., Clytia spp. or C. gracilis, and C. fallax was not present at all (Figure 16). In fact, L. tetraphylla, Obelia and Clytia have historically been the dominant hydromedusae in open shallow coastal and estuarine environments from the South Brazilian Bight (Vannucci, Reference Vannucci1957, Reference Vannucci1963; Teixeira et al., Reference Teixeira, Tundisi and Kutner1965; Navas-Pereira, Reference Navas-Pereira1980; Bardi, Reference Bardi2011; Nogueira Júnior, Reference Nogueira Júnior2012; Nagata et al., Reference Nagata, Nogueira Júnior and Haddad2014a, Reference Nagata, Nogueira Júnior, Brandini and Haddadb; Nogueira Júnior et al., Reference Nogueira Júnior, Brandini and Codina2014, Reference Nogueira Júnior, Nascimento, Maciel, Tilbert, Oliveira, Hoffmeyer, Sabatini, Brandini, Calliari and Santinelli2018). It is difficult to explain the reasons for such a replacement in the dominant species, particularly due to the poor historical knowledge of the Brazilian hydromedusan fauna, but unexpected high abundances of C. fallax have also recently been recorded at Patos Lagoon (Teixeira-Amaral et al., Reference Teixeira-Amaral, Amaral, de Ortiz, Agostini and Muxagata2017). Considering that the presence of C. fallax in the south-western Atlantic has only recently been detected, and now is known to occur in many estuaries along Brazilian coast between ~24 and 34°S (Teixeira-Amaral et al., Reference Teixeira-Amaral, Amaral, de Ortiz, Agostini and Muxagata2017; Nogueira Júnior et al., Reference Nogueira Júnior, Nascimento, Maciel, Tilbert, Oliveira, Hoffmeyer, Sabatini, Brandini, Calliari and Santinelli2018; present study), two mutually excluding hypotheses may be proposed:
(i) C. fallax may have been overlooked in the few previous studies of these estuaries, either due to taxonomic uncertainties and/or to the low abundances of the species. Although most of these historical estuarine studies focused on the zooplankton as a whole and the most abundant taxa such as copepods, some focused on or attempted to identify the estuarine Brazilian hydromedusae including a few with relatively satisfactory spatial and temporal coverage (Vannucci, Reference Vannucci1957; Teixeira et al., Reference Teixeira, Tundisi and Kutner1965; Montú & Cordeiro, Reference Montú and Cordeiro1988; Lopes, Reference Lopes1997; Bardi, Reference Bardi2011), and none has recorded C. fallax which is a very peculiar and easily identifiable species.
(ii) C. fallax appeared recently, perhaps introduced from tropical Atlantic African coastal estuaries which are the location of all historical records of the species (Vanhöffen, Reference Vanhöffen1911; Picard & Rahm, Reference Picard and Rahm1954 as Archaeoceania tournieri; Kramp, Reference Kramp1959). The evidence for this hypothesis would be the lack of previous records in spite of studies (albeit few) on these very same estuaries (e.g. Teixeira et al., Reference Teixeira, Tundisi and Kutner1965; Montú & Cordeiro, Reference Montú and Cordeiro1988; Lopes, Reference Lopes1997; Bardi, Reference Bardi2011) where the species is currently known to occur (see references above). Although speculative, it is not unlikely that C. fallax is an introduced species considering that along with its recent discovery, all sites with known populations are brackish water environments near ports (e.g. Vanhöffen, Reference Vanhöffen1911; Picard & Rahm, Reference Picard and Rahm1954; Kramp, Reference Kramp1959; Teixeira-Amaral et al., Reference Teixeira-Amaral, Amaral, de Ortiz, Agostini and Muxagata2017; this study), and the high national and international ship traffic in PES could be a potential vector, along with other cnidarian and non-cnidarian exotic species (e.g. Neves & Rocha, Reference Neves and Rocha2008; Van Ofwegen & Haddad, Reference Van Ofwegen and Haddad2011; Haddad et al., Reference Haddad, Bettim and Miglietta2014; Bettim & Haddad, Reference Bettim and Haddad2017).
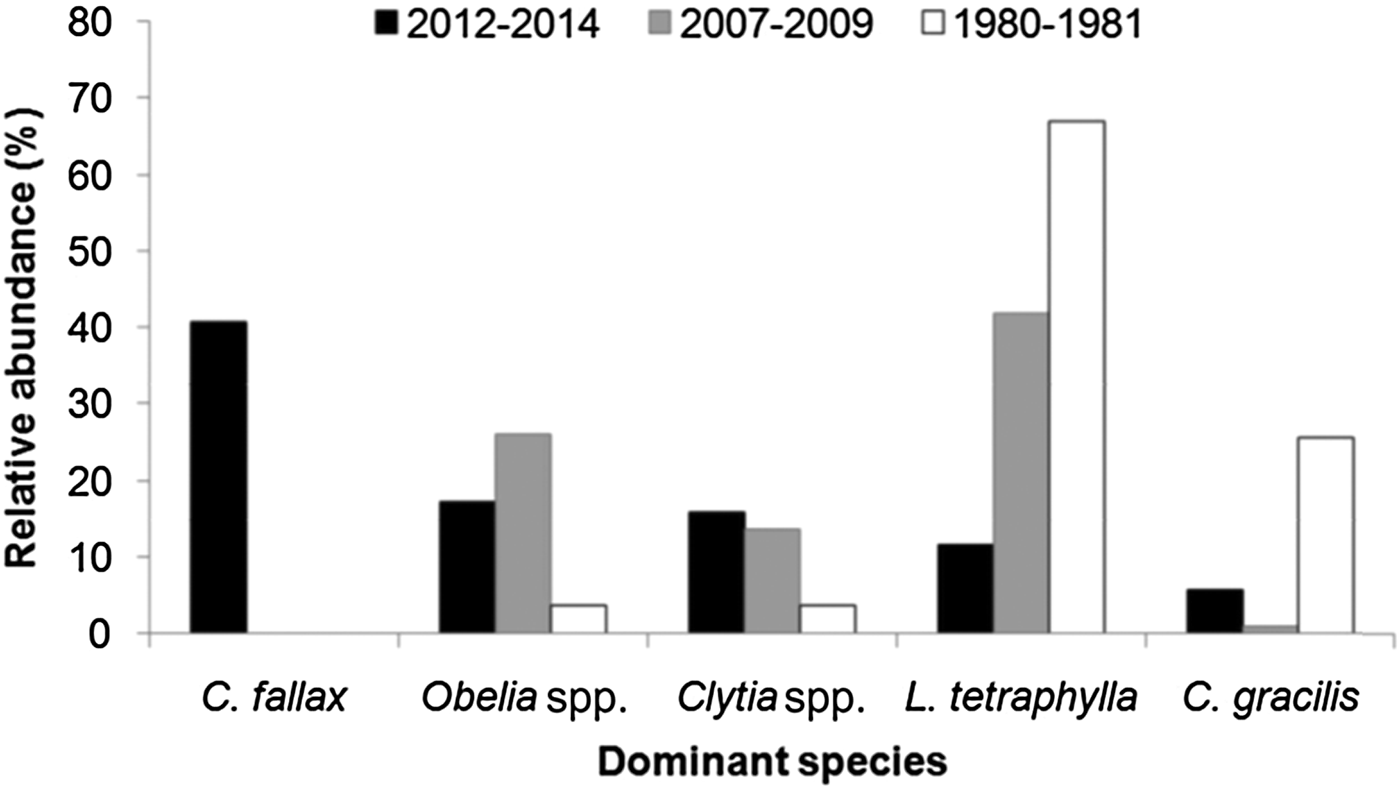
Fig. 16. Comparisons of the relative abundance (%) of dominant hydromedusae species from Paranaguá Estuarine System, southern Brazil from different periods. Data source: (a) 1980–1981 – taken from Montú & Cordeiro, Reference Möller, Fernandes, Seeliger and Odebrecht1989; (b) 2007–2009 – taken from Bardi, Reference Bardi2011; (c) 2012–2014 – present study. Lopes et al. (Reference Lopes, Vale and Brandini1998) did not provide quantitative data on hydromedusae but comment that Liriope tetraphylla was the dominant species.
Independently of the origin of the C. fallax population (hypothesis i or ii above), its massive presence during the present study is noteworthy and suggests a replacement of a historically dominant species. In the present study C. fallax dominated the total abundance and the typical previous dominant species L tetraphylla ranks fourth, representing only 11% of all hydromedusae. This contrasts with previous studies where L. tetraphylla has always been dominant, representing between 42 and 67% of the population (Figure 16). Considering that C. fallax and L. tetraphylla probably have different roles in the ecosystem – the former is meroplanktonic small-sized (<1 mm) and probably eats very small organisms and the latter is holoplanktonic larger-sized (>5 mm) and eats larger organisms like copepods (Figure 14), this shift in the dominance of these species could change the energy pathways of local food webs and the planktonic assemblage structure.
Species which produce medusa buds such as C. fallax can rapidly increase their population levels by asexual reproduction, typically under warm and specific temperature conditions (Carré & Carré, Reference Carré and Carré1990; Kawamura & Kubota, Reference Kawamura and Kubota2008). Thus, we question which environmental factors may have contributed to C. fallax population increase and what impacts it may cause. It occurred over three consecutive summers in the present study, but was mainly captured in summer 2012 (>16,000 medusae), which coincides with the swarm recorded from Patos Lagoon, ~500 km south (Teixeira-Amaral et al., Reference Teixeira-Amaral, Amaral, de Ortiz, Agostini and Muxagata2017); also suggesting that the changes reported here may not be restricted to PES. A hypothesis for the simultaneous occurrence of C. fallax swarms in these Brazilian estuaries may be related to large inter-annual variations in river discharge, circulation and salinization processes associated with ENSO events. March 2012 was at the end of a La Niña event (NOAA, 2017). On the southern Brazilian coast, these events are usually associated with lower precipitation and freshwater outflow, and higher salinity values (Grimm et al., Reference Grimm, Ferraz and Gomes1998; Möller & Fernandes, Reference Montú and Cordeiro2010; Nascimento Júnior & Sant'Anna Neto, Reference Nascimento and Sant'Anna Neto2015) and maybe this condition favoured a C. fallax outburst.
Although C. fallax abundance diminished from summer 2012 to the summer of 2014, it remained among the dominant species (Table 1), maintaining high levels and apparently persistent populations inside PES. Indeed, specimens of different sizes and development were observed every summer. We do not know if this change in assemblage structure is permanent or if it is part of an erratic and intermittent massive occurrence commonly registered for jellyfish around the world (e.g. Buecher et al., Reference Buecher, Goy, Planque, Etienne and Dallot1997; Malej & Malej, Reference Malej, Malej, Dumond, Shiganova and Nierman2004; Boero et al., Reference Boero, Bouillon, Gravili, Miglietta, Parsons and Piraino2008; Licandro et al., Reference Licandro, Souissi, Ibanez and Carré2012; Van Walraven et al., Reference Van Walraven, Langenberg, Witte and Van der Veer2015), including some invasive species (Greve, Reference Greve1994; Riisgård et al., Reference Riisgård, Madsen, Barth-Jensen and Purcell2012; Yilmaz, Reference Yilmaz2015). Thus, further monitoring programmes would be welcome to check if this peculiar hydrozoan species will maintain high population levels in the medium- to long-term. Moreover, future studies assessing the biology and ecology of C. fallax (e.g. genetics and evolution, life cycle constraints, preferential temperature and salinity ranges, effects of climatic events, reproductive potential and feeding rates) will be important to assess which environmental factors contributed to its population outburst as reported here, and what impacts it may cause. The life cycle of C. fallax is not known and its taxonomy is arguable and it was suggested to belong to the families Oceaniidae (Picard & Rahm, Reference Picard and Rahm1954), Cytaeididae (Kramp, Reference Kramp1961) and Hydractiniidae where it is currently placed (Schuchert, Reference Schuchert2016). In any case, it probably has a polyp stage (e.g. Bouillon et al., Reference Bouillon, Gravili, Pages, Gili and Boero2006) and therefore life-cycle studies and factors affecting its potential benthic stage should also be considered.
CONCLUSIONS
The present study constitutes a comprehensive survey of planktonic hydrozoans from PES, a Natural World Heritage site. Currently, a total of 39 species have been recorded from PES, 35 of them studied here. Given the high sampling effort employed (185 samples analysed and >49,000 organisms examined), it can be considered that the planktonic hydrozoan fauna from PES is relatively well represented in the present study, as also suggested by the species accumulation curves (Figure 15), although a few further species are likely to be found. The high biodiversity observed herein indicates that the estuary harbours a diverse aquatic fauna, being important for regional biodiversity conservation. The most abundant species (~40% of all specimens) was unexpectedly Cnidostoma fallax, indicating changes in the assemblage structure compared to previous decades when L. tetraphylla has always been dominant. It is difficult to deduce the reasons for these changes in the planktonic hydrozoan assemblage structure due to the low number of historical studies for Brazilian estuaries. The complete absence of C. fallax from these previous studies from PES and nearby estuaries may suggest it has been recently introduced, although it may have been overlooked. Further studies including molecular analysis (see for instance Harrison et al., Reference Harrison, Kim and Collins2013 for Blackfordia virginica) could help to clarify the origins of such populations. In any case, changes in the structure of the hydromedusae assemblage were clear when compared to previous decades when L. tetraphylla has always dominated. Such replacement may lead to changes in the local pelagic assemblage and food chain. Although C. fallax was abundantly sampled through three consecutive years, future surveys are important to check the permanency and the abundance levels of this population.
ACKNOWLEDGEMENTS
We are grateful for the Taxonline – Rede Paranaense de Coleções Biológicas, University of Paraná, for the resources used in the species photographs and to Franciele Betim for the assistance with the photographs; and Ronei Derengoski, Abrão Pereira de Campos, Bianca Salvador, Laura Straub, Yasmim Freitas and Fernanda de Felipe, partners in the fieldwork. We thank also referees for comments that substantially improved the paper.
FINANCIAL SUPPORT
This study is part of Biomar project, which was financed by Fundação Grupo O Boticário de Proteção à Natureza (BL0002_20111). The authors acknowledge Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (Capes; http://www.capes.gov.br) for funding a Master of Science scholarship for LSN (2014–2016) and Conselho Nacional de Pesquisa (CNPq; http://www.cnpq.br) for funding a scientific initiation scholarship for EMV (2014–2015).