Introduction
Speciation involves, among other reproductive barriers, the evolution of intrinsic postzygotic isolation (Coyne & Orr, Reference Coyne and Orr2004). The modern study of post-zygotic reproductive barriers began with Sturtevant's (Reference Sturtevant1920, Reference Sturtevant1921) analysis of interspecific genetic incompatibilities between Drosophila melanogaster and Drosophila simulans (Provine, Reference Provine1991; Barbash, Reference Barbash2010). One direction of the cross, D. melanogaster females×D. simulans males, produces sterile adult hybrid females and inviable hybrid males that die at the larval–pupal transition (Sturtevant, Reference Sturtevant1920; Hadorn, Reference Hadorn1961). The genetic basis of this hybrid male lethality is now well worked out. In an F 1 hybrid male genetic background, the wild-type D. melanogaster allele of the X-linked gene, Hybrid male rescue (Hmr), is incompatible with the wild-type D. simulans allele of the second chromosome gene, Lethal hybrid rescue (Lhr), causing lethality (Barbash et al., Reference Barbash, Siino, Tarone and Roote2003; Brideau et al., Reference Brideau, Flores, Wang, Maheshwari, Wang and Barbash2006).
The other direction of the cross, D. simulans females×D. melanogaster males, produces sterile adult hybrid males, while inviable hybrid females typically die as embryos (Sturtevant, Reference Sturtevant1920, Reference Sturtevant1921; Hadorn, Reference Hadorn1961). The genetic basis of hybrid lethality in this direction of the cross is, however, somewhat less well characterized. In F 1 hybrid female embryos, a D. melanogaster X-linked factor, Zygotic hybrid rescue (Zhr), is incompatible with an uncharacterized maternal factor from D. simulans, maternal hybrid rescue (mhr), causing lethality (Sawamura et al., Reference Sawamura, Yamamoto and Watanabe1993b). Zhr appears to correspond to a D. melanogaster-specific block of heterochromatin rich in 359-bp satellite repeats at the pericentromeric base of the X chromosome (Sawamura & Yamamoto, Reference Sawamura and Yamamoto1997; Ferree & Barbash, Reference Ferree and Barbash2009). In F 1 hybrid female embryos, the D. melanogaster X fails to condense properly when heterochromatin is first established, resulting in mitotic defects, lagging chromatin and missegregation (Ferree & Barbash, Reference Ferree and Barbash2009).
Progress in determining the genetic basis of F 1 hybrid lethality between these species has relied almost exclusively on the characterization of so-called hybrid rescue mutations – compatible alleles at otherwise incompatible loci. The hybrid rescue mutations, Hmr 1 and Lhr 1, are rare within D. melanogaster and D. simulans populations (Watanabe, Reference Watanabe1979; Hutter & Ashburner, Reference Hutter and Ashburner1987), respectively, and both alleles have large insertions in their 5′-regions and behave as partial loss of function mutations (Barbash et al., Reference Barbash, Siino, Tarone and Roote2003; Brideau et al., Reference Brideau, Flores, Wang, Maheshwari, Wang and Barbash2006). Similarly, the hybrid rescue mutation Zhr 1 corresponds to a rare deletion of a substantial part of the 359-bp satellite block (Sawamura et al., Reference Sawamura, Yamamoto and Watanabe1993b; Ferree & Barbash, Reference Ferree and Barbash2009). The wild-type alleles at all three loci cause hybrid lethality (Sawamura & Yamamoto, Reference Sawamura and Yamamoto1997; Barbash et al., Reference Barbash, Roote and Ashburner2000; Orr & Irving, Reference Orr and Irving2000; Ferree & Barbash, Reference Ferree and Barbash2009). Unlike these hybrid rescue mutations, the D. simulans alleles conferring F 1 hybrid female rescue are not rare, with four separate surveys finding that ![]() ,
, ![]() ,
, ![]() , and, surprisingly,
, and, surprisingly, ![]() D. simulans strains tested yield some level of hybrid female rescue (Bocquet & Tsacas, Reference Bocquet and Tsacas1969; Lachaise et al., Reference Lachaise, David, Lemeunier, Tsacas and Ashburner1986; Sawamura et al., Reference Sawamura, Taira and Watanabe1993a; Orr, Reference Orr1996). Hybrid female lethality, once considered the wild-type state (Sturtevant, Reference Sturtevant1921), appears to be rarer in more recent samples, raising the speculation that rescue has increased in frequency in D. simulans populations over the last century (Orr, Reference Orr1996; Carracedo et al., Reference Carracedo, Asenjo and Casares2000).
D. simulans strains tested yield some level of hybrid female rescue (Bocquet & Tsacas, Reference Bocquet and Tsacas1969; Lachaise et al., Reference Lachaise, David, Lemeunier, Tsacas and Ashburner1986; Sawamura et al., Reference Sawamura, Taira and Watanabe1993a; Orr, Reference Orr1996). Hybrid female lethality, once considered the wild-type state (Sturtevant, Reference Sturtevant1921), appears to be rarer in more recent samples, raising the speculation that rescue has increased in frequency in D. simulans populations over the last century (Orr, Reference Orr1996; Carracedo et al., Reference Carracedo, Asenjo and Casares2000).
Genetic and molecular characterization of the D. simulans mhr factor has lagged behind the other hybrid rescue mutations. One challenge is that sexual isolation in this direction of the cross is prohibitively strong – D. simulans females are reluctant to mate with D. melanogaster males (Sturtevant, Reference Sturtevant1920, Reference Sturtevant1929; Watanabe & Kawanishi, Reference Watanabe and Kawanishi1979) – making genetic mapping of the rescue phenotype difficult. Another problem, however, is that characterization of the D. simulans side of hybrid female rescue has yielded conflicting results. Sawamura et al. (Reference Sawamura, Taira and Watanabe1993a) inferred that hybrid female rescue depends on a single recessive maternal factor, mhr; Orr (Reference Orr1996) inferred that rescue depends on a dominant maternal factor(s), unnamed; and Carracedo et al. (Reference Carracedo, Asenjo and Casares2000) inferred that rescue depends on a dominant zygotically acting factor, Simulans hybrid females rescue (Shfr). All three rescue phenotypes map to the second chromosome.
Here, we survey genetic variability for hybrid female lethality among 37 D. simulans strains, sampled from a variety of geographic localities, in crosses with D. melanogaster males. We also assay levels of maternal effect hybrid female lethality resulting from crosses between D. simulans females heterozygous for wild-type chromosomes over previously described rescuing strains and D. melanogaster males. Our findings support the notion that F 1 hybrid female rescue is not rare. Furthermore, our findings suggest that hybrid rescue is not new, being common even in presumed ancestral populations of D. simulans from the Indian Ocean and Eastern Africa (Dean & Ballard, Reference Dean and Ballard2004; Baudry et al., Reference Baudry, Derome, Huet and Veuille2006; Schöfl & Schlötterer, Reference Schöfl and Schlötterer2006). Finally, our results suggest that the D. simulans side of hybrid female rescue differs from other described rescue mutations in potentially having a multigenic basis. Our results help explain inconsistencies in previous reports and have implications for how to identify the factors involved.
Materials and methods
We used isofemale lines derived from flies collected in natural populations from Zimbabwe (kindly provided by Todd Schlenke, Emory University), Seychelles, Mayotte and Eilat (kindly provided by Catherine Montchamp-Moreau, CNRS Gif-sur-Yvette). Laboratory stocks are available at the UC San Diego Drosophila species Stock Center except for net b py sd pm and y w f; mhr (kindly provided by H. Allen Orr, University of Rochester).
To assess the variability in hybrid female viability, we first crossed females from each D. simulans isofemale line to D. melanogaster Ore-R males. For most crosses, 2–13 independent replicates per line successfully produced more than ten hybrid progeny depending on the strain (Table 1). For interspecific crosses involving the D. simulans isofemale lines ZH65, ZH7 and E7, only one cross each yielded progeny despite several dozens of crosses attempted, due to the strong prezygotic isolation in this direction of the species cross. In general, the low number of replicates for some crosses reflects the challenge of strong sexual isolation. For isofemale line S0, one replicate cross that produced fewer than ten hybrids was included.
Table 1. Hybrid female lethality varies among crosses between D. simulans isofemale line females and D. melanogaster Ore-R males
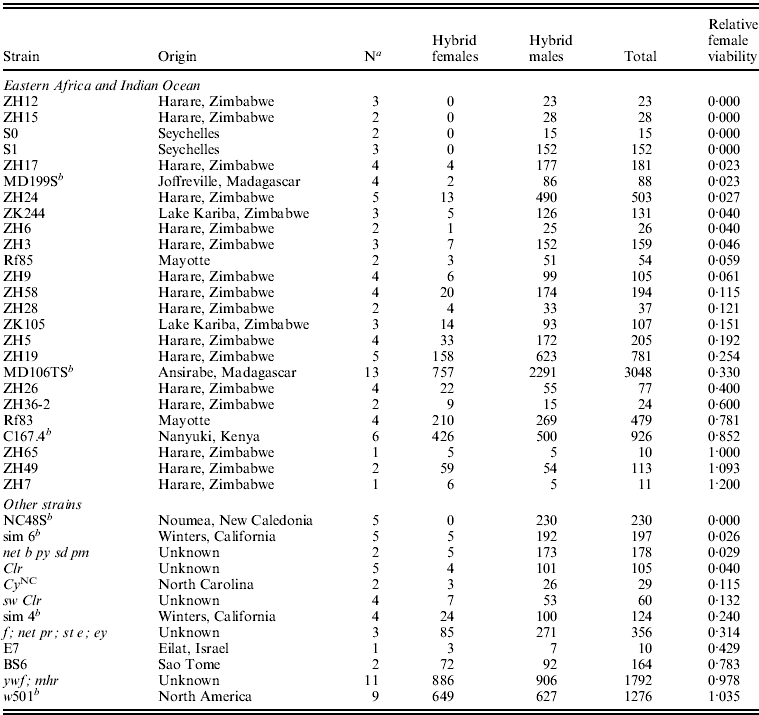
a Number of replicate crosses between each D. simulans isofemale line and Ore-R.
b Strains with genome sequence data (Begun et al., Reference Begun, Holloway, Stevens, Hillier, Poh, Hahn, Nista, Jones, Kern, Dewey, Pachter, Myers and Langley2007).
We performed further crosses to determine if hybrid female rescue was maternal or zygotic and to determine the dominance of rescue. We used reciprocal crosses between two known rescue strains (y w f; mhr and separately, C167.4: see Table 1) and nine strains with weak to no rescue (Table 2). The resulting F 1D. simulans females, heterozygous for the rescue factor(s), were then crossed to D. melanogaster Ore-R males to assess their hybrid female viability phenotype. All of these interspecific crosses were replicated independently between 2 and 5 times.
Table 2. Hybrid female rescue from crosses between heterozygous rescue/wild D. simulans females and D. melanogaster males
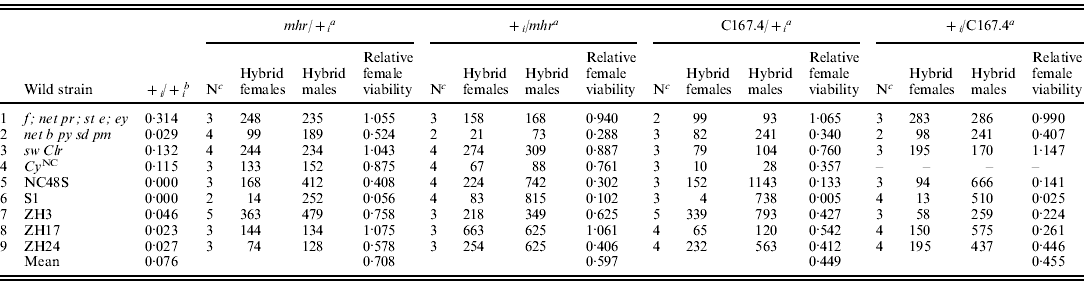
a Genetic background of heterozygous D. simulans females between a rescue strain (y w f; mhr or C167.4) and the ith non-rescue strain (1–9 ), resulting from a female/male cross.
b Relative viability of females from homozygous non-rescue D. simulans females crossed to D. melanogaster Ore-R males (data from Table 1).
c Number of replicate crosses between heterozygous D. simulans and Ore-R.
All interspecific crosses were performed under constant light on cornmeal-agarose medium at 18°C, as hybrid female viability is usually higher at low temperature for rescuing strains. Independent replicate crosses were set up with 5–10 virgin D. simulans females and 15–20 1-day old D. melanogaster Ore-R males, and parent flies were transferred to new vials every 3–4 days until all females had died. Statistical analyses were performed in R (R Development Core team).
Results and discussion
We crossed females from 25 Eastern African and Indian Ocean isofemale lines (Zimbabwe, Madagascar, Mayotte, Kenya and Seychelles) and 12 other lines (Israel, North America, New Caledonia, Sao Tome and four lab strains) of D. simulans to males from the D. melanogaster laboratory stock Ore-R (Table 1). The average viability of hybrid females relative to hybrid males from these crosses was 0·31, ranging from complete hybrid female lethality to complete rescue. Relative hybrid female viability from the Eastern African strains (mean=0·314) does not differ significantly from the other strains (mean=0·344; Kruskal–Wallis rank test: χ12=0·254, P=0·615). For the entire sample of 37 D. simulans strains, 15 (40%) show substantial hybrid female rescue (arbitrarily defined as relative viability >0·20). The frequency of such hybrid female rescue in Eastern Africa (![]() ) is similar to that outside of Eastern Africa (
) is similar to that outside of Eastern Africa (![]() ; Fisher's exact P=0·488). These crosses show that hybrid female rescue is common worldwide. Eastern African populations, especially those in Madagascar (Dean & Ballard, Reference Dean and Ballard2004) – frequency of rescue in Madagascar/Mayotte/Kenya is
; Fisher's exact P=0·488). These crosses show that hybrid female rescue is common worldwide. Eastern African populations, especially those in Madagascar (Dean & Ballard, Reference Dean and Ballard2004) – frequency of rescue in Madagascar/Mayotte/Kenya is ![]() – are believed to represent the ancestral range of D. simulans with new world populations having a relatively recent origin (Baudry et al., Reference Baudry, Derome, Huet and Veuille2006; Schöfl & Schlötterer, Reference Schöfl and Schlötterer2006). The similarly high frequency of hybrid female rescue in ancestral Eastern African and other populations of D. simulans thus argues against its sudden recent increase in frequency.
– are believed to represent the ancestral range of D. simulans with new world populations having a relatively recent origin (Baudry et al., Reference Baudry, Derome, Huet and Veuille2006; Schöfl & Schlötterer, Reference Schöfl and Schlötterer2006). The similarly high frequency of hybrid female rescue in ancestral Eastern African and other populations of D. simulans thus argues against its sudden recent increase in frequency.
To investigate the inheritance of hybrid female rescue, we made reciprocal crosses between the rescuing strains y w f; mhr and separately, C176.4, with five non-African strains (four lab strains and one from New Caledonia) and four African strains (three Zimbabwean strains and one from the Seychelles) showing moderate to no rescue in the initial crosses (Table 2). We then crossed the resulting heterozygous D. simulans female progeny to D. melanogaster Ore-R males. When we compare hybrid female rescue from heterozygous D. simulans females generated from reciprocal crosses (i.e. mhr/+ i vs. + i/mhr and C167.4/+ vs. + i/C167.4 in Table 2), the strength of rescue was highly correlated (Spearman's rank correlation for mhr crosses: ρ=0·82, P=0·007; for C167.4 crosses: ρ=0·89, P=0·003; see Table 2), demonstrating that hybrid female rescue does not depend on the cytoplasm.
If hybrid female rescue is zygotic and dominant, then heterozygous D. simulans females will transmit the rescue allele to half of their hybrid progeny, resulting in ~50% of the hybrid rescue effect produced by the homozygous rescuing strain. If, however, hybrid female rescue is maternal and dominant (recessive), then heterozygous D. simulans females should rescue 100% (0%) of the hybrid rescue produced by the homozygous rescuing strain. As Table 2 shows the pattern of rescue is complex. In some crosses, it seems that y w f; mhr rescue is maternal and recessive (Table 2, line 6, S1 strain: the relative hybrid female viability is close to 0), as originally reported by Sawamura et al. (Reference Sawamura, Taira and Watanabe1993a) . In other crosses, however, it seems that y w f; mhr rescue is maternal and dominant (Table 2, line 8, ZH17 strain: the relative hybrid female viability is close to 1). Still other crosses produce intermediate levels of rescue (Table 2, line 5, NC48S), making it difficult to formally distinguish zygotic vs. partially dominant maternal hybrid rescue. However, as the y w f; mhr strain clearly has a maternal hybrid rescue effect (Table 2, lines 6 and 8), it seems most parsimonious to infer that its dominance depends on genetic background. Similar results hold for the hybrid female rescue of C167.4 (Table 2).
In the original crosses, homozygous y w f; mhr females produced a slightly, albeit not quite significantly, stronger hybrid rescue than C167.4 females (Table 1; Fisher's exact P=0·097). The rescue produced by both strains when heterozygous was correspondingly different: the average rescue of y w f; mhr when heterozygous was higher than that of C167.4 when heterozygous. These findings suggest either that y w f; mhr and C167.4 have rescue alleles at the same locus with different strengths and/or dominance or, alternatively, that rescue is affected by multiple segregating factors that differ between the strains.
The data in Table 2 strongly suggest that hybrid female rescue involves more than a single locus. In particular, not only do y w f; mhr and C167.4 have different average rescue effects, but the strength of rescue in heterozygous state depends on the wild strains to which they were crossed. For example, strains showing no rescue when homozygous tended to produce the weakest rescue when heterozygous with y w f; mhr or C167.4 (e.g. Table 2, line 6), whereas strains showing moderate rescue when homozygous tended to produce the strongest rescue when heterozygous with y w f; mhr or C167.4 (e.g. Table 2, line 1). Indeed, there is a strong correlation between the level of rescue from homozygous D. simulans strains and the level of rescue when the same strains are heterozygous with y w f; mhr or C167.4 (Spearman's rank correlation for mhr crosses: ρ=0·86, P=0·002; for C167.4 crosses: ρ=0·92, P=0·001; see Fig. 1). This observation implies either that virtually every strain bears a different allele at a single hybrid rescue locus or, more plausibly, that the basis for hybrid female rescue is multigenic.
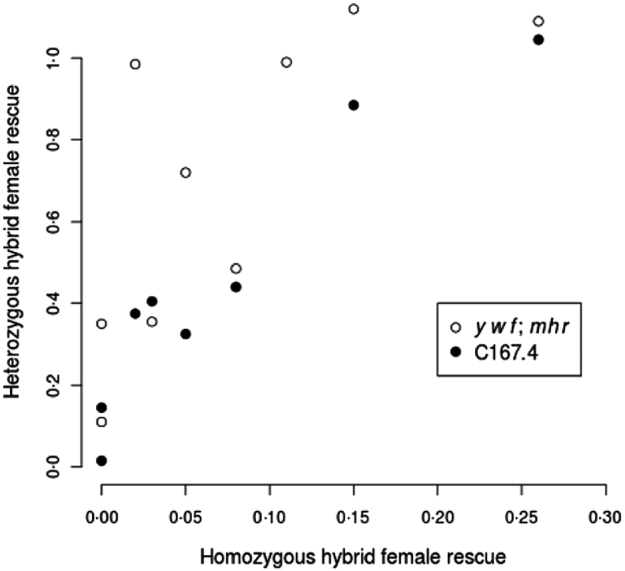
Fig. 1. Homozygous vs. heterozygous hybrid female rescue. Correlation between hybrid female rescue of non-rescue D. simulans strains and hybrid female rescue (averaged between reciprocal crosses) from heterozygous non-rescue/rescue strain (y w f; mhr (white circles) or C167·4 (black circles): data from Table 2). All relative viabilities of hybrid females vs. males result from crosses with D. melanogaster Ore-R males.
Conclusions
Our findings help explain apparent discrepancies among previous reports. In particular, our results are consistent with both Sawamura et al. (Reference Sawamura, Taira and Watanabe1993a) and Orr (Reference Orr1996), showing that D. simulans maternal effect hybrid rescue can appear either recessive (Sawamura et al. Reference Sawamura, Taira and Watanabe1993a) or dominant (Orr, Reference Orr1996) depending on genetic background. We also note that the previous finding that the D. simulans rescue mutation(s), Shfr, yields only partial (~50%) rescue when heterozygous is consistent not only with zygotic rescue, as inferred by Carracedo et al. (Reference Carracedo, Asenjo and Casares2000), but also with incompletely dominant maternal rescue, as observed here (e.g., Table 2, line 5).
The previous findings that mhr, Orr's (Reference Orr1996) unnamed rescue mutation(s), and Shfr all map to the second chromosome may, at face value, seem difficult to reconcile with the notion that maternal hybrid rescue is multigenic. After all, why should all of the multiple factors that contribute to hybrid rescue map to a single chromosome? While the previous studies showed that chromosome 2 had the largest effect on hybrid rescue, they did not demonstrate that all of the hybrid rescue phenotype could be explained by chromosome 2. Indeed, both Sawamura et al. (Reference Sawamura, Taira and Watanabe1993a) and Orr (Reference Orr1996) note that while chromosome 2 has the largest effect on rescue, their genetic analyses cannot exclude a role for other factors. We suggest that, indeed, multiple genetic factors contribute to hybrid female rescue, a fact that distinguishes D. simulans maternal hybrid female rescue from the other three rescue mutations, Hmr, Lhr and Zhr and a fact that will complicate future genetic mapping efforts.
Whatever the number of genes involved in hybrid female lethality, the abundant quantitative genetic variability described here may be useful in determining the molecular basis of hybrid lethality and, moreover, informative about the evolutionary history of the factors involved. First, as the incompatible locus Zhr on the D. melanogaster X chromosome comprises a large pericentromeric block of satellite DNA, the maternally transmitted factors from D. simulans that rescue hybrid female lethality are likely involved in the regulation of heterochromatin – e.g. chromatin-binding or -modifying proteins or small RNAs (Ferree & Barbash, Reference Ferree and Barbash2007, Reference Ferree and Barbash2009). We are testing the possibility that among strain variation in hybrid rescue correlates quantitatively with the amount of particular maternal products in the egg, thus providing a means for identifying candidate causative maternal factors.
Second, the variation in hybrid rescue found segregating among disparate geographic populations of D. simulans suggests that directional selection has been neither strong nor consistent species-wide. The rapid evolutionary turnover of heterochromatic satellite DNA sequences like the 359 bp repeats of Zhr – driven either by genetic conflict over transmission through the female germline (Henikoff et al., Reference Henikoff, Ahmad and Malik2001) or by nearly neutral processes (Charlesworth et al., Reference Charlesworth, Sniegowski and Stephan1994) – ought to elicit correspondingly rapid compensatory evolution at interacting loci, like those affecting maternal hybrid rescue. The abundant functional genetic variation for hybrid rescue in D. simulans is therefore surprising. One possibility is that genetic conflict-mediated arms races between selfish satellite DNAs in D. simulans and their interactors has, incidentally, maintained allelic variation that affects regulation of D. melanogaster Zhr-like repeats. Another possibility, however, is that the variability could reflect residual ancestral variation at loci historically involved in regulating a D. melanogaster Zhr-like satellite DNA that was subsequently lost (or simply diverged) in the lineage leading to D. simulans. Consistent with the latter model, some 359-bp satellite DNA exists in D. simulans but it is ~50-fold less abundant than in D. melanogaster and considerably diverged in sequence (Strachan et al., Reference Strachan, Webb and Dover1985; Lohe & Roberts, Reference Lohe, Roberts and Verma1988). Determining the molecular basis and evolutionary histories of the factors responsible for variable hybrid female rescue segregating in D. simulans may have general implications for the common observation of polymorphic interspecific incompatibilities, like those detected in plants, nematodes, insects, copepods and vertebrates (reviewed in Rieseberg & Blackman, Reference Rieseberg and Blackman2010; Cutter, Reference Cutter2012).
We thank Colin Meiklejohn, Allen Orr and Catherine Montchamp-Moreau for helpful discussion and comments on the manuscript. This work was supported by funds from the University of Rochester, the David & Lucile Packard Foundation and the NIH (GM79543) to DCP.





