Minerals are inorganic elements that are essential for normal physiologic function and are widely involved in a broad range of processes including cellular metabolism and antioxidant and anti-inflammatory defences( Reference Turnlund 1 , Reference Powell 2 ). They have critical roles in enzyme activity as co-factors or catalysts( Reference Garfinkel and Garfinkel 3 , Reference Culotta, Yang and Hall 4 ), and contribute to protein synthesis( Reference MacDonald 5 ) and the regulation of gene expression( Reference Ishikawa, Kudo and Suzuki 6 ). Mineral homoeostasis in the body system is tightly regulated, and deficiencies in essential minerals may in turn affect a wide range of physiological processes( Reference Moe 7 , Reference Blaine, Chonchol and Levi 8 ).
There is ample evidence showing that minerals are important for reproductive function in women. In particular, minerals may be associated with ovulation, as demonstrated by induced ovulation upon administration of Cu in animals following stimulation of the hypothalamus( Reference Murawski, Bydlon and Sawicka-Kapusta 9 ), or by acting as antioxidants in controlling oxidative stress, which has been shown to promote ovulation( Reference Ruder, Hartman and Goldman 10 ). In maturing mammalian oocytes, Zn accumulates as oocytes grow and regulates meiosis( Reference Kim, Vogt and O’Halloran 11 , Reference Bernhardt, Kong and Kim 12 ), and as such may also be important for reproductive health. Higher levels of Cu, Zn( Reference Kurdoglu, Kurdoglu and Demir 13 ), Ca and Mn( Reference Chakraborty, Ghosh and Goswami 14 ) were measured in women with polycystic ovary syndrome and an inverse association between Fe intake and ovulatory infertility was suggested( Reference Chavarro, Rich-Edwards and Rosner 15 ), all pointing to possible relations between essential minerals and reproductive health outcomes.
To date, most studies on minerals and reproductive function, particularly reproductive hormones, have been conducted using serum mineral concentrations( Reference Muneyyirci-Delale, Nacharaju and Altura 16 – Reference Zagrodzki and Ratajczak 18 ) or animal models( Reference Sanchez-Capelo, Cremades and Tejada 19 , Reference Basini and Tamanini 20 ). Although diet is a major source of essential minerals in the general population, there are very few studies using dietary assessments to explore associations between minerals and reproductive function. Therefore, our objective was to investigate the associations between the dietary intakes of ten minerals and two primary outcomes including (1) reproductive hormones and (2) anovulation in healthy, regularly menstruating women who were not taking dietary supplements. We hypothesised that intake of dietary minerals below the recommended levels would be associated with changes in reproductive hormone concentrations and an increased risk of anovulation. Although exploratory, our study provides a comprehensive examination of the influence of dietary minerals among healthy women.
Methods
Study design and sample collection
The BioCycle Study is a prospective cohort study that recruited 259 healthy regularly menstruating women from Western New York in 2005–2007. Details of the study design and sample collection are described elsewhere( Reference Wactawski-Wende, Schisterman and Hovey 21 ). In brief, women aged 18–44 years with self-reported menstrual cycle lengths of 21–35 d for at least 6 months before recruitment and self-reported BMI of >18 or <35 kg/m2 at screening were eligible for the study. Women were excluded if they used hormonal contraceptives in the past 3 months before screening, were pregnant or breast-feeding in the past 6 months, had a history of ovulatory disorders or uterine abnormalities or were taking vitamin or mineral supplements during the study period. Participants provided information on age, race, lifestyle and reproductive and health history by means of questionnaires at baseline. Each participant also completed the International Physical Activity Questionnaire (IPAQ) long form 2002, and we estimated their physical activity as high, moderate and low based on standard IPAQ cut-offs. Trained research staff measured weight and height according to standardised protocols at baseline.
Each participant provided fasting blood specimens up to eight times per cycle during her visits at specific phases of the menstrual cycle, including menstruation, mid-follicular phase and late follicular phase, luteinising hormone (LH) surge, estimated day of ovulation and early , mid- and late luteal phase. Fertility monitors (Clearblue Easy Fertility Monitor; Inverness Medical) and personal cycle length histories were used to determine the timing of visits( Reference Howards, Schisterman and Wactawski-Wende 22 ). In total, we collected data for two cycles from 250 women and one cycle from nine women; 94 % of the participants completed at least seven clinic visits per cycle. The University at Buffalo Health Sciences Institutional Review Board (IRB) served as the IRB designated by the National Institutes of Health under a reliance agreement and approved the study. All study participants provided written informed consent.
Dietary assessment
We obtained dietary data through 24-h dietary recalls completed by study participants when they visited the clinic during the menstruation, mid-follicular, ovulation and mid-luteal phases of each cycle. Approximately 87 % of participants completed four dietary recalls per cycle; 13 % of women completed three recalls per cycle; and 0·4 % of women completed only two. We used the Nutrition Data System for Research software version 2005 (the Nutrition Coordinating Center, University Minnesota) to estimate intakes of dietary minerals, including Ca (mg), P (mg), Mg (mg), Fe (mg), Zn (mg), Cu (mg), Mn (mg), Se (µg), Na (mg) and K (mg) from food and beverage intakes. Na intake captures amounts that were naturally occurring in foods and those that were added during food processing; however, it does not include Na from any added table salt. We categorised daily intakes of dietary minerals by their recommended dietary allowance (RDA). The RDA for each mineral are as follows: 1000 mg for Ca, 700 mg for P, 310 mg for Mg, 18 mg for Fe, 8 mg for Zn, 0·9 mg for Cu, 1·8 mg for Mn, 55 µg for Se and 1500 mg for Na( 23 ). Mean levels of K intake were below the RDA (4700 mg) in all of our participants, as such K intake was categorised by the US average of 2227 mg/d( 24 ). As intakes were not observed to change over the cycle, except for Zn, which showed minimal variability( Reference Gorczyca, Sjaarda and Mitchell 25 ), we averaged the intake of dietary minerals per cycle for these analyses. Total energy intake was also averaged per cycle given that we did not observe changes across the cycle( Reference Gorczyca, Sjaarda and Mitchell 25 ), although another study has noted fluctuations( Reference Barr, Janelle and Prior 26 ).
Reproductive hormone measurement
Details of hormonal analyses were previously reported( Reference Wactawski-Wende 27 ). Concentrations of total oestradiol, follicle-stimulating hormone (FSH), LH, progesterone and sex-hormone-binding globulin (SHBG) were measured using solid-phase competitive chemiluminescent enzymatic immunoassays (Specialty Laboratories Inc.) on a DPC Immulite 2000 analyzer (Siemens Medical Solutions Diagnostics) at the Kaleida Health Center for Laboratory Medicine. Total testosterone concentrations (ng/dl) were measured by liquid chromatography/tandem MS using a Shimadzu Prominence Liquid Chromatograph (Shimadzu Scientific Instruments, Inc.) with an ABSceix 5500 tandem mass spectrometer (AB SCIEX) at the University of Minnesota. Increased sensitivity was obtained using Mobile Phase B (100 % acetonitrile) with a low standard of 4 ng/dl added to the standard curve. Free (i.e. bioavailable) oestradiol and testosterone and free androgen index were calculated based on standardised methods( Reference Sjaarda, Schisterman and Schliep 28 ). The coefficient of variation (CV) was <10 % for oestradiol and SHBG, <5 % for LH and FSH, <14 % for progesterone and <7 % for total testosterone. We defined sporadic anovulatory cycles as cycles with peak progesterone concentrations ≤5 ng/ml and no observed serum LH peak during the mid-luteal or late luteal phase cycle visit (in the event that timing of progesterone measurement was too early)( Reference Lynch, Mumford and Schliep 29 ). In total, forty-two of the 509 cycles (8·3 %) in the study were considered anovulatory.
Statistical analysis
Distributions of demographic variables were characterised by intakes of dietary minerals below or above the RDA or the US average intake for K. We used Fisher’s exact test or Student’s t test to compare the demographic variables as appropriate. We calculated Pearson’s correlation coefficients among person-averaged dietary mineral intakes.
Associations between dietary minerals and serum reproductive hormones were assessed by weighted linear mixed models with random intercepts. Cycle-averaged dietary minerals were evaluated in quintiles (comparing lower intakes with the highest intake quintile) and as dichotomised by the RDA or US average. Models were adjusted for age, BMI, race, physical activity, Mediterranean diet score, intakes of total energy, fibre and protein, as well as other hormone concentrations. Inverse probability weights were used to account for the complex feedback mechanisms between changing reproductive hormone levels over the menstrual cycle( Reference Robins, Hernan and Brumback 30 , Reference Cole and Hernan 31 ). The results are presented as percent difference in hormone levels with 95 % CI. We adjusted all models of dietary mineral intake and reproductive hormones for the false discovery rate using the Benjamini–Hochberg procedure within each dietary mineral group (i.e. Ca, P, Mg and so on) to account for multiple comparisons.
We used Poisson regression with robust error variance estimates to investigate the risk of sporadic anovulation with dietary mineral intakes by quintiles and RDA. All sporadic anovulation models were adjusted for age, BMI, race, physical activity, Mediterranean diet score and intakes of energy, fibre and protein. We report estimates as risk ratios (RR) and 95 % CI. SAS version 9.4 (SAS Institute) was used for all statistical analyses.
Results
Average daily intake of minerals, including P, Cu, Mn, Se and Na, were above the RDA in more than half of our participants, whereas intakes of Ca, Mg and Fe above the RDA were observed in only about 10 % of women (Table 1). The mean age of our participants was significantly higher among women whose intakes of dietary P (27·7 (sd 8·3) years) and Zn (28·8 (sd 8·7) years) were greater than the RDA and whose K was greater than the US average (30·0 (sd 8·8) years), compared with those who consumed less (24·8 (sd 7·1) for P, 25·9 (sd 7·5) for Zn and 26·4 (sd 7·8) for K). Intakes of dietary Ca, P, Mg, Zn, Cu and K were significantly different by race. Dietary minerals assessed in our study were all significantly correlated to each other (P<0·0001; Table 2). The highest correlations were detected between Mg and Mn (r 0·85), whereas the least correlated minerals were Ca and Cu (r 0·33) and Zn and Mn (r 0·33).
Table 1 Characteristics of the study cohort by dietary mineral intakes less than or greater than daily RDAFootnote * (Mean values and standard deviations; numbers and percentages)

OC, oral contraceptive.
* Cycle-averaged daily intake of dietary K was categorised by the US average intake (e.g. 2227 mg). P values for continuous variables were calculated using Student’s t test, and for categorical variables using Fisher’s exact test.
† Statistically significant differences (P<0·05).
Table 2 Correlations among average dietary mineral intakes across both cycles (n 259)Footnote *
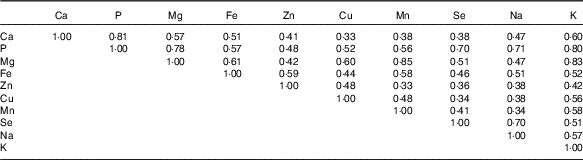
* All were statistically significant (P<0·0001).
Compared with Ca intake above the RDA (≥1000 mg), intake of Ca <RDA was associated with lower progesterone concentrations (−20·0 % difference; 95 % CI −34·9, −1·9; Table 3), adjusted for age, BMI, race, physical activity, intakes of energy, Mediterranean diet score, fibre and protein, as well as other hormones. Intake of Mg below the RDA (<310 mg) was inversely associated with testosterone (−4·7 % difference; 95 % CI −9·2, 0·0), relative to above the RDA. However, these results did not persist after adjusting for false discovery rate. We found that intake of Na below the RDA (<1500 mg) was associated with higher concentrations of FSH (21·3 % change; 95 % CI 7·5, 36·9) and LH (36·8 % change; 95 % CI 16·5, 60·5), and lower concentrations of progesterone (−36·9 % change; 95 % CI −56·5, −8·5), compared with Na intake above the RDA (≥1500 mg). Stratified by the US average intake, we detected inverse associations between K intake <2227 mg and free androgen index (5·7 % change; 95 % CI −0·1, 11·9), relative to intake of K ≥2227 mg, although the result was no longer significant after adjusting for false discovery rate. Compared with the highest quintile, the lowest quintiles of Fe and Zn were associated with lower progesterone (−36·4 % difference; 95 % CI −53·4, −13·4) and LH (−14·0 % difference; 95 % CI −24·1, −2·6), respectively (online Supplementary Table S1).
Table 3 Associations between dietary mineral intakes and reproductive hormone concentrations by daily RDAFootnote * (Percentage differences in hormone concentrations and 95 % confidence intervals)

FSH, follicle-stimulating hormone; LH, luteinising hormone; SHBG, sex-hormone-binding globulin.
* Models were adjusted for age, BMI, race, physical activity, Mediterranean diet score, intakes of energy, fibre and protein, as well as other hormones. For progesterone, only measurements during the luteal phase were included. Intakes of K were categorised by the US average intakes (e.g. 2227 mg/d).
† Statistically significant estimates and intervals.
Compared with intakes of minerals above the RDA, intakes of Mn <1·8 mg (RR 2·00; 95 % CI 1·02, 3·94) and Na <1500 mg (RR 2·70; 95 % CI 1·00, 7·31) were associated with an increased risk of sporadic anovulation, adjusted for age, BMI, race, physical activity, Mediterranean diet score and intakes of energy, fibre and protein (Table 4). Intake of Se <55 µg was associated with increased risk for sporadic anovulation (RR 2·66; 95 % CI 0·96, 7·36), relative to Se intake ≥55 µg. In general, no significant associations were detected when dietary minerals were assessed in quintiles (online Supplementary Table S2).
Table 4 Dietary mineral intake by RDA and risk of sporadic anovulationFootnote * (Risk ratios (RR) and 95 % confidence intervals)

* All models were adjusted for age, BMI, race, physical activity, Mediterranean diet score and intakes of energy, fibre and protein. Intakes of dietary K were categorised by the US average intakes (e.g. 2227 mg/d).
† Statistically significant estimates and intervals.
Discussion
Overall, our data suggest associations between intakes of specific dietary minerals, particularly Na intake, and reproductive hormones in normally menstruating women of reproductive age. Our data also indicate associations between insufficient intakes of Mn and Na and an increased risk of sporadic anovulation, although the majority of dietary minerals that we evaluated were not associated with the risk of anovulation. As our assessed minerals are abundant in easily accessible food items, our data suggest that specific dietary factors may influence reproductive hormones and ovulation among healthy women.
We observed associations between dietary Na intake below the RDA and higher FSH and LH concentrations, lower progesterone levels and an increased risk of sporadic anovulation. High intake of Na has been associated with increased levels of steroid hormones, such as glucocorticoids, in 370 adults( Reference Baudrand, Campino and Carvajal 32 ); however, studies evaluating the potential role of dietary Na on changes of other hormone levels are scarce, limiting comparison with our work. One experimental study conducted in male rats reported increased levels of FSH and testosterone and decreased levels of LH in rats fed a high-salt diet compared with controls fed a low-salt diet( Reference Iranloye, Oludare and Morakinyo 33 ). Sufficient levels of Na intake might be important for mammalian reproduction, as suggested in an animal study, which detected significantly lower mating and fewer litters in mice fed low-Na foods, compared with mice fed high-Na foods( Reference McBurnie, Blair-West and Denton 34 ). Specific biological mechanisms linking Na intake and ovulatory function are limited, although this could be perhaps similar to a relationship between Na and inflammation. Positive associations between Na and inflammation were suggested in a cross-sectional analysis of 1597 individuals in whom 24-h Na excretion was associated with increases in serum C-reactive protein concentration, a global marker of inflammation( Reference Fogarty, Lewis and McKeever 35 ). As ovulation is considered an acute inflammatory process( Reference Espey 36 ), our finding on the association between Na intake below the RDA and increased risk of anovulation could be partly related to this mechanism. Nonetheless, because Na intake assessed in our study does not reflect Na from salt added at the table, exposure misclassification is possible. Despite the fact that Na is an essential mineral, excessive consumption is related to adverse health conditions such as high blood pressure and CVD( Reference Whelton, Appel and Sacco 37 ), and thus our result should be interpreted carefully.
An association between Mn intake below the RDA and an increased risk for sporadic anovulation was indicated in our study, although we did not detect any associated changes in hormones. The impact of Mn on women’s reproductive health has been investigated in relation to pubertal development in prior work. Specifically, in animal studies using prepubertal female rats, a significant increase in serum LH levels was detected upon MnCl2 treatment( Reference Pine, Lee and Dearth 17 , Reference Kim, Jang and Han 38 ). Given that Mn is abundant in easily obtainable foods (e.g. teas, nuts, legumes and whole grains), further investigation into the particular sources of dietary Mn and improved ovulatory function could be beneficial for women of reproductive age.
Although our data do not support hormonal differences in relation to dietary Se, intake of Se below the RDA was associated with an increased risk for sporadic anovulation. Owing to its antioxidant properties, greater attention has been given to Se in relation to fertility. A significantly higher level of glutathione peroxidase activity, an enzyme containing Se, was observed in menstruating women compared with women without regular menstruation( Reference Massafra, Buonocore and Gioia 39 ), and plasma Se was related to oestradiol concentrations across the menstrual cycle( Reference Ha and Smith 40 ). In addition, Se was positively associated with progesterone concentrations in adolescent girls, a marker of ovulatory function, in research specifically investigating Se status, sex hormone secretion and thyroid metabolism( Reference Zagrodzki, Ratajczak and Wietecha-Posluszny 41 ). Combined with previous studies using Se measured in blood, our data using dietary Se may also perhaps underscore a potential role of Se in ovulatory function in healthy women.
Our data suggested an association between dietary Ca and progesterone, although the result was no longer significant after adjusting for false discovery rate. Nevertheless, this association is in line with an experimental study, which demonstrated an association between Ca and progesterone in ovarian follicle cells( Reference Francisco and Schuetz 42 ). Although we did not detect any associations between Ca and oestradiol, prior studies reported a relation between Ca and oestrogen metabolism in women( Reference Gallagher, Riggs and DeLuca 43 – Reference Napoli, Thompson and Civitelli 45 ), suggesting a possible interaction between Ca and oestrogen and link with other oestrogen-dependent conditions, such as endometriosis( Reference Harris, Chavarro and Malspeis 46 ). We recently reported no association between Ca consumption via dairy intake and sporadic anovulation( Reference Kim, Wactawski-Wende and Michels 47 ), and our findings regarding overall dietary Ca intake in this analysis were similar. In a separate analysis, we did not detect any significant changes in Ca measured in diet across the cycle overall and by anovulation status. Therefore, our null results might be owing to the fact that any changes in dietary Ca over the cycle are very small and are not subsequently associated with reproductive hormones and ovulation measured in our study.
We further observed that insufficient dietary intakes of Mg were associated with lower testosterone levels, although the association did not remain after adjustment for multiple comparisons. Influence of Mg on hormonal change has been suggested in an experimental study that showed that changes in Mg status were associated with changes in testosterone and SHBG( Reference Excoffon, Guillaume and Woronoff-Lemsi 48 ). Previously, a study of twenty-five post-menopausal women and fifteen premenopausal women reported no association between serum Mg concentrations and testosterone, although an inverse association with oestrogen was detected( Reference Muneyyirci-Delale, Nacharaju and Altura 16 ). Our measurement of Mg via dietary intake is different from the serum measurement, as the latter may reflect overall Mg status in the body system. Still, our data suggest the importance of Mg in maintaining the level of bioavailable testosterone for women of reproductive age.
Consumption of K was lower than the RDA for all participants in our study, which is consistent with the US general population, where average intakes of dietary K are substantially lower than the RDA (4700 mg) among both men and women( 23 ). Stratifying intake by the US average, we found that intake of K below the US average was associated with higher testosterone, free testosterone and free androgen index, although the results were no longer significant after adjusting for false discovery rate. In an experimental study, K demonstrated a regulatory role in plasma testosterone levels( Reference Sanchez-Capelo, Cremades and Tejada 19 ), potentially supporting our observation between K and androgen levels. Although we observed associations between testosterone levels and consumption of K, this did not translate into associations with the risk of anovulation in healthy regularly menstruating women.
Our study has several limitations. In an animal study, a role of reproductive hormones on Na consumption has been suggested, possibly through the sympathetic nervous system, which could be influenced by oestradiol and drive Na intake in turn( Reference Kensicki, Dunphy and Ely 49 ). Therefore, it is possible that the intakes of certain dietary minerals, particularly Na, could be affected by hormonal changes specific to cycle phase. However, this might be of less concern as we used longitudinal modelling with inverse probability weighting to account for changing levels of endogenous reproductive hormones over the menstrual cycle, and we did not observe changes in intakes over the cycle. As the women in our study were instructed not to take any mineral supplements or undergo any diet regimes during the study period( Reference Wactawski-Wende, Schisterman and Hovey 21 ), our data reflect the most common sources of minerals in a regular diet and the results are only generalisable to healthy regularly menstruating women not taking supplements. However, there are several strengths in our study as well. We used 24-h dietary recalls collected multiple times throughout the menstrual cycles to estimate usual intakes. This approach was used to reduce potential measurement error( Reference Subar, Kipnis and Troiano 50 ), although more recalls may have been necessary to more adequately assess intake for specific minerals such as Ca. Our data also capture a broad spectrum of the essential minerals via diet, and there is greater public health utility and easy interpretation of measures of daily mineral consumption.
Overall, our analyses suggest that intakes of specific dietary minerals, particularly Na and Mn, may influence reproductive hormone concentrations and ovulatory function in premenopausal women with regular menstrual cycles. Minerals are mostly obtained via diet in the general population, and a range of whole food diets will likely provide adequate micronutrients to support normal ovulatory function. Given that essential minerals are abundant in easily accessible food items, our data suggest that dietary factors likely have important influences on ovulation and fertility. Moreover, the reproductive hormones investigated in our study affect not only women’s reproductive health but general health across the life span. Thus, the associations with dietary minerals suggested in our study may be also of significance for other health outcomes related to reproductive organs or hormone levels.
Acknowledgements
The authors thank the BioCycle Study participants for their time and efforts.
This work was supported by the Intramural Research Program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health (Contract nos: HHSN275200403394C, HHSN275201100002I and Task 1HHSN27500001).
K. K. developed the analytic plan, performed statistical analyses and drafted and revised the manuscript. S. L. M. consulted on statistical analyses. J. W.-W., K. A. M., K. C. S., T. C. P., E. N. C. and S. L. M. reviewed and revised the manuscript for important intellectual content. J. W.-W. designed and conducted the BioCycle Study. All authors approved the final manuscript as submitted.
The authors declare that there are no conflicts of interest.
Supplementary material
For supplementary material/s referred to in this article, please visit https://doi.org/10.1017/S0007114518000818







