Introduction
Neisseria meningitidis is a gram-negative diplococcus that asymptomatically colonises the oropharynx and nasopharynx in ~10% of humans [Reference Pace and Pollard1, Reference Batista2], providing a reservoir for transmission. Although the bacteria are typically asymptomatic colonisers, bloodstream invasion can occur, resulting in invasive meningococcal disease (IMD). The mechanisms that lead from colonisation to invasive disease are still not entirely understood but are largely attributed to host susceptibility, environmental conditions and meningococcal virulence factors [Reference Pace and Pollard1, Reference Batista2]. Polysaccharide capsule expression protects N. meningitidis from opsonisation and phagocytosis by host immune cells [Reference Pace and Pollard1].
IMD has a rapid onset [Reference Guiddir3] and causes severe illness often associated with high mortality and morbidity, including long-term sequelae (e.g. amputations, hearing loss and neurodevelopment deficiencies) [Reference Pace and Pollard1, Reference Batista2]. Efficient clinical recognition of IMD, which often relies on principal signs and symptoms for diagnosis, and initiation of appropriate medical treatment are imperative in improving a patient's chances of a favourable outcome. Disease most frequently presents as either meningitis, septicemia or a combination of both. Classic symptoms of meningitis include fever, intense headache, stiff neck, vomiting or changes in consciousness. Purpura or petechial rash, asthenia or arterial hypotension are classic signs of meningococcemia [Reference Batista2]. Other less common forms of IMD include septic arthritis, pericarditis, gastroenteritis and invasive pneumonia, and present with symptoms different from those seen with meningitis or septicemia [Reference Guiddir3].
Meningococci are divided into serogroups by the type of polysaccharide capsule they produce [Reference Batista2]. Serogroups A, B, C, W, X and Y cause the majority of disease burden [Reference Pace and Pollard1]. The sequencing of similar housekeeping genes that are relatively well conserved allows for grouping into sequence types (ST; e.g. ST-11, ST-32) using the multilocus sequence typing (MLST) technique. A clonal complex (cc; e.g. cc11) is a group of similar STs and is named after the most genetically typical and persistent ST in the group. A single ST can belong to multiple serogroups (e.g. ST-11 can belong to serogroup B, C or W) [Reference Kelly and Pollard4].
Timeline of serogroup W meningococci emergence
Serogroup W meningococci (MenW), sometimes referred to as serogroup W-135 meningococci, were first identified in the 1960s [Reference Kelly and Pollard4]. Throughout the 1990s, MenW was responsible for only 2.6% to 4% of IMD cases in the United Kingdom (UK), France and the USA [Reference Kelly and Pollard4]. In 2000, an international outbreak of IMD due to MenW occurred during the annual Hajj pilgrimage to Mecca in Saudi Arabia. The outbreak was found to be caused by a MenW strain ST-11 [Reference Abad5, 6]. The Hajj pilgrimage in 2001 resulted in an almost identical outbreak of the same strain [Reference Kelly and Pollard4]. As the Hajj pilgrims returned to their home countries, hypervirulent MenW ST-11 strains likely disseminated throughout the world [Reference Tsang7, Reference Wilder-Smith, Chow and Goh8]. However, as of 2002, no further Hajj-related outbreaks were observed after the MenACWY meningococcal vaccine became a requirement for the Hajj visa in Saudi Arabia [Reference Wilder-Smith, Chow and Goh8].
In the following years, MenW ST-11 cases remained low at endemic levels through most of the early 2000s. The strain eventually reemerged as two predominant sublineages: the Anglo-French Hajj strain and the South American/UK strain [Reference Lucidarme9]. It is unclear whether these two different strains reflect the emergence of MenW ST-11 in two different geographic regions or ultimately resulted from one common ancestor in 2000 (i.e. the Hajj 2000 strain) [Reference Abad5]. The Hajj outbreak strain was found to be closely related to the Anglo-French Hajj strain but genetically and epidemiologically distinct from the South American/UK strain [Reference Lucidarme9]. Although there have been no reports of Hajj-associated MenW cases in Latin America, surveillance was largely absent in many countries in the years following the Hajj outbreaks. However, the South American/UK MenW ST-11 strain emerged across the Southern Cone of South America during the 2000s. Notable increases in confirmed cases were observed in Argentina, Chile and Brazil [Reference Abad5]. The South American/UK strain can be further characterised into two distinct strains: the ‘original UK strain’, which rapidly emerged in the UK beginning in 2009, and the ‘2013 UK strain’, a variant outbreak strain which emerged in 2013 and is currently expanding outside the UK. Both the original and 2013 strain belong to ST-11 [Reference Knol10]. The Anglo-French Hajj strain appears to have spread from 2003 onward and persists throughout Africa (2003–2013) and France (2012) [Reference Hong11].
In recent times, serogroups C and A (Africa) have been responsible for most epidemics. Disease caused by serogroups B, C and Y have historically been observed at both endemic and epidemic levels (Europe, North America and South America). However, the prevalence of disease caused by MenW has been increasing in Europe, Africa, North America, South America, Asia and the Middle East [Reference Abad5, Reference Hong11–Reference Iversen15]. The percentage of MenW cases in Australia increased from 8% in 2013 to 39% in 2016 [Reference Martin16] and from 3% in 2012 to 19% in 2016 in Canada [Reference Tsang7]. In France, the South American/UK strain was the most frequent MenW cc11 isolate found between 2010 and 2016. Of the two South American/UK MenW strains, a significant increase in the ‘2013 UK’ strain was observed in 2016 [Reference Hong11]; this strain has been associated with an atypical clinical disease presentation: some patients have been reported to initially present with acute gastrointestinal (GI) symptoms (i.e., abdominal pain, vomiting and diarrhoea), septic arthritis, severe respiratory infection and peritonitis. The unusual nature of these symptoms has led to delays in IMD diagnosis, with some reports of patients being sent home with a diagnosis of gastroenteritis or undergone appendectomies to address their symptoms [Reference Guiddir3]. The current review was conducted to further evaluate the reports of MenW IMD cases associated with atypical clinical symptoms on a global scale.
Methods
Literature search
A basic PubMed search was performed to identify commonly used terms in literature describing meningitis, serogroup W and atypical presentations. Terms referring to meningococcal disease due to serogroup W were selected in reference to the existing literature, and authors conducted a stepwise literature search with different combinations of these terms to optimise the search string and obtain the maximum number of publications. Terms that did not change the number of publications retrieved were omitted from the final search string. Following this, a literature search was conducted using the Ovid MEDLINE® database (January 1, 1946‒January 23, 2019). The official search terms were created to include all variations or synonyms, and no language limits were applied. The search query was ‘(1 or 2) and 3 and 4’ where 1, 2, 3 and 4 were defined as follows:
1) GI diseases or gastroenteritis
2) Pneumonia or septic arthritis or respiratory or epiglottitis or supraepiglottitis or abdominal or abnormal or unusual or atypical
3) Meningitis or meningococcemia or meningococcal or N. meningitidis
4) Serogroup W or group W or MenW or sequence type or ST-11
No filters were applied. Following the search, additional references were added from the authors' personal files if they were relevant to the topic and helpful for discussion.
Results
Search results
The search was performed on January 23, 2019 and yielded 45 results (Fig. 1). The citations were screened by reading their title and abstract. Their relevance to MenW epidemiology, characterisation or clinical presentation provided the basis for inclusion. Twelve articles were excluded for varying reasons; these articles included vaccine clinical trials (1), case studies with complement deficiency or properdin deficiency (2), molecular characterisation studies (3), unusual clones (1), genomic database comparison (1), humanised mice studies (1), Umrah pilgrim carriage studies (1), a comparison of MenA in Greece (1) and a 3-year surveillance study of meningococcal disease in Argentinian children (1). The remaining 33 articles [Reference Batista2–Reference Kelly and Pollard4, 6, Reference Barra12–Reference Iversen15, Reference Andersen and Solberg17–Reference Brawley41] were reviewed, and those that did not provide enough relevant information for discussion or for which an English translation was inaccessible were omitted [Reference Andersen and Solberg17, Reference Bisgaard, Fagerberg and Hjort19, Reference Harrison23–Reference Kriz25, Reference Olea29, Reference Puleston31, Reference Turhan37, Reference Wunderink40]. An additional 14 articles were included from the authors' personal files that were not retrieved by the literature search [Reference Pace and Pollard1, Reference Abad5, Reference Tsang7–Reference Hong11, Reference Martin16, Reference Cheddani42–Reference Villena and Santolaya47]. Table 1 provides a summary of the articles retained in this analysis.

Fig. 1. Flow diagram of the study.
Table 1. Key messages from articles included in the review
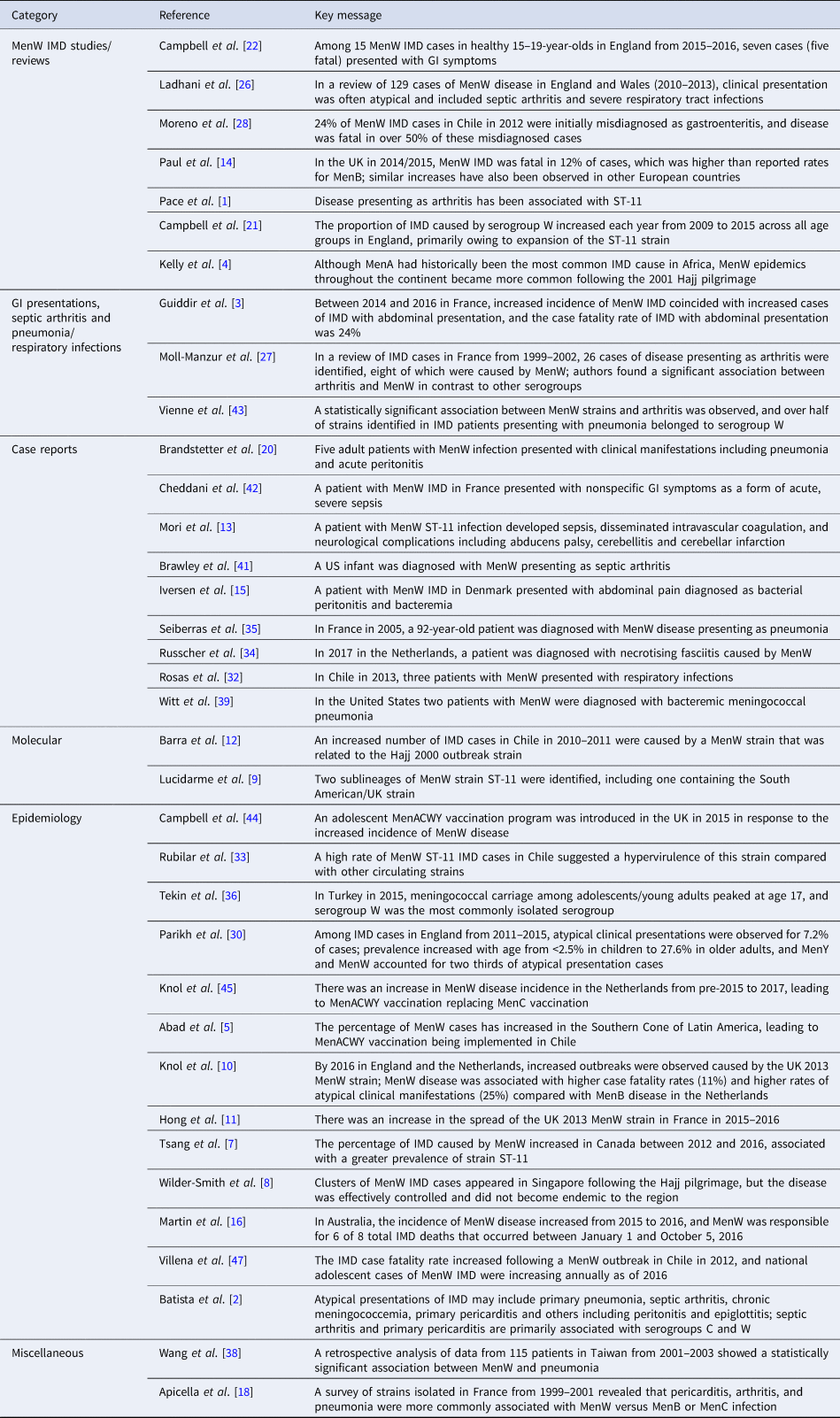
IMD = invasive meningococcal disease; MenACWY = meningococcal serogroups A, C, W-135 and Y; MenB = meningococcal serogroup B; MenC = meningococcal serogroup C; MenW = meningococcal serogroup W; MenY = meningococcal serogroup Y.
As expected, IMD caused by MenW was found to be associated with the presentation of atypical signs and symptoms. The most prevalent atypical symptoms are discussed in the following sections.
Acute GI symptoms
Although nausea, vomiting and diarrhoea are all symptoms associated with IMD, acute GI symptoms as a primary presentation are rare [Reference Campbell22] and considered nonspecific. These nonspecific symptoms may appear during the early stages of meningococcal disease progression but are rarely considered to be the principal signs of IMD. In 2015–2016 in the UK, there were seven MenW IMD cases that presented primarily with GI symptoms; of these, four were reviewed by a clinician and patients were sent home with a presumed diagnosis of gastroenteritis. Of the seven cases, six strains were identified as ST-11 and one strain was nontypeable; five patients died [Reference Campbell22]. In 2012, 60 cases of MenW were reported in Chile, comprising 57.7% of all characterised IMD cases. The most common primary clinical symptoms were fever (60.3%), diarrhoea (55.6%), respiratory/cold symptoms (52.5%) and nausea or vomiting (46.7%). Signs of meningeal irritation (Brudzinski sign and nuchal rigidity) appeared only 8.7% of the time. Diarrhoea was observed at a significantly higher rate in patients who died (55.6%) compared with survivors (26.8%). Of the 60 cases, 24% were originally diagnosed as gastroenteritis due to the primary presentation of abdominal pain and 12% of individuals underwent unwarranted surgery. The case fatality rate (CFR) for the MenW cases was 31.7% (19/60) but was 57% among cases initially diagnosed as gastroenteritis (8/14) [Reference Moreno28].
In a third study by Guiddir et al. [Reference Guiddir3], all confirmed IMD cases (n = 11 979) in France (1991–2016) were screened for abdominal presentation of IMD based on one or more of the following symptoms within 24 h of being diagnosed: abdominal pain, gastroenteritis with diarrhoea or vomiting or diarrhoea. There were 105/11979 (0.9%) total cases of IMD with abdominal presentation. Of these, MenC, MenB and MenW were responsible for 42%, 33% and 16% of cases, respectively (Fig. 2), and sequencing data were available for 96 strains. ST-11 (MenW and MenC) strains were responsible for 45% of the cases. Further characterisation of 92 of the 105 isolates using MLST showed that all MenW isolates with abdominal symptoms belonged exclusively to the South American/UK strain, suggesting an association between MenW ST-11 and abdominal presentations; this is consistent with the previous two studies demonstrating a significant association between the South American/UK MenW strain and abdominal presentations of IMD in the UK and Chile [Reference Campbell22, Reference Moreno28]. During a narrower timeframe of 2010–2016, the proportions of all IMD cases belonging to MenB and MenW (across all STs) vs. the proportions of cases exclusively with abdominal presentations belonging to these serogroups were 62% vs. 29% for MenB and 6% vs. 29% for MenW [Reference Guiddir3]. Due to the localisation of abdominal pain over the right iliac fossa, unnecessary abdominal surgery was performed in 20% (20/105) of cases in this study [Reference Guiddir3]. The reported CFR was 24%, compared with a CFR of 10.4% for all IMD cases in France over the same time period [Reference Guiddir3].

Fig. 2. Serotypes responsible for all IMD cases or cases with abdominal presentation in France, 1991–2016. Percentages are provided along with the 95% CIs (*P < 0.01, **P < 0.001, ***P < 0.0001). Adapted with permission from Guiddir et al., Clinical Infectious Diseases 2018; 67: 1220-1227 [Reference Guiddir3].
The causal relationship between MenW ST-11 and its presentation in IMD with abdominal pain remains unclear. Changes in genes responsible for metabolic functions in South American/UK and Anglo-French Hajj strains have been demonstrated. Guiddir et al. [Reference Guiddir3] suggest these changes may also affect genes responsible for virulence factors in the meningococcal bacterial wall (lipopolysaccharide and peptidoglycan), which are potent inducers of the inflammatory response; this in turn implies that the induction of an abdominal inflammatory response may be involved in abdominal pain. Previous studies have demonstrated postmortem intestinal inflammation due to MenW IMD [Reference Guiddir3]. In one case, a 46-year-old man presented to the emergency department with severe acute abdominal pain, vomiting and fever. Before clinicians diagnosed MenW meningococcemia, a computed tomographic scan of the patient's abdomen revealed severe inflammation of the duodenum, and further histologic characterisation showed partial villous atrophy. The authors concluded that abdominal pain experienced by patients with GI symptoms results from mesenteric hypoperfusion and can be improved with the administration of fluids [Reference Cheddani42].
Septic arthritis
Reports of meningococcal disease presenting as septic arthritis date back to the 1980s. Brawley et al. [Reference Brawley41] reported a 22-month-old arriving at the hospital with a swollen, painful knee. Following diagnosis of acute septic arthritis, the infectious agent was identified as MenW. The patient showed no rash or classic signs of meningococcemia, giving the clinician no reason to suspect meningococcal infection [Reference Brawley41]. Septic arthritis caused by N. meningitidis often presents as pain, erythema, local heat and/or immobilisation or impotence of the joint involved. Septic arthritis tends to be monoarticular, with the knee being the most frequently affected [Reference Pace and Pollard1], and occurs mainly at the extremes of age [Reference Ladhani26]. A 3-year clinical follow-up of 129 MenW cases in England and Wales (2010–2013) revealed that septic arthritis was overrepresented in MenW cases, with 9 of 129 IMD cases having MenW strains isolated from joint fluid [Reference Ladhani26]. A study conducted in France (1999–2002) reported 26 IMD cases with septic arthritis, with MenW ST-11 being responsible for 8 (30.8%) [Reference Moll-Manzur27].
Pneumonia and upper respiratory tract infections
Although uncommon, pneumonia caused by N. meningitidis can be the primary manifestation of meningococcal disease. Primary meningococcal pneumonia tends to be more common in adults (>50 years old) and associated with serogroups W or Y [Reference Pace and Pollard1]. Across all serogroups, IMD presenting as primary pneumonia occurs only 5% to 10% of the time [Reference Batista2]. In England and Wales (2010–2015), bacteremic pneumonia was observed in 12% of MenW IMD cases and was more common in individuals ⩾45 years old, at 20%. Surveillance of 3411 IMD cases in England (2010–2015) showed that approximately half (130/235; 55.3%) of the atypical presentations (non-meningitis/septicemia) were pneumonia, with MenW and MenY responsible for two-thirds of the atypical presentations [Reference Parikh30]. A study of more than 2000 isolates in France from 1999 to 2002 suggested that MenW is significantly associated with complications of arthritis (P < 0.002) and causes pneumonia at much higher rates (18 MenW cases out of 33 total acute pneumonia cases, 54.5%) compared with MenB or MenC [Reference Apicella18, Reference Vienne43]. The study also found six cases of meningococcal pericarditis, of which two were caused by MenW, three by MenC and one by MenY [Reference Vienne43]. In a Taiwanese study (2001–2003), there was a higher prevalence of pneumonia found in MenW IMD patients vs. all IMD patients (23.8% vs. 1.5%) [Reference Wang38]. In the United States, two isolated cases of meningococcal pneumonia were described and attributed to MenW [Reference Witt and Olans39]. Three cases were described in Chile (2013) in which MenW IMD presented as a primary respiratory tract infection; rapid clinical deterioration with an intense systemic inflammatory response soon followed [Reference Rosas32]. All of Chile's MenW IMD cases in 2013 were caused by ST-11 [Reference Rubilar33].
At early stages, meningococcal disease usually presents as cold-like symptoms, including sore throat, cough, coryza and otalgia [Reference Batista2]. This often results in IMD being indistinguishable from a viral infection in its first 4 to 6 h. Viral infection may make individuals more susceptible to invasion of N. meningitidis, leading to IMD. Additionally, damage to the nasopharyngeal epithelium from other factors such as temperature and humidity has been associated with a higher incidence of IMD [Reference Pace and Pollard1]. MenW IMD has also been associated with a primary presentation of severe upper respiratory tract infection, most notably epiglottitis [Reference Campbell22]. In 2010–2013 in England and Wales, five MenW cases presented with a severe upper respiratory tract infection (epiglottitis or supraglottitis), three of which were associated with ST-11 (the remaining two cases were non-ST11 and unknown) [Reference Ladhani26]. Incidences of severe respiratory tract infections, as well as septic arthritis, were found to be overrepresented among MenW cases. Epiglottitis and supraglottitis are highly rare IMD presentations, and this was the first report of their association with MenW infection [Reference Ladhani26].
Discussion
Hajj outbreaks in 2000 and 2001 played a role in disseminating MenW ST-11 across the world, contributing to a rising number of meningococcal disease cases and deaths. MenW continues to evolve as a growing cause of IMD on a global scale. Clinical recognition of meningococcal disease attributed to MenW ST-11 has also been a challenge for physicians because of the higher frequency of unusual clinical presentations compared with typical bacterial meningitis cases. In addition, many of these symptoms are nonspecific. MenW ST-11 was found to be associated with complications such as pericarditis, peritonitis, acute GI symptoms (vomiting, diarrhoea and nausea), septic arthritis and severe respiratory tract infections (pneumonia, epiglottitis) [Reference Ladhani26]. Although most of these presentations and symptoms are described in all IMD, they have always remained uncommon and rarely seen as the primary presenting sign of IMD.
What about other ST-11s?
For the Hajj MenW ST-11 outbreak strain (i.e., the current Anglo-French Hajj strain), no link with unusual clinical presentations has been noted. However, cases of MenB and MenC ST-11 have presented with abdominal symptoms, mostly abdominal pain. Of 105 French IMD cases displaying an abdominal symptom (1991–2016), MenC, MenB and MenW were responsible for the majority of cases [Reference Guiddir3]. The proportion of cases with abdominal presentations compared with all cases was overrepresented for MenC and MenW, but not for MenB (Fig. 2). MenC isolates belonged to several ST-11 lineages, while MenW isolates belonged exclusively to the South American/UK lineage. Thus, these unusual IMD symptoms do not appear to be exclusive to MenW, but may be exclusive to the currently expanding MenW South American/UK ST-11 lineage among MenW strains [Reference Guiddir3].
Why the South American/UK strain?
All MenW cases associated with atypical presentations appear to fall within the two sublineages of the South American/UK strain (the original UK strain and the UK 2013 strain); atypical presentations have not been seen in IMD cases caused by the Anglo-French Hajj strain. Whole genome sequencing of the South American/UK and Anglo-French Hajj strains in France (1991–2016) identified differences between the two strains among 119 loci. Of the loci with characterised function, those driving metabolic function (metabolism of carbohydrate, fatty acids, amino acids and nucleic acids) made up the largest proportion. This indicates that several genetic differences may have developed in the emergence of the Anglo-French Hajj and South American/UK strains, potentially resulting in increased transmission and/or expansion. This could explain the recent expansion of the South American/UK strain. Virulence and carbohydrate metabolism have been previously suggested as causative factors of this expansion [Reference Guiddir3].
What is causing these atypical presentations?
While several theories including mesenteric hypoperfusion, septic epiploic microinfarctions, immune complex deposition and others have been proposed to explain the pathophysiology behind the observed presentations of abdominal pain, inflammation is suspected to be the more likely culprit. Changes in a strain's virulence factors can induce a stronger inflammatory response. Inflammation of the duodenum has been observed in a previous MenW IMD case with abdominal pain, implying a possible relationship between the two. Polysaccharide W, which makes up the capsule of MenW, has been shown to generate a low immune response, making elimination from the host difficult [Reference Wang38]. This may be a reason why MenW ST-11 strains have been associated with unusual primary infection sites. The factors contributing to the recent emergence of MenW ST-11 characterised by unique clinical symptoms still remain largely unclear and are an area for further investigation.
Limitations of the included literature
Limited information is available in the literature regarding atypical presentation of MenW disease, and all included studies were either retrospective observational or case reports. In addition, the literature review was subject to selection bias, and although the literature search was designed to maximise the yield of relevant publications, phrasing inconsistencies in the literature likely prevented some relevant articles from being captured. Controlled, prospective studies are needed to inform any unbiased assumptions regarding the differences between serogroup disease presentations.
Conclusions
This review highlights IMD data from around the world linked with three atypical presentations: acute GI symptoms, septic arthritis and pneumonia. Physicians need to be aware of MenW ST-11's unusual presentations in IMD to allow for recognition and treatment in a timely manner. They should be particularly vigilant with infant and elderly patients, who have a higher incidence of IMD. Clinicians should also be mindful of the possibility when treating any patient in poor condition who resides in an area with a high incidence of MenW IMD. A routine blood or fluid culture may be prudent in any unusual case of gastroenteritis, bacteremic pneumonia or septic arthritis. GI symptoms are an early sign of IMD sepsis and although nonspecific may warrant an observational period in the emergency room. The delay in major differential diagnosis caused by these acute GI symptoms may be a contributor to the high CFR associated with MenW IMD [Reference Guiddir3] and suggest that atypical primary presentations cause a delay in time to treatment. Moreover, delayed IMD diagnosis or misdiagnosis of IMD cases as viral infections due to atypical primary presentations could consequently delay prescription of antibioprophylaxis and therefore increase risk of secondary cases.
Because the incidence of IMD caused by MenW continues to increase throughout the world, including in Europe, Africa, North America, South America, Asia and the Middle East [Reference Abad5, Reference Hong11–Reference Iversen15], vaccination with MenACWY conjugated vaccines as a protective measure against MenW disease should be considered by national vaccination policies depending on each country's epidemiology and other country-specific factors. Vaccination is also important in the case of MenW disease because of the possibility for missed or delayed diagnosis owing to atypical presentations. Adolescent MenACWY conjugate vaccine programs have been introduced in the UK, Australia and the Netherlands as a result of increasing rates of MenW cases [Reference Abad5, Reference Campbell and Ladhani21, Reference Campbell44–46]. Chile similarly modified its national immunisation program in 2014 to include a single MenACWY dose administered at 12 months of age [Reference Villena and Santolaya47].
In short, the information gathered through this review concludes the following:
• An increase in serogroup W IMD has been observed on a global scale in the past decade, with serogroup W ST-11 strains being largely responsible.
• Serogroup W ST-11 IMD cases have been observed to present with unusual clinical characteristics worldwide.
• These unique presentations include acute GI symptoms, septic arthritis, pneumonia and upper respiratory tract infections.
• As MenW IMD continues to elude rapid diagnosis by physicians due to its frequent atypical presentation, physicians need to be familiar with the atypical signs and symptoms associated with serogroup W meningococcal disease. Quick recognition of this rapidly progressing disease can promote faster treatment and a higher patient survival rate.
Acknowledgements
Paul Balmer, Pfizer Inc, provided guidance on the overall manuscript content and approach to literature search. Editorial/medical writing support was provided by Karen L. Zimmermann, of Complete Healthcare Communications, LLC (North Wales, PA), a CHC Group Company, and was funded by Pfizer Inc.
Financial support
This work was sponsored by Pfizer Inc.
Conflict of interest
All authors are employees of and may hold stock or stock options in Pfizer Inc.
Disclaimers
All authors are employees of and may hold stock or stock options in Pfizer Inc.





