Earlier puberty represents a risk factor for hormone-related cancers such as breast cancer and has been related to other adverse outcomes such as mortality(Reference Golub, Collman and Foster1–Reference Jacobsen, Oda and Knutsen3). Higher childhood body fatness may result in earlier onset and/or more rapid progression of puberty(Reference Sandhu, Ben-Shlomo and Cole4–Reference Buyken, Karaolis-Danckert and Remer6). Since higher adiposity can be a result of excess energy intake (EI), one can also assume a role of EI for pubertal timing. Several studies have addressed this question with respect to age at menarche with conflicting results. Higher EI has been associated with both earlier(Reference Meyer, Moisan and Marcoux7) and delayed(Reference Petridou, Syrigou and Toupadaki8) menarche, yet others have found no association(Reference Moisan, Meyer and Gingras9, Reference Merzenich, Boeing and Wahrendorf10).
Recently, interest in dietary energy density (ED), i.e. the ratio of the amount of food to total EI, has emerged. According to experimental studies that manipulated ED under laboratory conditions, ED is a major determinant of EI and potentially affects adiposity(Reference Rolls, Drewnowski and Ledikwe11). It has been postulated that increased body fat resulting from excess EI and higher ED in childhood could influence pubertal timing. EI, irrespective of adiposity, may also be a regulator of childhood growth (velocity) and associated hormones, which have been related to pubertal timing and/or cancer risk(Reference He and Karlberg12, Reference Uauy and Solomons13). In addition to these mechanisms, higher ED can be associated with lower diet quality and hence nutrient intake. Higher intakes of thiamin, Fe(Reference Kissinger and Sanchez14) and fibre(Reference Koo, Rohan and Jain15) have been related to delayed pubertal onset in girls. Children with lower diet quality, indexed by the Nutritional Quality Index, indeed experienced their pubertal growth spurt (age at take-off, ATO) earlier(Reference Cheng, Gerlach and Libuda16).
However, data on ED in children are limited, particularly if they choose their diet spontaneously and consume foods ad libitum. Its role on pubertal timing has not previously been addressed. The few observations on ED and body fat have yielded mixed results(Reference Johnson, Mander and Jones17–Reference McCaffrey, Rennie and Kerr19). In part, this may be explained by different methods of calculating ED and including beverages(Reference McCaffrey, Rennie and Kerr19). The aim of the present study was to examine the association between ED, body fat and ATO in the DOrtmund Nutritional and Anthropometric Longitudinally Designed (DONALD) Study participants. ED was calculated using four different approaches.
Methods
The DONALD Study is an ongoing, open cohort study started in 1985(Reference Kroke, Manz and Kersting20). Information on diet, growth and metabolism between infancy and adulthood is regularly collected: four visits in the first year of life; two in the second; then one annually. The Ethics Committee of the University of Bonn, Germany, approved the study. Examinations are performed with parental consent.
ATO could be estimated from repeated height measurements in 376 term singletons with a birth weight >2500 g(Reference Karaolis-Danckert, Buyken and Sonntag21). Of these, 219 had anthropometry measured, plausible dietary data 2 and 3 years before ATO, and information on potential confounders (maternal overweight/education, breast-feeding and baseline anthropometry). The time points represent mean age at ‘adiposity rebound’, a critical period in childhood. This sample size of 219 was sufficient to detect mean differences in ATO of 0·42 years or 0·47 BMI/fat mass index (FMI) Z-scores, between ED tertiles (80 % power, α = 0·05, two-tailed).
Anthropometry
Weight, height and skinfolds (triceps, biceps, subscapular and iliac) were measured at each visit as reported previously(Reference Kroke, Manz and Kersting20). From age 2 years onwards, standing height was measured using a digital stadiometer. BMI Z-scores were calculated using the German reference(Reference Kromeyer-Hauschild, Wabitsch and Kunze22). Percentage of body fat was estimated using the Slaughter et al. (Reference Slaughter, Lohman and Boileau23) equations for pre-puberty (baseline) and puberty (ATO), and converted to FMI. Z-scores of log-transformed FMI were obtained via standardisation by age and sex.
Puberty
ATO was estimated using the parametric Preece and Baines formula 1(Reference Preece and Baines24). As described previously(Reference Karaolis-Danckert, Buyken and Sonntag21, Reference Buyken, Bolzenius and Karaolis-Danckert25), Preece and Baines formula 1 was fitted on various age ranges of height-for-age, beginning with age 2 years, to determine the optimal data range. ATO represented the age at minimal height velocity at onset of the pubertal growth spurt. Best fit was determined by four criteria, including graphical inspection of growth curves and comparison of residual standard deviations(Reference Karaolis-Danckert, Buyken and Sonntag21, Reference Buyken, Bolzenius and Karaolis-Danckert25). Finally, data from age 5 to 13 years (girls) and age 6 to 13 years (boys) were selected. Preece and Baines formula 1 was also used to estimate age at peak height velocity (n 216). Menarcheal age was available in 104 girls.
Diet
Dietary intake was assessed by 3 d weighed dietary records, as described previously(Reference Kroke, Manz and Kersting20). Parents weighed all foods and beverages consumed by their children, including water, for three consecutive days with regularly calibrated electronic scales (32·6 % weekend days and 67·4 % weekdays). The records were checked for completeness and accuracy, and mean EI and nutrient intakes were calculated using the in-house nutrient database(Reference Sichert-Hellert, Kersting and Chahda26). The ratio of reported EI and calculated BMR was used to exclude two potential under-reporters(Reference Sichert-Hellert, Kersting and Schoch27). Habitual dietary intakes were calculated as means 2 and 3 years before ATO.
We calculated ED (kJ/g) using four approaches(Reference McCaffrey, Rennie and Kerr19, Reference Cox and Mela28, Reference Ledikwe, Blanck and Khan29): (1) ED_all included all foods and drinks, regardless of energy content; (2) ED_energy included all foods and energy-containing drinks (>21 kJ, or 5 kcal, per 100 g)(Reference McCaffrey, Rennie and Kerr19); (3) ED_milk included all foods and milk as a drink, but no other beverages; (4) ED_food included foods only (solid/liquid).
Other data
At study entry, parents were interviewed, weighed and measured. Parents of children were regularly asked which sports their children performed and how often. Using this crude information, level of physical activity at age 5 years was determined (low/moderate/high).
Statistics
Normality of continuous variables was assessed by statistical testing and graphical inspection. We calculated adjusted mean outcome levels (ATO or BMI/FMI Z-score at ATO) in tertiles of ED. P trend across tertiles was determined by multiple linear regression, with ATO or BMI/FMI Z-score at ATO as outcomes and ED as the independent variable. There was no significant interaction of ED with sex (P>0·1), and stratification did not indicate sex differences. Potential confounders were selected for their relationship with ED and the outcomes, or based on the literature(Reference Moisan, Meyer and Gingras9, Reference Koo, Rohan and Jain15, Reference Karaolis-Danckert, Buyken and Sonntag21, Reference Buyken, Bolzenius and Karaolis-Danckert25, Reference Günther, Karaolis-Danckert and Kroke30): sex; birth year; birth weight (>3000 g, yes/no); maternal overweight (BMI ≥ 25 kg/m2, yes/no); maternal education ( ≥ 12 years schooling, yes/no); maternal age at birth; full breast-feeding ( ≥ 4 months, yes/no); household smoking (yes/no); physical activity (low/moderate/high); protein, fat (percentage of EI, percentage of total energy intake); fibre (g/100 kJ); baseline BMI/FMI Z-score. Variables changing the ED estimate or associated with the outcome (P < 0·1) were retained in the models. Diet quality according to the Nutritional Quality Index (low/moderate/high) formed a potential mediator, since it could lie on the pathway between ED and ATO(Reference Cheng, Gerlach and Libuda16). Analyses were performed using SAS 9.1 (SAS, Inc., Cary, NC, USA).
Results
ATO took place approximately 1·5 years earlier in girls than in boys (Table 1). ED ranged between 4·1 and 7·0 g/kJ, depending on the calculation method.
Table 1 Puberty, family and dietary characteristics of the DOrtmund Nutritional and Anthropometric Longitudinally Designed Study sample
(Mean values, standard deviations, number of participants and percentages, n 219)

ATO, age at take-off; %E, percentage of total energy intake; ED, dietary energy density; ED_all, all foods and drinks; ED_food, foods and energy-containing drinks (>21 kJ/100 g); ED_milk, foods and milk as a drink, but no other beverages; ED_food, foods only.
* According to the International Obesity Task Force definition(Reference Cole, Bellizzi and Flegal32).
† BMI ≥ 25 kg/m2, yes/no.
‡ Based on the parental interview (which sports do children perform and how often).
ED_food at baseline was not associated with ATO, BMI or FMI Z-score at ATO in either model (P>0·1; Table 2). ED_all, ED_energy and ED_milk were not significantly associated with any of the outcomes either. A higher ED_all was initially associated with a higher BMI Z-score at ATO in a model only adjusting for sex (P trend = 0·01). However, this was explained by baseline BMI Z-score (P trend = 0·3; data not shown).
Table 2 Adjusted mean age at take-off (ATO, in years), BMI Z-score and fat mass index (FMI) Z-score at ATO in tertiles of pre-pubertal dietary energy density (ED) based on foods only, DOrtmund Nutritional and Anthropometric Longitudinally Designed Study
(Least square mean (lsmean) values and 95 % confidence intervals, n 219)
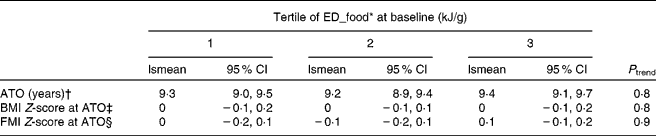
* ED_food corresponds to ED based on food intake only.
† Adjusted for sex, maternal age at birth (years), birth weight ( < 3000 g, yes/no), protein percentage of total energy intake (%E), fibre (g/100 kJ) and baseline BMI Z-score.
‡ Adjusted for sex, maternal overweight (BMI ≥ 25 kg/m2, yes/no), birth weight ( < 3000 g, yes/no), protein (%E), fibre (g/100 kJ) and baseline BMI Z-score.
§ Adjusted for sex, maternal overweight (BMI ≥ 25 kg/m2, yes/no), birth weight ( < 3000 g, yes/no), protein (%E), fibre (g/100 kJ) and baseline FMI Z-score.
Further adjustments for other potential confounders or Nutritional Quality Index category, a possible mediator(Reference Cheng, Gerlach and Libuda16), did not change these results. ED was not related to later pubertal markers either, i.e. age at peak height velocity (P trend = 0·5–0·8) and age at menarche in girls (P trend = 0·9–0·99), irrespective of the ED calculation method (data not shown).
Discussion
The present results suggest that healthy children with higher pre-pubertal ED do not experience their pubertal growth spurt earlier.
One mechanism by which ED could influence ATO would be increased body fatness. In another DONALD subsample (n 215), neither BMI nor FMI were clearly associated with ATO(Reference Buyken, Karaolis-Danckert and Remer6), which supports the present results. However, ED was not related to later pubertal markers (age at peak height velocity, menarche) either – and these were predicted by pre-pubertal body composition in the previous analysis. Another mechanism could be that higher ED lowers diet quality, related to ATO in 222 DONALD Study participants(Reference Cheng, Gerlach and Libuda16). Nutritional Quality Index scores were inversely related to ED in the present study (data not shown), but considering diet quality had no impact.
Similar to puberty onset, ED was not related to body fatness at ATO, i.e. at a distinct physiological age in a child's development. This is a worthwhile addition since previous studies have relied on chronological age. However, at a given chronological age in childhood differences in body composition may just stem from different developmental stages. In one study(Reference McCaffrey, Rennie and Kerr19), ED (excluding beverages) at 6–8 years was related to FMI gain at 13–17 years. However, no association existed for percentage of body fat, BMI or waist circumference Z-score. Johnson et al. (Reference Johnson, Mander and Jones17) found that only higher ED at 7 years, not at 5 years, was associated with a higher risk of a FMI >80th percentile at 9 years.
ED may have been below a threshold above which unfavourable effects (increased adiposity, earlier puberty onset) might operate; it was lower than in other paediatric studies when similar calculations were compared(Reference Johnson, Mander and Jones17–Reference McCaffrey, Rennie and Kerr19). DONALD Study participants have a higher socio-economic background, a general interest in health and might have healthier dietary behaviour(Reference Kroke, Manz and Kersting20). Besides the ED range, this could be important since an effect of ED may exist on excess adiposity in particular(Reference Johnson, Mander and Jones17, Reference McCaffrey, Rennie and Kerr19). However, extremes of anthropometry were not represented in this analysis either. Correspondingly, the risk for overweight at ATO did not differ significantly between ED tertiles (data not shown). The homogeneity of our sample by contrast makes it less susceptible to residual confounding.
An additional limitation lies in the dietary assessment, since 3 d might not be sufficient to derive valid estimates of EI(Reference Lanigan, Wells and Lawson31). We addressed this by considering two records per child, reflecting habitual intake, and excluded potential under-reporters. Lastly, only crude measures of physical activity were available.
In conclusion, a higher habitual ED in childhood was not associated with an earlier puberty onset or higher body fatness at that time point in healthy, free-living children with a low overall ED.
Acknowledgements
The DONALD Study is funded by the Ministry of Innovation, Science and Research of North-Rhine-Westphalia, Germany. The present study was funded by the World Cancer Research Fund International. A. K. and A. E. B. conceived the study; A. L. B. G. and L. J. S. conducted the analysis; A. L. B. G. wrote the manuscript. The authors declare no conflicts of interest.




