Scientific Classification
Domain: Eukaryota
Kingdom: Plantae
Phylum: Spermatophyta
Subphylum: Angiospermae
Class: Dicotyledonae
Order: Solanales
Family: Solanaceae
Genus: Solanum
Species: Solanum elaeagnifolium Cav.
EPPO Code: SOLEL

Names and Taxonomy
The family Solanaceae lists about 1,400 species worldwide, of which 143 are considered weeds (Sheppard et al. Reference Sheppard, Shaw and Sforza2006). The genus Solanum is the most numerous of the family Solanaceae. Solanum elaeagnifolium Cav. belongs to the clade Leptostemonum, commonly known as the “spiny solanum” clade. Solanum elaeagnifolium is native to northern Mexico and the American Southwest. A revision for the S. elaeagnifolium clade and analytic phytokeys are provided by Knapp et al. (Reference Knapp, Sagona, Carbonell and Chiarini2017). The silvery color of its leaves and their resemblance to the leaves of the olive tree (Elaeagnus) were the reasons for naming the species elaeagnifolium (Heap and Carter Reference Heap and Carter1999). Nowadays, it is commonly known as silverleaf nightshade (Boyd et al. Reference Boyd, Murray and Tyrl1984). According to Krigas et al. (Reference Krigas, Tsiafouli, Katsoulis, Votsi and van Kleunen2021), in northern Greece S. elaeagnifolium is also called “Lernaean Hydra,” due to its intense regrowth after herbicide treatment. Solanum elaeagnifolium is known in South Africa as silverleaf bitter apple or Satansbos (Satan’s bush), indicating how harmful it is to the country (Wilson et al. Reference Wilson, Ivey, Manyama and Nanni2013). In America and other parts of the world, it has received various names over the years, such as white horsenettle, bullnettle, tomatillo, meloncillo, and trompillo (Davis et al. Reference Davis, Smith and Hawkins1945; Kwong et al. Reference Kwong, Sagliocco, Weiss, Hunt, Morfe and Kularatne2006). In Algeria, farmers call it echouka, which means thorn, because of the multiple spines on the stem (Adjim and Kazi Tani Reference Adjim and Kazi Tani2018). In South Korea, it received the name Eun-bit-kka-ma-jung, which is a combination of its silvery coloration and a common plant in the country (Hong et al. Reference Hong, Joo, Hong, Jo and Kim2014).
Importance
Solanum elaeagnifolium is considered one of the most noxious weeds worldwide, negatively affecting a broad spectrum of agricultural and livestock activities, ecosystem services, and environmental parameters (Tataridas et al. Reference Tataridas, Jabran, Kanatas, Oliveira, Freitas and Travlos2022a). Various plant tissues possess toxic substances, specifically the alkaloids solasodine and solanine, which both affect the gastrointestinal tract of animals (Sforza and Jones Reference Sforza and Jones2007). The poisoning is induced by the combination of the alkaloids with sugars, leading to the synthesis of glycoalkaloids (Mellado et al. Reference Mellado, García, Arévalo and Pittroff2008). The most toxic parts are the berries, the consumption of which causes distention, flatulence, respiration problems, and other issues (Smith and Faithful Reference Smith and Faithful1998). According to Qasem (Reference Qasem2014), the most vulnerable animals are cows (Bos taurus) and, to a lesser extent rabbits (Oryctolagus spp.), sheep (Ovis aries), and goats (Capra hircus).
Solanum elaeagnifolium invades fields where cotton (Gossypium spp.), wheat (Triticum spp.), maize (Zea mays L.), vegetables, olives (Olea europaea L.), citrus, vines, and other similar crops are intensively cultivated (FAO 2011). It has allelopathic potential and negatively affects cultivated crops such as wheat and cotton (Balah and AbdelRazek Reference Balah2020). Solanum elaeagnifolium adversely affects both common and durum wheat, but more susceptibility is observed in durum wheat (Albarni et al. Reference Albarni, Al-Mouemar and Ibrahim2012). In Jordan, pepper (Capsicum annuum L.) growers reported that the costs for control were exorbitant, as resprouts were observed to lead to low yields (Qasem Reference Qasem2014). In the United States, it was observed to cause up to a 50% reduction in wheat yields (Sforza and Jones Reference Sforza and Jones2007). Cotton is similarly vulnerable, as only one S. elaeagnifolium plant in 10 m of the cotton row can cause a 0.31% to 0.35% loss in yield (Smith et al. Reference Smith, Pawlak, Murray, Verhalen and Green1990) and a 1.54% reduction in fiber yield (Green et al. Reference Green, Murray and Verhalen1987). Reductions in groundnut (Arachis hypogaea L.) yields amount to 17% if the competition lasts for 4 wk, 53% if competing for 8 wk, and 66% if competing for 12 wk or more (Hackett et al. Reference Hackett, Murray and Weeks1987). In Greece, alfalfa (Medicago sativa L.) yields were suppressed 8% to 26% due to competition with S. elaeagnifolium (Travlos et al. Reference Travlos, Gatos and Kanatas2013). In Egypt, it caused a 30% reduction in barley (Hordeum vulgare L.) yields (Amer Reference Amer, Pullaiah and Ielmini2021). In Tunisia, the weed was found in a very large area of irrigated crops (>2,300 ha) causing yield reductions in annual summer crops (Sayari et al. Reference Sayari, Khebour Allouche, Laarif and Mekki2021). In Morocco, the weed can cause up to 64% yield reduction in maize, while the 1,000-grain weight and the number of grains per bushel are negatively affected (Baye and Bouhache Reference Baye and Bouhache2007), and since its introduction in the country, land value has decreased by 25% (Gmira et al. Reference Gmira, Douira and Bouhache1998). Solanum elaeagnifolium is host of the parasitic weed hemp broomrape (Orobanche ramosa L.) and it has been found that this parasitism leads to a high subsequent infestation of tomato (Solanum lycopersicum L. plants (Qasem Reference Qasem2019).
In Australia, S. elaeagnifolium has been shown to cause up to a 25% reduction in land use value (Stanton et al. Reference Stanton, Wu and Lemerle2011) and up to a 70% reduction in grain yields (Hawker Reference Hawker2004). In the Eyre Peninsula region, yield losses ranged between 5% and 15% on heavy soils, while they reached 30% to 50% on light and sandy soils (Feuerherdt Reference Feuerherdt2010). In South Australia, the annual cost caused by S. elaeagnifolium reached A$10 million (Kwong et al. Reference Kwong, Sagliocco, Weiss, Hunt, Morfe and Kularatne2006). Overall, farmers in Australia spend around A$1,730 yr−1 for S. elaeagnifolium control and experience $7,786 in losses due to loss in production (McLaren et al. Reference McLaren, Morfe, Honan and Holtcamp2004).
Other problems caused by S. elaeagnifolium include the blockage of irrigation canals and allergies reported in Iraq, and problems in harvesting, and biodiversity loss reported in Syria (Bouhache and Gbẻhounou Reference Bouhache and Gbẻhounou2014). It has also been reported that in S. elaeagnifolium invasion zones, reduced visitation of pollinators (mainly those that vibrate anthers such as the genus Amegilla) to flowers of native plants has been observed, thereby reducing seed production from desirable plants (Tscheulin and Petanidou Reference Tscheulin and Petanidou2013; Tscheulin et al. Reference Tscheulin, Petanidou, Zografou and Pantelis2008).
Solanum elaeagnifolium is a primary or secondary host to insects, fungi, bacteria, viruses, and parasitic plants. In areas where cotton is grown in the United States, S. elaeagnifolium appears to be an important host of nymphs and adults of the cotton flea (Pseudatomoscelis seriatus) (Esquivel and Esquivel Reference Esquivel and Esquivel2009), posing a secondary risk to cotton production. In the same country, it was found to be infested by the fungus Alternaria solani, while the insects Planococcus citri, Heliothrips sp., Tetranynchus sp., Coccinella sp., and Musca domestica were found in the aboveground plant tissues. Records from Texas, USA, indicate that the insects tobacco moth (Manduca sexta), an enemy of plants of the family Solanaceae, and groundnut aphid (Aphis craccivora) can successfully complete their biological cycle on S. elaeagnifolium (Chavana et al. Reference Chavana, Singh, Vazquez, Christoffersen, Racelis and Kariyat2021). The highly damaging insect Tuta absoluta has S. elaeagnifolium as its secondary host (Baldé Reference Baldé2013). The Colorado potato beetle (Leptinotarsa decemlineata), which is the most significant defoliator pest of potato (Solanum tuberosum L.) worldwide, may have S. elaeagnifolium as an intermediate host before or after potato harvest for opportunistic feeding (Tscheulin et al. Reference Tscheulin, Petanidou and Settele2009), also posing a problem for summer sowing, as demonstrated on the island of Lesvos in Greece (Tscheulin et al. Reference Tscheulin, Petanidou, Zografou and Pantelis2008). It is also host to both the potato-infecting bacterium ‘Candidatus Liberibacter solanacearum’ (Lso) and the potato psyllid Bactericera cockerelli (Thinakaran et al. Reference Thinakaran, Pierson, Kunta, Munyaneza, Rush and Henne2015). In Tunisia, S. elaeagnifolium was found to be naturally infected by Potato virus Y, being a host and reservoir of the virus in the potato crop through its transmission by the aphids Myzus persicae and Aphis fabae (Boukhris-Bouhachem et al. Reference Boukhris-Bouhachem, Hulle, Rouzé-Jouan, Glais and Kerlan2007). Moreover, S. elaeagnifolium is a host of Cucumber mosaic virus, Tomato yellow leaf curl Sardinia virus, Tomato yellow leaf curl virus, and Pepper mottle virus (Rodriguez-Alvarado et al. Reference Rodriguez-Alvarado, Fernandez-Pavia, Creamer and Liddell2002; Zammouri and Mnari-Hattab Reference Zammouri and Mnari-Hattab2014). Finally, it can also be a refuge for snakes and rodents (Qasem et al. Reference Qasem, Al Abdallat and Hasan2019) and plays host to the parasitic weed field dodder (Cuscuta pentagona Engelm. var. pentagona) (Qasem Reference Qasem2014).
Despite the plant’s multiple negative impacts, there are several uses of various plant parts of S. elaeagnifolium in the fields of food, medicine, animal husbandry, and plant breeding. Solasodine is one of the two known alkaloids of S. elaeagnifolium berries that have led to its export and commercial exploitation for corticosteroids and steroid products in India and Argentina (Roy et al. Reference Roy, Scalera, Booy, Branquart, Gallardo, Genovesi, Josefsson, Kettunen, Linnamagi, Lucy, Martinou, Moore, Pergl, Rabitsch and Solarz2015). The seeds have a high content of flavonoids and phenolics, which demonstrate high antioxidant activity (Feki et al. Reference Feki, Koubaa and Damak2014). The presence of flavonoids and phenolics, combined with the inhibition of glycation end products, has raised the possibility of using the plant against diabetic complications such as atherosclerosis and cataracts (Houda et al. Reference Houda, Derbré, Jedy, Tlili, Legault, Richomme, Limam and Saidani-Tounsi2014). The plant has also been found to contain compounds that appear to limit the growth of cancer cell lines, paving the way for the exploitation of these compounds as anticancer agents (Hernández et al. Reference Hernández, Carranza, Cobos, López, Ascasio and Silva2017). Leaf extracts are considered effective for the containment of various human pathogenic bacteria (Balavivekananthan et al. Reference Balavivekananthan, Xavier, Kumar and Sabitha2021).
Although the consumption of various plant parts by ruminants should be avoided due to the tannins and low digestibility, selective grazing by goats in dry conditions, and always before the onset of flowering at an early vegetative stage, is a practice that can have positive effects on the animals’ nutrition and can provide weed control where there are high infestations (Mellado et al. Reference Mellado, García, Arévalo and Pittroff2008). Solanum elaeagnifolium has also been used over the centuries as a food ingredient, as the Pima Indians used the ripe fruits as a milk coagulant (Néstor et al. Reference Néstor, Dely Rubí and Héctor2012). Successful crosses with S. elaeagnifolium have been made, and new generations of eggplant (Solanum melongena L.) hybrids have been generated that may be more resistant to drought and adapted to low nitrogen levels (Villanueva et al. Reference Villanueva, Rosa-Martínez, Şahin, García-Fortea, Plazas, Prohens and Vilanova2021). Solanum elaeagnifolium can occasionally be used as a “trap crop” for infestation by the parasitic weed O. ramosa, although special care is needed if tomato is grown after S. elaeagnifolium, as reported earlier (Qasem Reference Qasem2019). Other uses of the plant include the utilization of the oil from its seeds for biofuel production and for the production of soap and hair shampoo, as reported by Feki et al. (Reference Feki, Koubaa, Jaber, Makni and Damak2013). It is also a source of lignin and cellulose and can be used as a substrate in mushroom (Pleurotus ostreatus) cultivation (Monsivais et al. Reference Monsivais, Aviles and Martínez2021).
Description
Solanum elaeagnifolium is a summer perennial, deep-rooted species that propagates both sexually through seeds and asexually through rhizomes (Figure 1). The root system of S. elaeagnifolium consists of two parts: (1) the primary root that grows vertically in the soil, is more than 1 cm in diameter, and is the main storage organ of the plant; and (2) the horizontal secondary roots or creeping perennial roots (rhizomes) that emerge from the primary root, grow horizontally in the soil up to 2 m, and then also turn downward. Multiple smaller secondary roots sprout horizontally to the ground and do not have a long life span. All root fragments have the potential to form buds (EPPO 2007). The central root can reach up to 2 m in depth and can produce multiple rhizomes. The shoots have spines, usually orange or brown in color, extending all the way to the flower calyxes. The leaves of S. elaeagnifolium are amphistomatic, elongated, frequently curly, and contain a layer of dense trichomes on the lower surface and a less dense layer on the upper surface. The flowers are hermaphroditic, nectarless, and white to dark-purple colored. Fruit formation takes place in the calyx of the flowers. The size of the fruit, which is a berry, is normally 1.0 to 1.5 cm. Berries are initially green in color, with characteristic vertical lines, turning to dark yellow and brown as the fruit and seeds mature. A berry can bear from 60 to 120 seeds. The size of the seeds is 2.5 to 4.0 mm, and they are held together by a sticky substance contained within the seed.

Figure 1. Vegetative and reproductive growth stages of Solanum elaeagnifolium: (A1) germinated seed, (A2) roots, (B1) seedlings, (B2) sprouts, (C1) trichomes in leaf, (C2) vegetative growth stage, (C3) prickles in shoot, (D) flowering, (E) immature green to mature yellow and ripe brown berry, (F1) mucilaginous substance coating the seeds, (F2) ripe seeds in a berry.
The plant germinates in waves from spring to summer and has an extensive flowering and fruiting period. As a result, in the same spot in a field, plants can be identified at different growth stages, from seedling to the onset of flowering, the green immature berry, and the mature brown berry. Solanum elaeagnifolium populations differ in height, number and shape of leaves, number of fruits, presence and abundance of spines, abundance of trichomes, flower color, and number and size of berries. The enormous genetic variation has recently been suggested to be due to cryptic genetic diversity (Chiarini et al. Reference Chiarini, Scaldaferro, Bernardello and Acosta2018). The genome of S. elaeagnifolium is diploid (2n = 24). Diploid populations have been observed in North America, while polyploidy has been recorded in Argentina, which may indicate that the plant has invaded the country (Chiarini Reference Chiarini2014). In particular, tetraploid (2n = 48) and hexaploid populations (2n = 72) have been recorded in Argentina (Scaldaferro et al. Reference Scaldaferro, Chiarini, Santiñaque, Bernardello and Moscone2012).
Distribution
Europe
According to Dana et al. (Reference Dana, Sanz-Elorza and Sobrino2001), S. elaeagnifolium is not considered invasive in Spain today. In the Balkans, S. elaeagnifolium has been introduced in North Macedonia, Bulgaria, Serbia, Croatia, Montenegro, and Bosnia and Herzegovina. In Bosnia and Herzegovina, it is found in abandoned sites in the city of Mostar (Maslo Reference Maslo2016). In Croatia, it is mainly found in abandoned areas within the urban fabric and across the road network, as well as on two islands (Milovic Reference Milovic2001). In France, attempts have been made to eradicate S. elaeagnifolium (Fried Reference Fried2011), and it is found in fewer than 10 locations, with the most recent being in the Étang de l’Or area in the Valergues commune in southern France (Andrieu et al. Reference Andrieu, Coste and Delaumone2017). It is found on roadsides and in disturbed sites and was first recorded in 2010 in a vineyard in the Montpeyroux area in Hérault (EPPO 2012). In Italy, it is widely distributed. Solanum elaeagnifolium is considered an invasive species in Greece (Arianoutsou et al. Reference Arianoutsou, Bazos, Delipetrou and Kokkoris2010), mainly found across the road network, whether this includes rural and small roads or highways, but also in agricultural areas, especially where durum wheat is grown (Krigas et al. Reference Krigas, Tsiafouli, Katsoulis, Votsi and van Kleunen2021). A 15-yr survey of the weed’s distribution showed that about 60% of the country’s populations are located in just two provinces (Krigas et al. Reference Krigas, Tsiafouli, Katsoulis, Votsi and van Kleunen2021). Solanum elaeagnifolium occurs in 16 different land cover types (or CORINE land cover types), with agricultural land accounting for about 65% of its distribution in Greece (Krigas et al. Reference Krigas, Votsi, Katsoulis and Tsiafouli2016).
Africa
Solanum elaeagnifolium is found in many provinces in South Africa, with reports from 1985 to 2004 describing that the largest infestations are found in the north of the country (Viljoen and Wassermann Reference Viljoen and Wassermann2004). In Lesotho, it is also widespread and invades arable land, forest plantations, pastures, and the road network (Henderson Reference Henderson2001). Knapp et al. (Reference Knapp, Vorontsova and Särkinen2019) classifies the weed taxonomically in Namibia, although there are no other records. In Morocco, the invasion started in 1950 in the cotton cultivation zone and quickly expanded to many areas in the central part of the country (Taleb and Bouhache Reference Taleb and Bouhache2006). Its spread suggests that it crossed 800 km in 45 yr between 1950 and 1995 (Adjim and Kazi Tani Reference Adjim and Kazi Tani2018), and in 55 yr of invasion it has now invaded about 15,000 ha (Brunel Reference Brunel2011). In northeastern Morocco, the spread was slow, because the weed was in uncultivated land, hence the pressure exerted under conditions of intense farming activity was not present (Chafik et al. Reference Chafik, Bouhache, Berrichi and Taleb2013). In Egypt, the spread has been rapid, and within 82 yr of first being reported, it has been detected 420 km west of the first site located near the eastern border of the country and has become established in cropland and perennial crops, across the railway network, along roadsides, and within abandoned sites (Amer Reference Amer, Pullaiah and Ielmini2021). In the El Alem region of Tunisia, where semiarid conditions prevail, S. elaeagnifolium covers about 40% to 60% of the soil in irrigated areas, occurring in scattered spots in 54% of surveyed zones and in uniform infestations in 30% of the zones (Sayari et al. Reference Sayari, Khebour Allouche, Laarif and Mekki2021). In the survey zone set by Sayari and Mekki (Reference Sayari and Mekki2021), it was found that S. elaeagnifolium infestation increased by 72.5 ha in just 6 yr. In Algeria, the weed has spread over hundreds of hectares in the area where it was first recorded and continues to spread and form dense populations, especially near the Mediterranean coast (Adjim and Kazi Tani Reference Adjim and Kazi Tani2018). The same authors recorded that within 35 yr (1980 to 2015) the weed extended it range by 800 km within the country.
Middle East and Asia
According to FAO (2011), about 60% of the arable land in Syria is infested with S. elaeagnifolium, while increased invasions are also observed in areas of Iraq. According to Bouhache and Gbẻhounou (Reference Bouhache and Gbẻhounou2014), infested areas up to 2014 in Iraq amounted to 148 ha; in Jordan, 43 ha; in Syria; at least 27,500 ha; while in Lebanon, there is a limited spread, with less than 1 ha. In Japan, there is only one record indicating that the weed is simply present in the country (Mito and Uesugi Reference Mito and Uesugi2004). In Pakistan, the weed is found in the Tindo Central Kurram area, where its presence is rare (Ali et al. Reference Ali, Yar, Salman Khan, Hussain, Hussain, Hussain, Aneva, Yue Phin Tng and Bussmann2022). The weed has been recorded in China, but no further information is available.
South, Central, and North America
In North America, which includes the native range of S. elaeagnifolium, very large infestations have been recorded for dozens of years. As early as 1979, more than 800,000 ha of cotton in Texas were recorded as infested by the weed (Abernathy and Keeling Reference Abernathy and Keeling1979). In 2010, 1.2 million ha were reported infested by the weed in Texas (Feuerherdt Reference Feuerherdt2010). In South America, the weed has been recorded in Argentina, Chile, Uruguay, and Paraguay. In Chile, populations are found between 18° and 34° latitude (Kwong et al. Reference Kwong, Sagliocco, Weiss, Hunt, Morfe and Kularatne2006), while in Argentina, populations are found between 23° and 41° latitude (Vigna et al. Reference Vigna, Fernández and Brevedan1981). The weed is also recorded in the Bahamas and the Greater and Lesser Antilles Islands, where it is exotic (Knapp Reference Knapp2009).
Australia
According to Hawker (Reference Hawker2004), the weed has invaded more than 210,000 ha in Australia, becoming a problem mainly for cereals and pastures. Kwong et al. (Reference Kwong, Sagliocco, Weiss, Hunt, Morfe and Kularatne2006) reported that the weed has invaded 40,000 ha where cereals are grown. A more recent record reports that in South Australia alone, the areas infested by S. elaeagnifolium are at least 210,000 ha, and in total across the country, they amount to more than 350,000 ha (Feuerherdt Reference Feuerherdt2010) and have the potential to invade 398 million ha (Kwong et al. Reference Kwong, Sagliocco, Weiss, Hunt, Morfe and Kularatne2006). Siebert (Reference Siebert1975) cited an unpublished study that reported that in South Australia the weed expanded from 120 ha to 20,240 ha in 10 to 12 yr. In New South Wales, the affected area nearly quintupled in 15 yr from 20,000 ha to 139,000 ha (Dellow Reference Dellow1993). According to Gopurenko et al. (Reference Gopurenko, Wang, Zhu, Lepschi and Wu2014), the genetic diversity of S. elaeagnifolium populations in Australia compared with populations from areas of its native distribution suggests that it is probably at the stage of successful establishment and dominance, rather than at the beginning of its invasion.
Habitat
Solanum elaeagnifolium has the potential to tolerate extreme environments with heavy drought events and growth limiting factors, such as coastal areas, urban environments, marginal lands, or contaminated soils. Solanum elaeagnifolium thrives in areas with mild winters and warm summers and in both arid (annual precipitation >200 mm) and semiarid bioclimatic zones, as can be observed on our map (Figure 2; Structured Appendix). Typically, it is also found in humid areas with up to 1,200 mm yr−1 of precipitation (Adjim and Kazi Tani Reference Adjim and Kazi Tani2018). In Argentina, diploids and hexaploids are found in areas that record annual precipitation of <500 mm, while hexaploids are found only in areas with >500 mm of annual precipitation (Scaldaferro et al. Reference Scaldaferro, Chiarini, Santiñaque, Bernardello and Moscone2012). It has been suggested that the plant is also native to Argentina (Chiarini et al. Reference Chiarini, Scaldaferro, Bernardello and Acosta2018). One argument used to refute this hypothesis is that more monophagous insect species are observed in North America than in Argentina, suggesting that the invasion in South America occurred after the plant was already established in North America (Olckers and Zimmermann Reference Olckers and Zimmermann1991). The adaptability of S. elaeagnifolium as a member of the subclade Elaeagnifolium of the clade Leptostemonum in arid climate zones is likely due to events that occurred millions of years ago, specifically during the Pliocene (Echeverría-Londoño Reference Echeverría-Londoño2017).
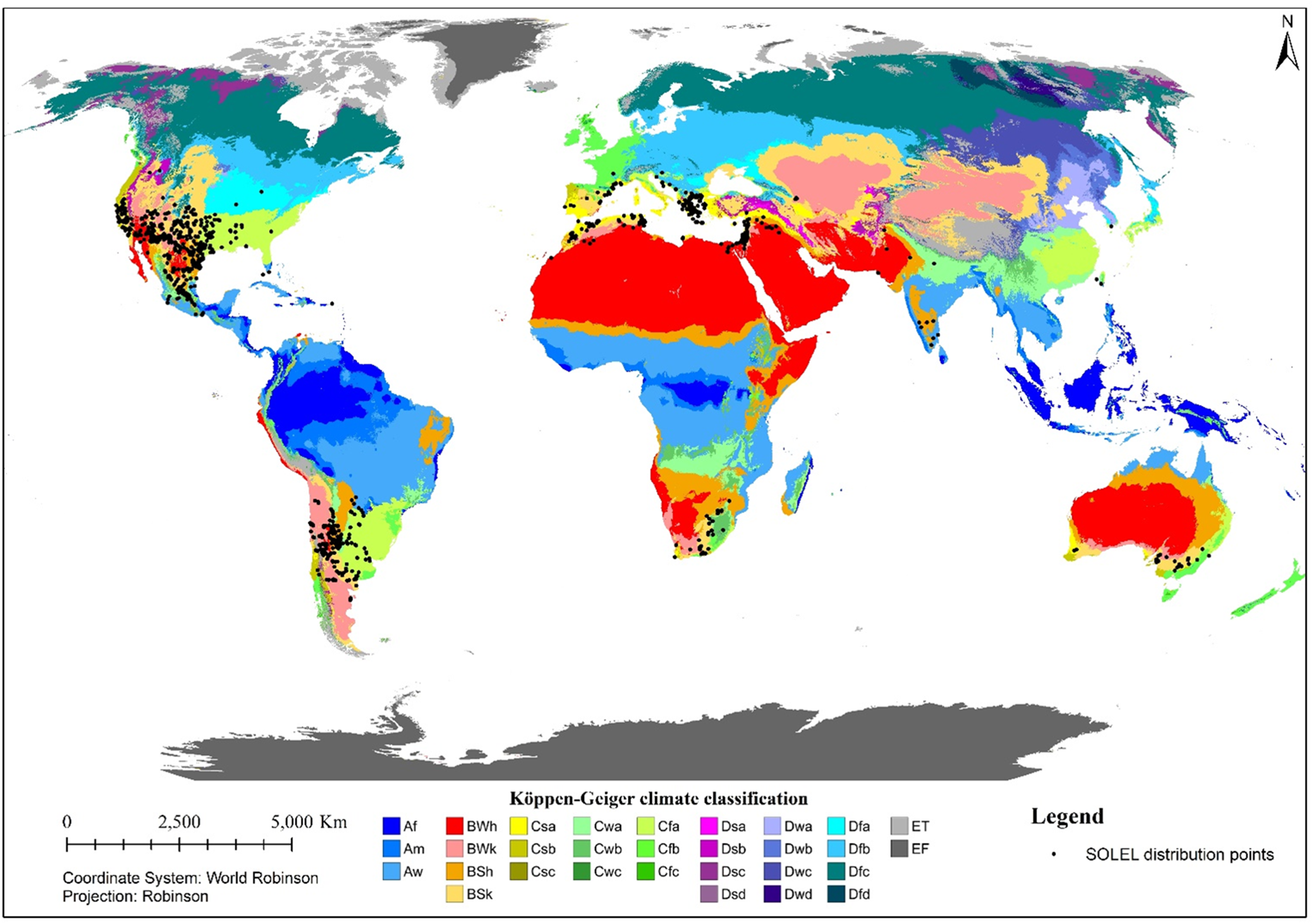
Figure 2. Global records of Solanum elaeagnifolium in relation to the Köppen-Geiger climate classes.
It has been observed to form dense and large populations in coastal areas, flower beds and parks in urban environments, pastures, and perennial crops such as olive or citrus orchards and vineyards, as well as along roadsides, and is generally associated with environments affected by human intervention (Figure 3). Its abundance and dispersal have been found to be positively correlated with anthropogenic activities, highway networks, and ports (especially on islands) (Dimitrakopoulos et al. Reference Dimitrakopoulos, Koukoulas, Michelaki and Galanidis2022). In Jordan, the weed is found both near watercourses and in dry and saline soils, and even in the desert within relatively moist microhabitats (Qasem et al. Reference Qasem, Al Abdallat and Hasan2019). It was found that only diploid populations thrive in saline soils (Scaldaferro et al. Reference Scaldaferro, Chiarini, Santiñaque, Bernardello and Moscone2012). According to Formozis et al. (Reference Formozis, Tsakaldimi and Ganatsas2021), the plant is found on the margins of forests and not inside them, as dense vegetation, high shading by tree canopy, and residues on the ground (bark) do not allow the penetration of the necessary solar radiation and precipitation for the establishment of S. elaeagnifolium. Its resistance to adverse conditions and various abiotic factors makes it a successful and problematic invasive plant for native flora and fauna and human activities. According to Mekki (Reference Mekki2006), it occurs in a wide range of soil types, but not in soils that are deep and sandy or under flooded conditions. It establishes satisfactorily on molisols, vertisols, and alfisols (Vigna et al. Reference Vigna, Fernández and Brevedan1981). In Greece, a large survey of weed populations showed that a very high proportion (>60%) is found in soils with high saturation and low soil-surface organic matter (Krigas et al. Reference Krigas, Tsiafouli, Katsoulis, Votsi and van Kleunen2021). In France, it is mainly found in clay and calcareous soils (Fried Reference Fried2011). In Australia, it was observed to be absent on roadsides where eucalyptus species are found (Zhang et al. Reference Zhang, An, Wu, Li Liu and Stanton2012). In Texas, USA, plants were found to be taller on clay soils than on sandy soils, because plants in sandy soils produced more spines to defend against pests such as insects and therefore did not have the resources to grow as tall (Kasper et al. Reference Kasper, Chavana, Sasidharan, Racelis and Kariyat2021).

Figure 3. Representative habitats infected by Solanum elaeagnifolium. (A) urban areas, (B) agricultural land, and (C) disturbed habitats and abandoned places.
Invasion History
Europe
In Spain and worldwide, the first to characterize the plant was Cavanilles in 1795 in the botanical garden of Madrid (Casasayas i Fornell 1989), but S. elaeagnifolium spread slowly in the country, or perhaps the invasion did not originate in Madrid. In France, it was introduced as a seed contaminant and has been established in the Montpellier area since 1967 (Fried Reference Fried2011). Bouhache and Gbẻhounou (Reference Bouhache and Gbẻhounou2014) report that the weed was deliberately introduced into the Montpellier botanical garden in 1855. In Portugal, the presence of the weed was confirmed in 2022 by Tataridas et al. (Reference Tataridas, Oliveira, Frazão, Moreira, Travlos and Freitas2022d). In Aragon, Spain, it is thought that the plant was introduced as an ornamental because of its beautiful flowers (Cirujeda et al. Reference Cirujeda, Pardo, Anzalone, Leon, Fernández-Cavada, Ochoa and Zaragoza2005). In Bulgaria, the first record was made in 2015, with the most likely method of introduction via transport of seeds by vehicular traffic or as seed contaminant transported via this route (Vladimirov et al. Reference Vladimirov, Bancheva and Delcheva2015). In Bosnia and Herzegovina, the first record was from 2010 in the city of Mostar (Lasić et al. Reference Lasić, Jasprica and Ruščić2010) via accidental introduction. In Croatia, it was first recorded on the island of Plavnik in 1978 (Gaži-Baskova and Šegulja Reference Gaži-Baskova and Šegulja1978). In Montenegro, the introduction was most likely to have occurred through the transfer of weed seeds as contaminants in crop seeds as recorded in three locations in 1999 (Hadžiablahović et al. Reference Hadžiablahović, Karaman and Bulić2004). In Turkey, the first record was from 2007 in a wetland, possibly introduced by birds carrying seeds (Ilcim et al. Reference Ilcim, Uludug and Uremis2016). The point of introduction in Greece is considered to be in northern Greece, specifically Thessaloniki, where S. elaeagnifolium was introduced in 1927 (Krigas and Kokkini Reference Krigas and Kokkini2004), probably from Texas (Krigas et al. Reference Krigas, Tsiafouli, Katsoulis, Votsi and van Kleunen2021), while another probable means of introduction was as a contaminant on tobacco (Nicotiana tabacum L.) seeds (EPPO 2006 from Yannitsaros and Economidou Reference Yannitsaros and Economidou1974).
Africa
The invasion of S. elaeagnifolium on the African continent is divided into two main zones: Northern and Southern Africa. In Morocco, S. elaeagnifolium is estimated to have been introduced either in 1939 as a contaminant on cotton seeds from North America (Gmira et al. Reference Gmira, Douira and Bouhache1998) or in 1949 (Taleb and Bouhache Reference Taleb and Bouhache2006). In 1950, its presence in Morocco coincided with the introduction of cotton cultivation in the Tadla region (Taleb and Bouhache Reference Taleb and Bouhache2006), and it is now considered perhaps the worst weed in the country (Baye et al. Reference Baye, Ameur, Bouhache and Taleb2007) and the most difficult to control. In Tunisia, S. elaeagnifolium was observed around 1985 in the Kairouan area, when it had already begun to have a negative impact on the environment and was a major threat to irrigated land in the semiarid areas of Sbikha (Mekki Reference Mekki2006). Its introduction into the country was probably accidental through trade and tourism (Mekki Reference Mekki2006). In Egypt, the first record was made in 1930 near the border with Palestine (Amer Reference Amer, Pullaiah and Ielmini2021). In Algeria, S. elaeagnifolium was officially recorded in 1999, but it was present in the country at least 15 yr before (Adjim and Kazi Tani Reference Adjim and Kazi Tani2018). In South Africa, the first official record of the weed was reported in 1952, although the first records appear from 1905 with the accidental introduction of the weed in feed intended for pigs (Sus domesticus). In Zimbabwe, the first record was made in 1969 (Pitso Reference Pitso2010).
Middle East and Asia
The most likely means of introduction of the weed into Middle Eastern countries were via sheep (Jordan and Lebanon) or as a contaminant in cotton seeds and via camels (Camelus spp.) in Syria (Bouhache and Gbẻhounou Reference Bouhache and Gbẻhounou2014). In Lebanon, one of the first records was made only recently in 2015 in the north Beqaa region, with its introduction as a contaminant in the soil of tree seedlings (Haidar and Sabra Reference Haidar and Sabra2015). In Jordan, S. elaeagnifolium was recorded in 1970, and the first systematic records were made in the early 21st century on the sides of highways with a possible means of dispersal being via the digestive tracts of sheep (Qasem Reference Qasem2014). Solanum elaeagnifolium was first recorded in Iran in 2013 in cotton fields (Arabsalmani et al. Reference Arabsalmani, Farahani and Saeedi2014). The first records in Azerbaijan were made in 2016 and verified in 2021 near habitable areas (Zernov and Mirzayeva Reference Zernov and Mirzayeva2016). Russia, which borders Azerbaijan, includes S. elaeagnifolium in its quarantined weed lists, although it has never been recorded there. In South Korea, S. elaeagnifolium was observed on a roadside near the sea on an island in 2014 (Hong et al. Reference Hong, Joo, Hong, Jo and Kim2014).
South, Central, and North America
Although no official records of S. elaeagnifolium invasion appear in Brazil, Taleb and Bouhache (Reference Taleb and Bouhache2006) include Brazil in the list of countries where S. elaeagnifolium has been introduced. A 1916 study highlights that S. elaeagnifolium had by then been recognized as a common weed between Argentina and New Mexico (Standley Reference Standley1916), and in 1893 as a native species of Argentina (Philippi Reference Philippi1893). In Mexico, official records of the plant are old, for example, in the Nuevo Leon region (Loesener Reference Loesener1913). In the United States, some of the oldest records are from Ohio, Mesilla, NM, and other areas of the U.S. Southwest. In California it is estimated to have been introduced through cattle bedding transported on trains in 1890.
Australia
In Australia, the S. elaeagnifolium invasion began in the early 20th century, probably in 1901 in the Bingara region (Stanton et al. Reference Stanton, Heap, Carter and Wu1999) or in 1909 in north Melbourne (Smith and Faithful Reference Smith and Faithful1998), where weed propagules were transferred as a contaminant in seeds and feed (Wu et al. Reference Wu, Stanton and Lemerle2016).
Dispersal and Establishment
According to FAO (2011), the dispersal of weed seeds worldwide is facilitated by global and local trade, wherein the plant’s propagative material (mainly seeds) is found as a contaminant in crop seeds or in storage facilities. Its transport via straw for animal feed was recorded in Morocco (Chafik et al. Reference Chafik, Bouhache, Berrichi and Taleb2013). Alfalfa has been suspected as a means of long-distance transport of S. elaeagnifolium seeds, as animal feed is a commercial product transported through global trade. In Algeria, weed propagules were transported to two oases north of the Sahara, probably by farmers who came from nearby destinations (Adjim and Kazi Tani Reference Adjim and Kazi Tani2018). Qasem (Reference Qasem2014) claims that the seeds can be transported over long distances through specific irrigation sources. Indeed, in Algeria, rivers are considered a means of transporting the weed southward in the country to new areas, while in the west of the country, a large area in Sebkha with high salinity is an “ecological barrier” to the spread of the weed (Adjim and Kazi Tani Reference Adjim and Kazi Tani2018). Agricultural machinery is a means of weed dispersal that can rapidly multiply populations of S. elaeagnifolium, as farm machinery is usually not adequately cleaned and is used on multiple fields (Chafik et al. Reference Chafik, Bouhache, Berrichi and Taleb2013). Vehicular traffic has generally been blamed for dispersing weed seeds and invasive plants to new areas. The sticky mucilaginous substance between S. elaeagnifolium berries provides an ideal medium for the seeds to adhere to vehicles, facilitating transport via roads.
Sheep and camels have been recorded as means of dispersal to new areas (Bouhache and Gbẻhounou Reference Bouhache and Gbẻhounou2014). Specifically, seeds were found to be shed in sheep manure up to 31 d after consumption, while the highest proportion was shed up to 9 d after consumption (Heap and Honan Reference Heap and Honan1993). Birds are another means of dispersing the weed, as they can carry the seeds over distances of more than a kilometer. Recently, ground squirrels (Spermophilus citellus) were reported to feed on the shoots, leaves, flowers, and seeds of S. elaeagnifolium, possibly increasing the dispersal of the weed (Rammou et al. Reference Rammou, Astaras, Migli, Boutsis, Galanaki, Kominos and Youlatos2022). Also, in Barcelona, Spain, S. elaeagnifolium seeds were found in the stomach of gulls (Larus michahellis), indicating that birds are a means of dispersal over long distances, this being a particular problem in urban environments (Martín-Vélez et al. Reference Martín-Vélez, Montalvo, Afán, Sánchez-Márquez, Aymí, Figuerola, Lovas-Kiss and Navarro2022). Undigested manure is a medium that enhances invasions in new areas (Adjim and Kazi Tani Reference Adjim and Kazi Tani2018), as demonstrated in the eastern regions of Morocco (Chafik et al. Reference Chafik, Bouhache, Berrichi and Taleb2013), where seeds survived a few weeks within the gastroesophageal system of animals.
Invasion Risk and Pathways
Kriticos et al. (Reference Kriticos, Crossman, Ota and Scott2010) modeled the climatic suitability for S. elaeagnifolium using CLIMEX (Kriticos et al. Reference Kriticos, Maywald, Yonow, Zurcher, Herrmann and Sutherst2015). The annual Growth Index (GIA) highlights the potential for ephemeral populations of S. elaeagnifolium to grow throughout most continental regions aside from extremely cold and dry regions (Figure 4). The limitations are mostly due to insufficient moisture in the desert regions of Africa, Asia, and Australia (Figure 5). In northwestern South America, continuous excessive moisture prevents growth of S. elaeagnifolium. The lack of suitable moisture for growth of S. elaeagnifolium is also reflected in the Dry (Figure 6) and Wet Stress maps (Figure 7). Cold stress limits the poleward range in the midlatitudes in the Northern Hemisphere. In the Southern Hemisphere, lethal cold stress occurs in the Andes Mountains and many parts of the South Island of New Zealand (Figure 8). The Ecoclimatic Index (EI) describes the climatic suitability as a number between 1 and 100, calculated from the GIA and Stress indices (Figure 9).

Figure 4. Potential global distribution of Solanum elaeagnifolium. Annual Growth Index (GIA) for Solanum elaeagnifolium modeled using CLIMEX. Meteorological data from CliMond 1995H, v. 2.

Figure 5. Potential global distribution of Solanum elaeagnifolium. Moisture Index (MI) for Solanum elaeagnifolium modeled using CLIMEX. Meteorological data from CliMond 1995H, v. 2.

Figure 6. Potential global distribution of Solanum elaeagnifolium. Dry Stress (DS) for Solanum elaeagnifolium modeled using CLIMEX. Meteorological data from CliMond 1995H, v. 2.

Figure 7. Potential global distribution of Solanum elaeagnifolium. Wet Stress (WS) for Solanum elaeagnifolium modeled using CLIMEX. Meteorological data from CliMond 1995H, v. 2.

Figure 8. Potential global distribution of Solanum elaeagnifolium. Cold Stress (CS) for Solanum elaeagnifolium modeled using CLIMEX. Meteorological data from CliMond 1995H, v. 2.

Figure 9. Potential global distribution of Solanum elaeagnifolium. Global climatic potential distribution of Solanum elaeagnifolium (Kriticos et al. Reference Kriticos, Crossman, Ota and Scott2010). Climate suitability (CLIMEX Ecoclimatic Index [EI]) is mapped as a composite of natural rainfall and irrigation.
Under current climate conditions, central Europe, the Middle East, and China are climatically suitable for S. elaeagnifolium but as yet uninvaded (Figure 9). According to Kriticos et al. (Reference Kriticos, Crossman, Ota and Scott2010), S. elaeagnifolium is expected to spread toward the poles in the future due to climatic changes that will temper cold stress limits. Climate change is expected to bring warmer summers, increasing the impact of the weed (Stanton et al. Reference Stanton, Wu and Lemerle2011), as it will be able to adapt to the adverse conditions of water shortage and increasing temperatures (Kriticos et al. Reference Kriticos, Crossman, Ota and Scott2010). The prevalence of semiarid and arid conditions is expected to create time frames within which the weed will have an advantage in establishment, growth, and competition over native vegetation (Mekki Reference Mekki2006). In addition to anthropogenic activities and the expansion of road networks that favor the spread of S. elaeagnifolium, it is believed that high mean and maximum temperatures in summers and high minimum temperatures in winters favor its further spread (Krigas et al. Reference Krigas, Votsi, Katsoulis and Tsiafouli2016). However, how far north in Europe S. elaeagnifolium can spread depends on its sensitivity to frost and waterlogging (Krigas et al. Reference Krigas, Tsiafouli, Katsoulis, Votsi and van Kleunen2021). Rising CO2 levels are expected to reduce the effectiveness of herbicides overall, thereby affecting the management of plant invasions.
Life-Form and Life History
Solanum elaeagnifolium is a stress-tolerant perennial shrub (see section “Ecophysiology”). Its life lasts about 6 to 8 mo, followed by a period of dormancy in winter (Figure 10). There is, however, the possibility that the life cycle may be shorter or longer depending on soil and environmental conditions, cultural practices, competition with native vegetation or crops, and the influence of various biotic and abiotic stress factors. In the Northern Hemisphere, including the conditions in Greece, the germination of seeds and the emergence of new seedlings derived from weed rhizomes begins in March to April, coinciding with the spring rains and the elevation of temperatures. In countries such as Syria, Algeria, and Morocco, seed germination starts in February. Germination occurs in waves and lasts until the end of summer, when it declines as autumn approaches, as temperature conditions are not favorable for the germination of weed seeds or sprouting. Solanum elaeagnifolium flowers early in the summer with rising temperatures. Berry formation begins in midsummer with the appearance of the first immature, green-colored berries with characteristic dark green lines. Over a period of about 1 to 2 mo the berry ripens and turns yellow, at which point the leaves of the plant begin to shed, and the seeds of the fruit gradually ripen. By October, most of the fruits have taken on a dark-yellow or brown coloring and usually remain on the shoots over winter. Frequently, the opening of the berries and the dispersal of the seeds is observed while they are on the shoots or have fallen to the ground. The seeds go through a dormancy stage in winter (after November), and new shoots are observed the following spring, following the perennial cycle of the weed’s life cycle. Shortening the life cycle through mowing or stem cutting leads to a reduction in plant height, biomass production, and a reduction or failure of berry and seed production (Zhu et al. Reference Zhu, Wu, Stanton, Raman, Lemerle and Burrows2012, Reference Zhu, Wu, Stanton, Burrows, Lemerle and Raman2013c).
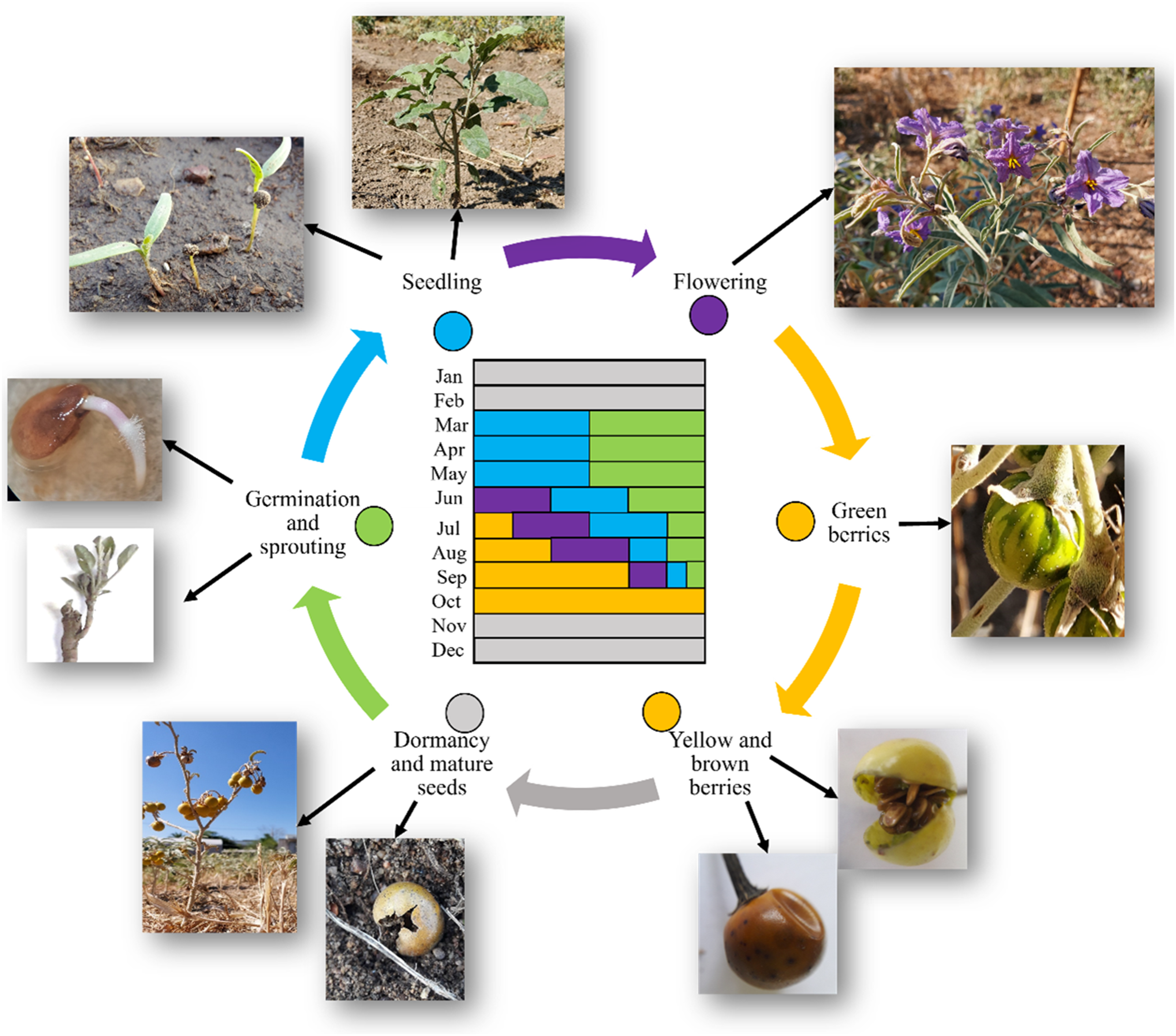
Figure 10. The life cycle of Solanum elaeagnifolium (in the Northern Hemisphere). Green, initiation of seed germination and vegetative reproduction from roots; blue, first growth stages of the new shoots, which are either from seeds or lateral roots; purple, flowering period; orange, berry formation and the gradual maturity of berries and the change of color from green to yellow and brown. Plants are dormant in the winter (gray), and the cycle is repeated in spring.
Growth and Development
Morphology
Roots
A single S. elaeagnifolium plant can produce up to 15 to 20 new plants in a single growing season. During the first year of rootstock establishment, sprouts can grow to a 25-cm radius (Sayari and Mekki Reference Sayari and Mekki2021), and new shoots can arise from dormant root fragments for up to 18 mo. In undisturbed soils, a large proportion of roots develop at depths of up to 5 cm, while in disturbed soils (e.g., where roots have been fragmented by soil tillage), root fragments are distributed to a depth of up to 40 cm in the ground (Cuthbertson Reference Cuthbertson1976). Davis et al. (Reference Davis, Smith and Hawkins1945) report that roots of S. elaeagnifolium can reach a depth of more than 274 cm in sandy soil: 45% of the rhizomes were found up to the first 30 cm near the soil surface, 80% up to 120 cm, and 99% up to 274 cm. In heavy clay soil, the roots have been found to extend up to 120 cm in depth (Tideman Reference Tideman1960).
Shoot and Leaves
The dry weight of the plant is about 8 to 10 g at 70 d after emergence (Bryson et al. Reference Bryson, Reddy and Byrd2012). Root fragments placed at 8 cm in the soil produced new shoots 13 d later (Boyd and Murray Reference Boyd and Murray1982b). Christodoulakis et al. (Reference Christodoulakis, Lampri and Fasseas2009) describes the morphology of the leaf in detail. Leaf size varies between 2.5 and 16 cm in length and 1 and 4 cm in width (EPPO 2006). The trichomes are stellate hairs with 8 to 16 rays and are found on both leaves and shoots. The hairs on the upper leaf surface have deep “roots” that pierce the mesophyll, making them particularly difficult to detach from the leaf. The thickness of the leaf is determined to be about 800 µm, and a dense parenchymal mesh of elongated cells is formed on the adaxial leaf surface, occupying 40% of the mesophyll (Christodoulakis et al. Reference Christodoulakis, Lampri and Fasseas2009). The epidermal cells of the abaxial leaf surface are smaller in size and have thinner cell walls than the epidermal cells of the adaxial surface. The anomocytic stomata are located on both leaf surfaces (Christodoulakis et al. Reference Christodoulakis, Lampri and Fasseas2009). Mesophyll cells and trichomes were found to secrete secondary metabolites such as flavonoids, alkaloids, and phenolics (Christodoulakis et al. Reference Christodoulakis, Lampri and Fasseas2009), which could be used for medicinal purposes (Bouslamti et al. Reference Bouslamti, El Barnossi, Kara, Alotaibi, Al Kamaly, Assouguem, Lyoussi and Benjelloun2022).
Flower
The corolla is 2.5 to 3.5 cm in diameter (Knapp et al. Reference Knapp, Vorontsova and Särkinen2019). Anthers are 6- to 10-mm long (Knapp et al. Reference Knapp, Vorontsova and Särkinen2019). There are five stamens that consist of short cylindrical filaments supporting elongated poricidal anthers. The pollen is particularly rich in nitrogen and protein, making it an attractive source for pollinators. The number of flowers is positively correlated with plant height (Petanidou et al. Reference Petanidou, Price, Bronstein, Kantsa, Tscheulin, Kariyat, Krigas, Mescher, De Moraes and Waser2018).
Berries and Seeds
Viljoen et al. (Reference Viljoen, Stoltsz and Rijst2011) report that a plant can produce up to 200 berries in 1 yr. Depending on the maturity stage of the berry, the levels of phenolic substances and flavonoids vary (Houda et al. Reference Houda, Derbré, Jedy, Tlili, Legault, Richomme, Limam and Saidani-Tounsi2014). Fruit yield is positively correlated with the number of lateral shoots per plant and number of seeds per fruit (Khanna and Singh Reference Khanna and Singh1987). Solasodine levels are negatively correlated with the number of fruits per plant, number of lateral shoots per plant, and number of seeds per fruit (Khanna and Singh Reference Khanna and Singh1987). Overall, it has been found that plants germinating in March (for the Northern Hemisphere) and derived from rhizomes produced >10,000 seeds per plant, while seed-derived plants produced 1,200 seeds (Zhu et al. Reference Zhu, Wu, Stanton, Burrows, Lemerle and Raman2013c). Delayed germination leads to reduced berry and seed production, and seeds that germinated in July did not result in berry formation in the Northern Hemisphere (Boyd and Murray Reference Boyd and Murray1982b).
Stress Tolerance
Solanum elaeagnifolium exhibits high phenotypic plasticity and genetic diversity, factors that each assist it in adapting to different environments and resisting a range of stresses (Singleton et al. Reference Singleton, Mangat, Shim, Vavra, Coldren and Angeles-Shim2020). Drought can act as an inhibitor of the weed’s reproductive and invasive potential. The amphistomatic leaf type is a characteristic that makes S. elaeagnifolium desiccate and allows it to absorb CO2 more efficiently and to adapt to the specific conditions of semiarid regions, such as the Mediterranean region (Christodoulakis et al. Reference Christodoulakis, Lampri and Fasseas2009). In environments where adverse conditions prevail (such as roadsides or where drought or high salinity prevails), the plant usually displays pronounced trichomes and dense prickles, while plant height remains low (Qasem et al. Reference Qasem, Al Abdallat and Hasan2019). In contrast, S. elaeagnifolium bears fewer prickles under conditions of water sufficiency (Qasem Reference Qasem2014). Increased shade leads to a reduction in height, leaf number, dry weight accumulation, fruit production, photosynthetic capacity, and total nonstructural carbohydrate concentration in the roots (Boyd and Murray Reference Boyd and Murray1982a). However, the negative effect of increased shading is more visible in seed-derived plants than in plants derived from the plant’s rhizomes, as the latter are more tolerant of shading due to replenishment of nutrients stored in the underground storage organs. Dense hairs on both the upper and lower leaf surfaces have been documented as a factor that probably moderates herbicide uptake, as there is poor wetting of the leaf cuticle; reduces insect damage by acting as a natural barrier; and probably causes leaf “chilling” during the summer months, when high temperatures act as evapotranspiration rates are reduced (Burrows et al. Reference Burrows, White, Harper, Heady, Stanton, Zhu, Hu and Lemerle2013). Plant injury (either through mowing or biological agents) has been found to induce the expression of specific genes that increase levels of secondary metabolites, such as terpenes, which are associated with plant defenses against biotic stress factors (Tsaballa et al. Reference Tsaballa, Nikolaidis, Trikka, Ignea, Kampranis, Makris and Argiriou2015).
Ecophysiology
In the native range of S. elaeagnifolium (Mexico and the southern United States), dry winters and wet summers prevail. In Mexico, S. elaeagnifolium even thrives in the Chihuahuan Desert, where annual rainfall is 250 mm, arid conditions prevail, and total rainfall is about 75 mm in the summer months of July and August (Contreras-Cisneros Reference Contreras-Cisneros, Mata-González, Trejo-Calzada, Pedroza-Sandoval, Prado-Tarango and Abdallah2022). In Victoria in Australia, S. elaeagnifolium occurs frequently in areas where annual rainfall ranges between 360 and 560 mm, preferring light soils (Smith and Faithful Reference Smith and Faithful1998), whereas throughout the country it is found in warm areas with annual rainfall of 250 to 600 mm (Feuerherdt Reference Feuerherdt2010). Sudden and heavy summer rainfall is capable of triggering a new wave of weed seed germination (Rutherford Reference Rutherford1978). Typically, S. elaeagnifolium spreads in many areas of Tunisia where summers are hot and arid, and the average maximum annual temperature is 36 C (Mekki Reference Mekki2006), and typically in the El Alam region, where annual rainfall is 300 to 400 mm, winters are mild and rainy, summers are hot and dry, and soils are light (Sayari et al. Reference Sayari, Khebour Allouche, Laarif and Mekki2021). In Algeria, it was observed that S. elaeagnifolium does not thrive in areas where the Saharan climate prevails and is mainly found near Mediterranean climates where the lowest average temperature (3 C) is observed in January (Adjim and Kazi Tani Reference Adjim and Kazi Tani2018). However, a few years ago, S. elaeagnifolium was detected on the northern border of the Sahara in two oases (600 m above sea level), which is a concern for the further spread of the weed farther south in irrigated areas and relatively moist microhabitats (Adjim and Kazi Tani Reference Adjim and Kazi Tani2018). In the northwest of the country, it also occurs in stony areas with alkaline pH.
A greenhouse experiment demonstrated that under water-sufficient conditions, S. elaeagnifolium produced more than 3,400 seeds per plant, accumulated 49% to 66% more biomass, and developed >60% more leaf area than plants grown under low-irrigation conditions (Travlos Reference Travlos2013). These responses suggest that water availability is critical to S. elaeagnifolium growth and are indicative of its ability to spread, establish, and dominate in areas with adequate water supply and high soil moisture. According to Adjim and Kazi Tani (Reference Adjim and Kazi Tani2018), winter dormancy of S. elaeagnifolium starts when temperatures are less than 10 C and is accompanied by the necrosis of the aboveground part of the plant.
Phenology and Population Dynamics
Solanum elaeagnifolium exhibits high genetic diversity that allows the plant to develop new biotypes that may be better adapted to the environment or be more resistant to management practices (Zhu et al. Reference Zhu, Wu, Raman, Lemerle, Stanton and Burrows2013b). The prickles that are formed along the shoots make hand weeding and grazing difficult (Qasem Reference Qasem2014).
In Australia, fruit formation and seed production has been reported twice in one season, as a 75-mm rainfall following a hot and dry summer caused resprouting (McKenzie Reference McKenzie1976). In Syria, flowering starts in early May and extends to the end of August, while fruiting starts in July (Bakkour et al. Reference Bakkour, El-Meamar and El-Naser2021). In a greenhouse experiment in the United States, flower emergence in S. elaeagnifolium began 63 d after emergence, and the plants reached 97 cm in height, having 38 leaves 70 d after emergence (Bryson et al. Reference Bryson, Reddy and Byrd2012). The emergence of the first flowers is prolonged in cases where seeds germinate or rhizomes sprout early in spring; however, the extended life cycle leads to the production of more berries and, consequently, seeds (Zhu et al. Reference Zhu, Wu, Stanton, Raman, Lemerle and Burrows2012). The same authors observed that rhizome-derived plants flower 20 to 30 d earlier than seed-derived plants, provided that growth starts in spring (September to November for the Southern Hemisphere). Specifically, overwintering rhizome-derived plants need about 80 and 63 d to flower if growth starts in March or April, respectively, while seed-derived plants need 110 and 84 d, respectively (Zhu et al. Reference Zhu, Wu, Stanton, Burrows, Lemerle and Raman2013c). Plants growing from early spring (March and April) need about 1,006 and 872 growing degree days (GDD), respectively, to flourish at a base temperature of 10 C, while rhizome-derived plants need 706 and 643 GDD, respectively (Zhu et al. Reference Zhu, Wu, Stanton, Burrows, Lemerle and Raman2013c). The earlier germinating/sprouting plants do not require supplementary GDD; rather, they are exposed to a higher cumulative sum of GDD before the onset of anthesis. About 12.7 to 25.4 mm of rainfall has been reported to be required for seed germination in spring in cultivated areas (Turner et al. Reference Turner, Sanchez, Vavra, Dhaliwal, Emendack, Coldren and Angeles-Shim2021). The seeds of S. elaeagnifolium experience somatic heterochrony, which is a risk-spreading trait to allow the plant to persist in areas with low and erratic summer rainfall.
Reproduction
Floral Biology
Solanum elaeagnifolium is generally an obligate crossing species with gametophytic self-incompatibility. However, under conditions where foreign pollen is lacking or flowers have aged, plants can partially convert to be self-compatible. In the native range in Arizona, USA, plants were found to be self-compatible, while in the invasion zone in Thessaloniki, Greece, plants were found to be self-incompatible (Petanidou et al. Reference Petanidou, Godfree, Song, Kantsa, Dupont and Waser2012). Allelic redistribution, as it is necessarily cross-pollinating, creates genomic recombinations leading to high genetic variation of plants within and between populations (Singleton et al. Reference Singleton, Mangat, Shim, Vavra, Coldren and Angeles-Shim2020).
One of the main characteristics of S. elaeagnifolium is the very high variability in flower coloration, which occurs in populations that may be in very close proximity (Qasem et al. Reference Qasem, Al Abdallat and Hasan2019). In Jordan, it was observed that darker purple and blue flowers were found in fertile and heavy soils, while flowers of other colors were found in less fertile or saline soils, where adverse conditions for plant growth prevailed (Qasem Reference Qasem2014; Qasem et al. Reference Qasem, Al Abdallat and Hasan2019).
Pollination is carried out almost exclusively by buzz pollinators, which perform multiple flights and can visit hundreds of flowers within a few hours. Buzz pollinators vibrate the anthers during their visit to pick up valuable pollen. Their flights are intensive in the early morning hours and diminish during the day and as temperatures rise. Flowers face downward, preventing the stamens and corolla from being moistened by rainwater and preventing pollen drop from the vibrations of the buzz pollinators. Anthers are arranged in a loose arrangement that allows for vibration by pollinators and optimal pollen release (Vallejo-Marín et al. Reference Vallejo-Marín, Nunes and Russell2021). In an experiment conducted in the United States, it was found that the amount of pollen extracted with an electric toothbrush in an artificial pollen extraction experiment was positively correlated with the duration of anther vibration (Tayal and Kariyat Reference Tayal and Kariyat2021). In the native range in Texas, USA, visits of Xylocopa tabaniformis (Apidae) and Xylocopa varipuncta (Apidae) were observed on the flowers of S. elaeagnifolium (Rubio et al. Reference Rubio, Wright and Longing2022), while in south Texas, visits from the genera Exomalopsis, Halictus, Megachile, and Bombus were observed (Tayal and Kariyat Reference Tayal and Kariyat2021). In Lesvos, Greece, more than 70% of the visits were from buzz pollinators of the genus Amegilla, much less from pollinators of the family Halictidae, and almost negligible from pollinators Megachilidae and Xylocopa spp. (Tscheulin et al. Reference Tscheulin, Petanidou, Zografou and Pantelis2008). The flowers were visited an average of 1.91 times within 1 h.
Seedbank and Seed Production
Boyd and Murray (Reference Boyd and Murray1982b) reported that dense populations of S. elaeagnifolium have seed production potential of as much as 250 million seeds ha−1. One plant produces from 1,500 to 36,000 seeds according to Bouhache (Reference Bouhache2010). There are reports that S. elaeagnifolium seeds maintain their viability in soil for at least 6 yr and possibly longer (Mekki Reference Mekki2007; Stanton et al. Reference Stanton, Wu and Lemerle2011). Although seeds have reduced germination capacity, a large weed seedbank is created in the soil. In Morocco, more than 44,000 seeds m−2 were found at a depth of 60 cm, although not all of them were viable (Adjim and Tani Reference Adjim and Kazi Tani2015). Soil treatment causes changes in the seedbank. It has been found that seeds buried at 10 cm retained higher viability and germination than seeds buried more superficially at 3 yr after burial (Stanton et al. Reference Stanton, Wu and Lemerle2012). The same authors observed that seeds located within the fruit maintained viability and germination capacity at high levels for up to 2 yr, regardless of the depth at which the fruit was buried. Seedling emergence was found to be significantly reduced when the seeds were more than 1-cm deep in the soil (Boyd and Murray Reference Boyd and Murray1982b).
The application of phytoregulatory substances and phytohormones has been proposed as a strategy to promote germination potential and rate of germination in the soil seedbank by breaking the dormancy, so that it becomes possible to manage the plants at a young seedling stage and reduce the potential for replenishing the soil seedbank with new seeds. Several studies reported that seeds responded positively to gibberellic acid application (Balah et al. Reference Balah, Hassany and Mousa2021), whereas application of abscisic acid resulted in a reduction of seed germination (Turner et al. Reference Turner, Sanchez, Vavra, Dhaliwal, Emendack, Coldren and Angeles-Shim2021).
Seed Germination, Viability, and Dispersal
The seeds of S. elaeagnifolium are covered by a mucilaginous substance that inhibits germination (Mekki Reference Mekki2007). Chavana et al. (Reference Chavana, Singh, Vazquez, Christoffersen, Racelis and Kariyat2021) observed that most germination occurs up to 5 wk after sowing, but the rate of germinated seeds over total seeds planted is about 50%. Seed germination is favored by varying temperatures (mainly 15/25 C night/day, respectively), while constant temperatures between 20 and 40 C result in a very low seed germination level of less than 5% (Stanton et al. Reference Stanton, Wu and Lemerle2012). In a controlled condition experiment conducted in Greece, the constant temperature of 25 C and photoperiod of 12 h led to germination of ∼22% of seeds, starting on day 8 to day 22; beyond this day no further seed germination was observed (Formozis et al. Reference Formozis, Tsakaldimi and Ganatsas2021). In a greenhouse experiment in the United States, the first emergence of a seedling from an S. elaeagnifolium seed occurred at 8 d when temperatures were 22/30 C (Bryson et al. Reference Bryson, Reddy and Byrd2012). Light does not seem to affect the germination of S. elaeagnifolium seeds, as they germinated satisfactorily in both the presence and absence of light under conditions of alternating temperatures of 10/25 C (Podda et al. Reference Podda, Santo, Puddu, Biagini and Bacchetta2015). Seed germination decreases with increasing osmotic pressure (> −0.2 MPa) and temperatures also play an important role, as the germination level was found to be 26% under alternating temperatures of 15/25 C, a 12-h photoperiod, and 0.24 MPa, compared with alternating temperatures of 10/25 C and 15/30 C, where the germination level was less than 10% (Stanton et al. Reference Stanton, Wu and Lemerle2012).
In another experiment, osmotic pressure at −0.5 MPa led to complete inhibition of seed germination (Balah et al. Reference Balah, Hassany and Mousa2021). The germination level of seeds also decreases with increasing NaCl levels, as the germination level reaches 70% in distilled water, while it decreases to less than 20% at 80 mM NaCl, and finally reaches 5% germination at 160 mM NaCl (Stanton et al. Reference Stanton, Wu and Lemerle2012). Seeds were able to germinate in up to 125 mM NaCl in another experiment conducted using seeds from populations from Sardinia, Italy (Podda et al. Reference Podda, Santo, Puddu, Biagini and Bacchetta2015). CaCl2, MgCl2, NaCl, and KCl salts at concentrations of 80 or 160 mM significantly reduced germination of S. elaeagnifolium seeds compared with the control (Turner et al. Reference Turner, Sanchez, Vavra, Dhaliwal, Emendack, Coldren and Angeles-Shim2021).
An increase in pH leads to an increase in seed germination level from almost 50% at pH 4 to more than 75% at pH 10 under conditions of alternating temperatures of 15/25 C and a photoperiod of 12 h (Stanton et al. Reference Stanton, Wu and Lemerle2012). The optimal pH range for seed germination is between 7 and 9 (Balah et al. Reference Balah, Hassany and Mousa2021). Seeds retained their germination capacity even after consumption and excretion by sheep, as a significant proportion (18% to 67%) germinated under alternating 10/30 C and 12-h photoperiod conditions (Heap and Honan Reference Heap and Honan1993).
Digestion for 48 h by cattle has been shown to increase both germination and viability of the seeds, while ensiling them almost completely prevents germination (Piltz et al. Reference Piltz, Stanton and Wu2017). Subjecting S. elaeagnifolium seeds to 80 C increased seed germination from 36% to 45% when placed under alternating 20/30 C light conditions for 16 h, while the average germination time was measured at 2 wk (Chou et al. Reference Chou, Cox and Wester2012).
In general, seed germination can be considered as a factor directly influenced by the environmental conditions in the parental environment that determine the speed, rate, and germination potential of S. elaeagnifolium seeds. As an example, seeds harvested from plants located in cultivated land and seeds from plants located in an urban environment showed higher germination capacity compared with seeds from plants located in grasslands (Turner et al. Reference Turner, Sanchez, Vavra, Dhaliwal, Emendack, Coldren and Angeles-Shim2021). This difference is likely due to population pressure and competition with native vegetation. Specifically, in cultivated areas, S. elaeagnifolium plants have better access to nutrients and water due to fertilizers and irrigation, respectively, and the high germination capacity is justified by the advantage the plant receives over other weeds and crops.
Vegetative Reproduction
According to Adjim and Kazi Tani (Reference Adjim and Kazi Tani2018), S. elaeagnifolium pays a cost for having high clonal reproduction and dominating invaded environments: it exhibits reduced seed germination capacity. Plants arising from root fragments have been found to produce many more fruits per plant (∼120) than plants arising from seeds (∼20 to 25) at 6 mo after emergence and completion of their life cycle (Zhu et al. Reference Zhu, Wu, Stanton, Raman, Lemerle and Burrows2012). Root pieces 20-cm long showed a significantly higher number of new shoots produced than root pieces 5- to 10-cm long and also accumulated more dry weight and produced more berries (Boyd and Murray Reference Boyd and Murray1982b).
Management Options
Specific morphological characteristics of the plant tissues and the extended network of rhizomes are major constraints to the effective short- and long-term management of this species. Despite the scarce literature on S. elaeagnifolium management, several integrated methods, including efficient herbicides, mowing, and an increase in crop competitiveness, have been recognized as promising strategies (Tataridas et al. Reference Tataridas, Kanatas and Travlos2022c). The long-term management of S. elaeagnifolium requires acting beyond tactics toward systems, where efficient systemic herbicides, mowing, and narrow row spacing may deplete the resources for the weed (re)growth (Tataridas et al. Reference Tataridas, Kanatas and Travlos2022c). This strategy might be more challenging for European farming systems, where EU Green Deal is in force and targets the reduction of chemicals by 50% by 2030 and aims to keep soils healthy by adopting minimum-disturbance practices (Tataridas et al. Reference Tataridas, Kanatas, Chatzigeorgiou, Zannopoulos and Travlos2022b). Farm managers should focus fundamentally on the prevention of seed set. For instance, the control of S. elaeagnifolium stands with mowing may be ineffective to limit the regrowth, but the weed may not complete its life cycle, thus reducing the seedbank. Uncultivated areas are the ideal environment for the unhindered reproduction of the weed and the spread of its propagules, acting as a reservoir for future infestations in abandoned fields and agricultural areas. One issue of concern to scientists is whether common management protocols can be designed for populations in different invadable environments. It is particularly important to analyze the genetic similarity of S. elaeagnifolium populations in the native and invasion zones in order to develop effective strategies for managing the weed globally. One method being developed to analyze genetic similarities of plant populations involves the extraction of plant genomic DNA from dehydrated or polysaccharide-rich leaves and the use of microsatellite markers (Ripoll et al. Reference Ripoll, Bon and Jones2011). Recently, modern methods have been exploited to detect the genome through DNA or RNA fragments in weed seedlings or seeds for early detection or identification in new regions. These technologies involve (1) reverse transcription–polymerase chain reaction (RT-PCR) or PCR after transcriptase reaction (Zhang et al. Reference Zhang, Fan, Zhu, Zhao and Fu2013), which is though, an expensive and time-consuming method, and (2) recombinase polymerase amplification or recombinase polymerase amplification technology, which was found to be able to identify segments of S. elaeagnifolium DNA in as little as 1 h, while remaining a significantly cheaper method than RT-PCR (Lei et al. Reference Lei, Yan, Hu, Zhu, Xiong and Fan2017). Such a method, which enables the detection of the weed through genomic screening of seeds or seedlings in the field, is an important tool for early detection of the weed in new areas and the design of effective measures for integrated management. The identification of S. elaeagnifolium using microsatellite markers and electron microscopy to analyze its specific morphological characteristics is particularly important where there are plants of the Solanaceae family that resemble S. elaeagnifolium and may cause problems in their identification and management. In Australia, the use of microsatellite markers and analysis of leaf trichome morphology facilitated the identification of S. elaeagnifolium relative to the nearly identical quena (Solanum esuriale Lindl.) (Zhu et al. Reference Zhu, Burrows, Wu, Raman, Stanton and Lemerle2011). In 2013, reliable tools were developed to compare the genomes of S. elaeagnifolium with genomes of other plants to distinguish species and improve their management (Zhu et al. Reference Zhu, Raman, Wu, Lemerle, Burrows and Stanton2013a).
Grazing and Physical, Cultural, and Mechanical Methods
The use of competitive cover crops in pastures has been proposed as a means of reducing seed production and depleting the root system of S. elaeagnifolium, allowing it to be managed following optimized targeted herbicide applications (Stanton et al. Reference Stanton, Wu and Lemerle2011). Livestock could be an alternative to the release of natural enemies against S. elaeagnifolium, as frequent grazing reduces the growth of its root system and suppresses seed set (Hawker Reference Hawker2004). Extensive grazing in these areas is a critical point for discussion, because it has been shown that livestock can significantly reduce weed growth but can also disperse weed seeds. Adjim and Kazi Tani (Reference Adjim and Kazi Tani2018) suggest a ban on grazing in infested areas as a component of integrated management strategies to reduce S. elaeagnifolium pressure. Moreover, the application of ruminant grazing is a controversial method for weed control, as the plant parts are toxic to animals (Wu et al. Reference Wu, Stanton and Lemerle2016).
The establishment of allelopathic trees, such as eucalyptus, and the development of effective natural herbicides based on phytotoxic substances have also been proposed as novel methods to suppress S. elaeagnifolium (Zhang et al. Reference Zhang, An, Wu, Li Liu and Stanton2012).
In Australia, regular surveying for new infestations is proposed as an alternative to eradication of already established populations where the aim is to limit further spread of the weed (Carter Reference Carter1992). When infestations are less than 2 ha, then systematic control and survey of the weed limits its spread, whereas when infestations are greater than 4 ha, local extirpation is impossible (Carter Reference Carter1992).
Irrigated lands are considered an ideal environment for the establishment and spread of S. elaeagnifolium, and for this reason, systematic surveys of the weed flora in these areas should be carried out (Kriticos et al. Reference Kriticos, Crossman, Ota and Scott2010). Adjim and Kazi Tani (Reference Adjim and Kazi Tani2018) suggest that composting of manure should be compulsory so that it does not carry seeds that can cause new invasions.
Solanum elaeagnifolium competes strongly with annual crops such as cotton, soybean [Glycine max (L.) Merr.], and maize. In cotton, immediate control of S. elaeagnifolium at an early stage is recommended, as failure to control it will lead to rapid spread and reduced yields in a short time (Choudhary and Bordovsky Reference Choudhary and Bordovsky2006). Irrigated cotton has been found to be more competitive with S. elaeagnifolium than dryland cotton (Green et al. Reference Green, Murray and Verhalen1987). In Oklahoma, USA, S. elaeagnifolium became a major problem in cotton production when, after years of continuous applications of herbicides and reduced tillage, no weed control was achieved (Smith et al. Reference Smith, Pawlak, Murray, Verhalen and Green1990). In areas where S. elaeagnifolium invasion is moderate or high, deep tillage (>30 cm) and the introduction of alfalfa is recommended to deplete the weed’s root system reserves due to frequent mowing and competition with the weed (Ameur et al. Reference Ameur, Baye, Bouhache and Taleb2007). Selecting crops with rapid growth or choosing dense and narrow planting provides the necessary shading and ground cover to limit the growth of S. elaeagnifolium (Davis et al. Reference Davis, Smith and Hawkins1945). Frequent mowing is used periodically throughout the growing season to deplete the root resources of S. elaeagnifolium. Nonetheless, the most appropriate growth stage to cut the stems of the weed is before or during the flowering, when nutrients are translocated from the roots to the reproductive organs and seed set may be inhibited.
Soil tillage effectiveness is controversial, as this mechanical method and several other means (such as hoeing and harrowing) are considered ineffective and should, therefore, be avoided, because they result in the fragmentation of the root system, which multiplies the new shoots in invaded fields. However, in Jordan, a country where the weed is widespread, the management includes deep ploughing, mowing, herbicide application, weeding, soil tillage, and use of plant residues to suppress S. elaeagnifolium (Qasem Reference Qasem2014).
Chemical Methods
The application of herbicides and continuous control of new S. elaeagnifolium shoots in spring and autumn may not be justified due to the high cost of applications, reduced effectiveness due to resprouting, and environmental degradation due to increased chemical inputs and greenhouse gases. Herbicides are proposed to be applied at flowering to inhibit seed production, and it is suggested that herbicide labels include applications of effective formulations targeting underground propagules (Stanton et al. Reference Stanton, Wu and Lemerle2010). Bouhache and Gbẻhounou (Reference Bouhache and Gbẻhounou2014) suggest that contact herbicides should be applied at flowering and systemic herbicides after flowering, where non-structural carbohydrate levels increase as the plant approaches berry formation and maturity. Baye (2007) concluded that glyphosate has increased efficacy against the weed when applied at the full flowering stage and before fruiting, and control is higher in irrigated areas and under conditions of water sufficiency. In contrast, Sayari and Mekki (Reference Sayari and Mekki2021) suggest that glyphosate should be applied at the green immature berry stage, because it is the optimal stage for mobilizing stored hydrocarbons. Where weed control is required in annual crops, the applied herbicide doses should be high to sufficiently control the weed, targeting especially the rhizomes that are resprouting.
In pastures in Australia, the application of fluroxypyr or aminopyralid + 2,4-D is recommended (Kidston et al. Reference Kidston, Ferguson and Scott2010). In Morocco, the herbicides metolachlor and atrazine are proposed preemergence in maize cultivation, and fluometuron, pendimethalin and trifluralin in cotton cultivation, while synthetic auxins up to the 4- to 6-leaf stage are proposed postemergence for maize cultivation (Baye et al. Reference Baye, Ameur, Bouhache and Taleb2007). In general, the herbicides glyphosate, triclopyr, fluroxypyr, aminopyralid, picloram, 2,4-D, MCPA, tembotrione, glufosinate, and pyraflufen-ethyl, as well as some of their mixtures, provide good control of S. elaeagnifolium (Gitsopoulos et al. Reference Gitsopoulos, Damalas and Georgoulas2017; Wu et al. Reference Wu, Stanton and Lemerle2016).
Biological Agents
The selection of natural enemies of S. elaeagnifolium that target aboveground vegetation is considered an effective option to prevent seed production and further spread of the weed (Goeden Reference Goeden1971; Sforza and Jones Reference Sforza and Jones2007). Biological control agents such as nematodes and insects that feed on the leaf surface of S. elaeagnifolium could be used in management programs (Sforza and Jones Reference Sforza and Jones2007). Based on the steps needed to conduct a biological control program for the weed in Australia, it has been determined that in each country it is possible to (1) conduct molecular studies of populations of S. elaeagnifolium compared with populations from the native zone (United States and Mexico); (2) analyze the genetic variability, ecology, and population dynamics; (3) evaluate the native species of natural enemies and their selectivity and effectiveness; and finally, (4) evaluate the natural enemies in the weed’s native range (Kwong et al. Reference Kwong, Sagliocco, Weiss, Hunt, Morfe and Kularatne2006). It has been suggested that dry cropland be converted to pasture by sowing perennial cover crops that compete with S. elaeagnifolium, which combined with the perennial presence of natural enemies and continuous defoliation will reduce S. elaeagnifolium’s vigor (Klein Reference Klein2007). However, caution is required during winter tillage to avoid destroying the insect population in the soil. Klein (Reference Klein2007) argues that the release of biological control agents can be carried out seamlessly on uncultivated land to control weed populations. Among the recognized natural enemies of S. elaeagnifolium, two the most successful are the Texas potato beetle (Leptinotarsa texana) and the nematode Orrina phyllobia (EPPO 2007). While L. texana does not feed directly on the seeds, the leaf damage it causes could still significantly reduce the seed production of S. elaeagnifolium and deplete the resources of the underground organs for regrowth, assisting in its long-term management, (Hoffmann et al. Reference Hoffmann, Moran and Impson1998). The release of L. texana in sunflower (Helianthus spp.) in South Africa provided equal efficacy to chemical methods and resulted in lower costs for the farmer (Pitso Reference Pitso2010). In South Africa, S. elaeagnifolium defoliation has been observed in dozens of hectares (Klein Reference Klein2007). It has been reported that more oligophagous than polyphagous insects attack S. elaeagnifolium (Hill et al. Reference Hill, Hulley and Olckers1993). Frumenta nephelomicta was introduced into South Africa but failed to establish due to the dry conditions (Adamski and Brown Reference Adamski and Brown2002; Sheppard et al. Reference Sheppard, Shaw and Sforza2006).
General Outlook
Solanum elaeagnifolium can potentially invade new areas worldwide (Figures 2 and 4–9), such as central and eastern Africa, and has already invaded every continent except Antarctica. However, there are substantial areas of potentially invadable regions in Africa, Asia, Europe, and Oceania. Future actions should be focused on the transboundary prevention of introduction, the early detection and eradication of S. elaeagnifolium, and the integrated management of invaded areas. Preventing or slowing the spread of S. elaeagnifolium in Africa with its notoriously porous borders and Europe with its open borders policy is likely to be fraught with difficulty. An analysis of the sensitivity of the potential distribution of S. elaeagnifolium to future climate scenarios reveals areas of concern for future potential spread, providing a basis for targeted monitoring programs to underpin adaptive biosecurity management programs to prevent the spread of this pernicious weed.
Moving from continents to countries, it is evident that national protocols and frameworks should be developed for the management of S. elaeagnifolium invasions in invaded countries and prevention of dispersal in uninvaded countries. The local-scale management of S. elaeagnifolium is a substantial challenge for farmers and conservation managers due to its morphological and reproductive characteristics. The application of a single control tool (such as broadcast and repeated spraying) is considered ineffective, and a combination of agroecological-based tools, strategies, and methods including different chemical, mechanical, and physical means is required to achieve adequate control in the short and long term. The primary objectives for managing S. elaeagnifolium are to prevent seed production and to limit new germination or resprouting from underground propagules.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/inp.2023.21
Acknowledgments
This research received no specific grant from any funding agency or the commercial or not-for-profit sectors. No competing interests have been declared.
Structured Appendix
Distribution Data
We collected georeferenced occurrence data for Solanum elaeagnifolium species from the Global Biodiversity Information Facility (GBIF.org 2022). Records were downloaded using the functionocc_search() from the R package rgbif (Chamberlain et al. Reference Chamberlain, Barve, Mcglinn, Oldoni, Desmet, Geffert and Ram2021), then we used the R package CoordinateCleaner for data cleaning (Zizka et al. Reference Zizka, Silvestro, Andermann, Azevedo, Duarte Ritter, Edler, Farooq, Herdean, Ariza, Scharn, Svantesson, Wengström, Zizka and Antonelli2019). Some functions of this package include automatic flagging of common spatial and temporal errors in biological collection data. This package includes automated tests to flag (and exclude) records assigned to country or province centroid, the open ocean, the headquarters of the GBIF, urban areas, or the locations of biodiversity institutions (museums, zoos, botanical gardens, universities). Furthermore, it identifies per species outlier coordinates, zero coordinates, identical latitude/longitude, and invalid coordinates. It also implements an algorithm to identify data sets with a significant proportion of rounded coordinates (for details, see Zizka et al. Reference Zizka, Silvestro, Andermann, Azevedo, Duarte Ritter, Edler, Farooq, Herdean, Ariza, Scharn, Svantesson, Wengström, Zizka and Antonelli2019). We only used coordinates from GBIF after 1980 to avoid low precision and misidentification of the species. In addition, all records underwent a thorough visual inspection and quality check according to available literature and our own experience with S. elaeagnifolium. A database of distributional data of the species S. elaeagnifolium was constructed by compiling information from specimens from the literature and data collected by species distribution databases (Supplementary Table S1). Solanum elaeagnifolium distribution points according to Köppen-Geiger climate classification are depicted on Figure A1.

Figure A1. Solanum elaeagnifolium distribution points according to Köppen-Geiger climate classification.
Potential Distribution
We applied the CLIMEX ecological niche modeling package (Kriticos et al. Reference Kriticos, Maywald, Yonow, Zurcher, Herrmann and Sutherst2015; Sutherst and Maywald Reference Sutherst and Maywald1985) to estimate the global climate suitability patterns for S. elaeagnifolium. We used the Compare Locations module to run the model of S. elaeagnifolium described by Kriticos et al. (Reference Kriticos, Crossman, Ota and Scott2010). We used CM30_1995H_V2 (Kriticos et al. Reference Kriticos, Webber, Leriche, Ota, Macadam, Bathols and Scott2012) for climate data. We developed a composite invasion risk map using the method described by Yonow et al. (Reference Yonow, Ramirez-Villegas, Abadie, Darnell, Ota and Kriticos2019) by running the model with two scenarios: natural rainfall and irrigation of 2.5 mm d−1 as top up. We created the composite climate hazard map by using data of irrigation area by Siebert et al. (Reference Siebert, Henrich, Frenken and Burke2013). For each 30’ cell, if the irrigation area is greater than 10 ha, the greater value among the two scenarios is used. Otherwise, the natural rainfall scenario value is used.














