“My whole mental power has disappeared, I have sunk intellectually below the level of a beast”(a patient with schizophrenia, quoted by Reference KraepelinKraepelin, 1919, p. 25).
Traditionally, significant cognitive impairment was thought to be evident only in elderly deteriorated patients with schizophrenia. However, over the past 25 years, evidence has accrued to challenge this view. It is becoming evident that marked cognitive impairment is, in fact, the norm and often pre-dates the illness. The recent literature has attempted to characterise the prevalence, degree and nature of neuropsychological abnormality in schizophrenia (see Box 1). In this brief review, I will attempt to summarise the current state of knowledge with regard to cognitive impairment in schizophrenia.
Box 1. Cognitive impairment in schizophrenia – summary of recent research findings
Significant cognitive impairment is common in schizophrenia, affecting up to 75% of patients
A wide range of cognitive functions are affected, particularly memory, attention, motor skills, executive function and intelligence
The cognitive impairment often pre-dates the illness onset
It is an intrinsic part of the illness and is observed in young, drug-naïve patients
Efforts to explain core features of schizophrenia as a consequence of specific neuropsychological abnormalities have been disappointing
Cognitive impairment is related to social and functional outcome
The evidence is mixed regarding the efficacy of newer atypical antipsychotics in improving cognitive functioning in schizophrenia
Although some laboratory-based studies of cognitive rehabilitation have provided promising results, convincing evidence regarding generalisation to lasting improvements in day-to-day functioning is, as yet, lacking
Nature and degree of cognitive impairment
This field evolved from the traditional ‘lesion’-based approach in neuropsychology, that is, inferring regional brain dysfunction based on poor performance on putatively localising neuropsychological tests. On the basis of such an approach, various authors have concluded that schizophrenia is characterised by cognitive test profiles indicative of dysfunction of the frontal lobe, temporal lobe, left or right hemisphere, basal ganglia, etc. (Reference Blanchard and NealeBlanchard & Neale, 1994). This lack of consensus may reflect the heterogeneity of schizophrenia, and may also be a result of the relatively poor localising ability of many standard neuropsychological instruments. A variety of candidate brain regions and associated cognitive functions have thus been implicated in the psychopathology that characterises schizophrenia. In general, the strongest camps to emerge have been those that claim a disproportionate impairment of memory functioning (Reference McKennaMcKenna, 1991; Reference Saykin, Shtasel and GurSaykin et al, 1994) and those arguing for a relatively selective executive dysfunction (Reference Weinberger, Berman and ZecWeinberger et al, 1986). Others have reported more widespread neuropsychological dysfunction (Reference BuchsbaumBuchsbaum, 1990). An extreme case is put by Meehl who stated:
“I conjecture that whatever is wrong with the schizotaxic CNS is ubiquitous, a functional aberration present throughout, operating everywhere from the sacral cord to the frontal lobes” (Reference MeehlMeehl, 1990, p. 14).
In many ways, it now seems an old-fashioned approach to try to localise central nervous system (CNS) dysfunction using cognitive tests that were largely devised to identify specific brain damage, and were developed in an era before high-resolution neuroimaging was possible. Many current workers would agree with Shallice et al (1991) who proposed that from a neuropsychological perspective, an attempt to understand the nature of the information-processing impairment of schizophrenia should precede an attempt to localise it.
Over the years, many authors have questioned whether or not schizophrenia is characterised by genuine cognitive impairment. However, recent studies have clearly demonstrated its presence. To take an example, Heinrichs & Zakzanis (1998) carried out a large-scale comprehensive quantitative meta-analysis of cognitive impairment in schizophrenia, which involved comparisons of patients with schizophrenia v. controls. Their results are summarised in Table 1. As can be seen, the greatest impairment is observed in global verbal memory functioning. However, given the multitude of tests that show significant impairment, clearly any specific impairment (e.g. memory or executive functioning) exists within a more widespread reduction in general cognitive functioning. In accordance with this view, Palmer et al (1997) recently posed the question, “Is it possible to be schizophrenic yet neuropsychologically normal?” They gave a comprehensive neuropsychological battery to 171 out-patients with schizophrenia and compared them with 63 healthy controls. Two experienced neuropsychologists conducted blind ratings of the test results. Only 27% of the patients with schizophrenia were classified as neuropsychologically ‘normal’. This indicates that significant cognitive impairment in schizophrenia is, in fact, the norm. However, this study also highlights the fact that a proportion of patients with schizophrenia appear to remain neuropsychologically intact. This suggests that the pathophysiology underlying the cognitive deficits often associated with schizophrenia may be distinct from that causing some of the core clinical features. Evidence in support of this latter view can also be derived from the study by Goldberg et al (1993), who reported symptomatic improvement following treatment with clozapine, but with no accompanying improvement in neuropsychological functioning.
Does the cognitive impairment pre-date the illness?
The temporal relationship between cognitive impairment and illness has been tackled via studies where neuropsychological data have been collated retrospectively from individuals who later developed schizophrenia. The aim has been to determine whether any pre-existing abnormalities in cognitive functioning are apparent before the onset of schizophrenia. Jones et al (1994) used the subjects from the Medical Research Council National Survey of Health and Development, a random sample of over 5000 births in England, Scotland and Wales during the first week of March 1946. Out of this sample, 30 cases of schizophrenia arose between the ages of 16 and 43. All the subjects had been tested for non-verbal, verbal and reading abilities at the ages of 8, 11 and 15 years and arithmetic was measured at the ages of 11 and 15 years, with vocabulary being assessed at the ages of 8 and 11 years. The main results are shown in Table 2. As can be readily observed, children who developed schizophrenia in later life were significantly impaired on non-verbal and verbal intelligence tests from the age of 8, and on arithmetic/mathematic skills from the age of 11. This result clearly indicates the presence of detectable cognitive abnormalities in childhood which pre-dated the development of the illness.
In a Swedish study, David et al (1997) were able to capitalise on a remarkable sample of 50 000 males conscripted to the Swedish Army between 1969 and 1970. Tests of cognitive functioning were recorded at conscription. In later life, 195 subjects were admitted to hospital with schizophrenia. Low IQ emerged as a clear risk factor for those later diagnosed with schizophrenia, and poor performance on verbal tasks and a mechanical knowledge test conferred a significantly increased risk of schizophrenia, even after taking into account general intellectual ability.
Taken together, the results of these and other studies provide strong supportive evidence for the view that neuropsychological abnormalities pre-date the development of schizophrenia. It is tempting to interpret these findings as evidence of a neurodevelopmental abnormality in those individuals who develop schizophrenia in late adolescence or early adulthood. This association could be directly causal, that is, with cognitive impairment leading to false beliefs and perceptions, or, alternatively, could act via an indirect mechanism with any factors which cause low IQ (such as abnormal brain development) increasing later risk for schizophrenia (Reference David, Malmberg and BrandtDavid et al, 1997).
Is the cognitive impairment progressive?
The assumption of intellectual decline in schizophrenia is evident from the earliest writings on the disorder – for example, the term ‘dementia praecox’ (Reference KraepelinKraepelin, 1919) implies a continuing cognitive decline. Other writers have proposed that psychotic episodes may be neurotoxic, with increasing length of initial untreated illness associated with a poor prognosis. However, as reviewed above, there is increasing evidence that cognitive impairment often precedes the illness. One of the outstanding issues in this area is whether or not the cognitive impairment observed in patients with schizophrenia declines over time. There are opposing views on this subject. Russell et al (1997) recently proposed that intellectual decline in schizophrenia was a nothing more than a ‘myth’. They followed up patients who had received an intelligence test as a child, who then developed schizophrenia, and had their intelligence re-tested some 19 years later. There were no differences between the child and adult IQs. However, others have criticised this study on the grounds that the sample was unrepresentative in that the individuals had presented to child psychiatry units and were far more intellectually impaired than subjects in other studies of children who subsequently developed schizophrenia.
Rund (1998) has reviewed a number of longitudinal studies and concluded that the cognitive deficits appear to be relatively stable in schizophrenia, consistent with a static encephalopathy rather than a degenerative process. However, most clinicians are able to identify individual patients who appear to have severely declined cognitively since the onset of their illness. It may be that subgroups of patients show a particularly poor cognitive outcome. Further longitudinal studies are required in order to settle this issue.
‘Real-life’ consequences
It has been claimed that impaired cognitive test performance in patients with schizophrenia may be an epiphenomenon, for example, reflecting lack of motivation or distraction by hallucinations. In order to convince sceptics that the neuropsychological impairment is important, one would have to demonstrate a clear relationship between cognitive test performance and ‘real-life’ functional outcome. An important review of this area was published by Green (1996), who evaluated studies that used cognitive measures as predictors and correlates of functional outcome. The most consistent finding to emerge was that verbal memory functioning was associated with all types of functional outcome. (It is notable that verbal memory was the cognitive domain that showed the greatest impairment in the meta-analysis byReference Heinrichs and ZakzanisHeinrichs & Zakzanis (1998) – see Table 1.) Sustained attention or vigilance was also found to be related to social problem-solving and skill acquisition. Interestingly, psychotic symptoms were not significantly associated with outcome measures in any of the studies that were reviewed. Green (1996) concluded that deficiencies in verbal memory and vigilance may prevent patients from attaining optimal adaptation and hence may act as rate-limiting factors in terms of rehabilitation. It is interesting that Goldberg et al (1993), who reported symptomatic improvement with clozapine treatment, with no associated improvement in neuropsychological functioning, concluded:
“This suggests that certain cognitive deficits are relatively independent of psychotic symptoms in schizophrenia, and are probably central and enduring features of the disorder. Cognitive disability appeared to have been rate-limiting in the sample's rehabilitation” (p. 43).
We have, therefore, moved from a position where cognitive impairment was not considered to be particularly important in schizophrenia, to the current view, that it may be a central and rate-limiting feature in terms of rehabilitation.
Velligan et al (1997) has confirmed a poor correlation between symptomatology and ability to perform daily living tasks. However, cognitive impairment predicted over 40% of the variance in scores on a functional needs-assessment rating scale. Addington & Addington (1999) used a novel video-taped measure of interpersonal problem-solving skills. In a study of 80 out-patients with schizophrenia, they found that better cognitive flexibility and verbal memory were positively associated with interpersonal problem-solving ability.
Taken together, the evidence strongly supports the view that cognitive impairment in schizophrenia is directly related to social deficits and functional outcome for many patients.
Does the neuropsychological impairment relate to clinical symptoms and signs?
One of the few features of schizophrenia that most authors agree on is its heterogeneity. It is possible that part of the difficulty in detecting a consistent ‘neuropsychological signature’ of schizophrenia (Reference Blanchard and NealeBlanchard & Neale, 1994) is that there is no such thing as ‘schizophrenia’. Syndromes or symptoms may more clearly relate to disordered patterns of information processing. Liddle & Morris (1991) conducted a seminal study in this area where they assessed a group of patients with chronic schizophrenia using a battery of neuropsychological tests allegedly sensitive to frontal lobe dysfunction. Signs and symptoms were clustered into three syndromes: psychomotor poverty, disorganisation and reality distortion. Scores for the disorganisation syndrome were associated with impairment on tests that required the subject to inhibit a well-established but inappropriate response. Ratings for the psychomotor poverty syndrome were found to be associated with slowness of mental activity. More recently, Baxter & Liddle (1998) confirmed that the psychomotor poverty syndrome was associated with psychomotor slowing, and disorganisation was associated with impaired performance on the Stroop attentional conflict task, but not with other tests of cognitive inhibition. This led the authors to conclude that the disorganisation syndrome might be associated with a specific difficulty in suppressing irrelevant verbal responses. This approach is appealing, because it tries to integrate neuropsychology with the clinical features of schizophrenia. Pursuing this approach to a more specific level would result in an attempt to explain specific signs or symptoms in terms of aberrant information processing. As an illustration of this approach, McKenna (1991) proposed that delusions may arise as a consequence of a dysfunctional semantic memory system. Again, this hypothesis has intuitive appeal, as delusions by definition must represent false knowledge. However, efforts to try to provide convincing evidence of a causal relationship between a specific neuropsychological abnormality and a particular sign or symptom have, as yet, been disappointing.
As mentioned above, one way to simplify the heterogeneity of schizophrenia is to factor-analyse clinical ratings in an attempt to produce syndromes. An alternative to this approach is to try to extract neuropsychological factors from a battery of cognitive tests. This is the approach adopted by Heinrichs & Awad (1993). Patients with schizophrenia (n=104) performed the Wisconsin Card Sorting Test to assess executive function, the California Verbal Learning Test to tap memory functioning, the Purdue Peg Board to assess motor function and the Wechsler Adult Intelligence Scale – Revised to measure general intelligence. The resulting cognitive data were subjected to cluster analysis and five cognitive clusters emerged:
-
(a) selective executive dysfunction;
-
(b) normative function;
-
(c) executive and motor deficits;
-
(d) dementia/multi-focal disturbance; and
-
(e) relatively selective motor deficits.
Heinrichs & Awad (1993) proposed that cluster analysis of cognitive test data may thus have promise in reducing and clarifying the heterogeneity of schizophrenia, and concluded that several patterns of neurocognitive dysfunction may underlie schizophrenia, thus contributing to the heterogeneity of the illness and its variable functional outcome.
Frith (1992) has also proposed a fascinating theoretical model, where he relates specific signs and symptoms to particular information processing abnormalities. For example, he proposes that the inability to generate spontaneous (willed) intentions can lead to poverty of action, perseveration and inappropriate action. In contrast, the inability to monitor the beliefs and intentions of others can lead to delusions of reference, paranoid delusions, certain kinds of incoherence and third-person hallucinations.
Is the cognitive impairment in schizophrenia caused by medication?
Most neuropsychological studies in schizophrenia have been conducted on medicated patients. It has become increasingly clear that neuroleptic medication and the anticholinergic drugs that are given to treat extrapyramidal syndromes can have marked cognitive-impairing effects in patients with schizophrenia. Do drug-free patients with schizophrenia demonstrate significant cognitive impairment? The answer is a definitive yes. Several studies have appeared over the past few years which have clearly indicated marked and severe cognitive impairment in patients who have been taken off their medication, or in patients who have never been prescribed any neuroleptic or anticholinergic medication (e.g. Reference Blanchard and NealeBlanchard & Neale, 1994; Reference McCreadie, Latha and TharaMcCreadie et al, 1997; Reference Saykin, Shtasel and GurSaykin et al, 1994). Although drugs may make a contribution, they do not account for the cognitive impairment that is observed in schizophrenia.
The newer atypical antipsychotics and cognitive function
Negative features and neuropsychological impairments can cause the greatest problems in terms of rehabilitation and are generally considered to be minimally responsive to conventional neuroleptics. The recent development of novel antipsychotics raised the hope that these may help alleviate both negative symptoms and neuropsychological deficits. However, Hawkins et al (1999) state:
“Despite some reports of positive findings, the grounds for thinking that the novel antipsychotics will exert direct and significant effects on neurocognition nevertheless remain inferential, and infirmly so” (p. 6).
To date, only a few well-controlled studies of the effects of novel antipsychotics (e.g. clozapine, risperidone and olanzapine) on cognitive functioning have been undertaken. The newer drugs are thought to have a primary mode of action that consists of a combination of serotonergic and dopaminergic blockade. It has been proposed that it is the serotonergic antagonism that may particularly benefit cognition (Reference SharmaSharma, 1999). Breier (1999) also speculates that cognitive enhancement with atypical antipsychotics may represent a consequence of N-methyl-D-aspartate antagonism. In relation to neuropsychological outcome, the best-studied agent is clozapine and the results have been mixed. As stated previously, Goldberg et al (1993) reported clinical improvement despite no improvement in neuropsychological status, whereas other studies have reported cognitive enhancement. Information is also emerging for other atypicals. For example, Green et al (1997) reported that risperidone improved verbal working memory, and this effect was maintained after controlling for changes in symptoms. As Hawkins et al (1999) warn us, however, we still do not have convincing evidence that newly developed antipsychotic drugs have a lasting beneficial impact on cognitive status that, in turn, leads to an improvement in everyday functioning. This is not to say that the newer atypicals do not have such an effect, rather that the evidence has not yet been obtained. Sharma (1999) also cautions that it is possible that some of the positive effects reported on cognitive functioning may be a result of wash-out of previous drugs that were impairing cognition, rather than of a true beneficial effect of the atypical neuroleptic. He also recommends that in order to evaluate the effects of drugs on cognitive functioning, the duration of treatment should preferably last one year. A further problem with interpreting this literature is that positive effects on a specific neuropsychological test may be presented but, in fact, may actually represent the one positive finding out of a multitude of neuropsychological comparisons that were conducted. Finally, there is the well-recognised tendency for researchers not to submit for publication studies that produced negative findings, coupled with the reluctance of journals to publish such studies.
Cognitive rehabilitation
Given the increasing recognition of cognitive impairment in schizophrenia, together with the general failure of conventional drug treatment to treat this impairment, several recent studies have evaluated the potential for cognitive rehabilitation in schizophrenia. Early studies in this area focused primarily on training patients in specific neuropsychological tasks, for example, providing coaching or monetary performance incentives for performing the Wisconsin Card Sorting Test. However, improving cognitive tests with practice or money is unlikely to have a significant impact on more general day-to-day activities. In order for cognitive rehabilitation in schizophrenia to prove itself, large-scale properly controlled trials are necessary, focusing on: (a) adequacy of the control condition that matches for the therapist's time, contact and enthusiasm; and (b) demonstration of cognitive gains that extend beyond the training sessions and materials. Adopting this degree of methodological rigour can lead to sobering results. For example, Field & Galletly (1997) recently reported a controlled trial using computer-aided cognitive rehabilitation in attempting to remedy the attentional deficit in schizophrenia. The treatment resulted in significant improvement on a letter cancellation task. However, the control condition (playing computer games) led to a similar improvement, suggesting that the improvement was the result of a non-specific practice effect.
One approach to cognitive rehabilitation in schizophrenia is to try to: (a) focus on subgroups of patients who have specific cognitive abnormalities; and (b) be guided by the rehabilitation literature from brain-damaged patients. O'Carroll et al (1999) recently reported a study where they selected a group of patients with schizophrenia who had significant memory impairment. Drawing on the literature from patients who suffer from the classic amnesic syndrome, they evaluated the potential benefits of an ‘errorless learning’ approach (see Box 2). Put simply, patients with the classic amnesic syndrome are thought to have a preserved implicit memory system in the presence of a devastatingly impaired explicit memory system (explicit memory refers to memories of a specific episode, e.g. where one was yesterday, whereas implicit memory refers to memory without conscious awareness). During learning, it is proposed that amnesic patients implicitly remember the mistakes they made during learning. Lacking a functional explicit memory system, when tested after a delay, they cannot distinguish between errors made during learning and correct responses. It has been shown that such amnesic patients benefit from an errorless approach to learning, that is, they learn better when they are prevented from making mistakes during learning (Reference Baddeley and WilsonBaddeley & Wilson, 1994). Adopting this approach, O'Carroll et al (1999) showed that memory-impaired patients with schizophrenia benefited significantly from a learning approach where they were not allowed to make any mistakes during learning. However, this study was restricted to a single session, and further work is required in order to test whether errorless learning approaches in schizophrenia will have any lasting benefit beyond the laboratory.
Box 2. Errorless learning
Patients with memory problems appear to have a particular difficulty distinguishing between errors and correct responses made during learning
Training using errorless learning principles involves preventing patients from making any mistakes during learning
This is achieved by starting with very easy tasks, and very gradually increasing the difficulty level
Preliminary results suggest that memory-impaired patients with schizophrenia may benefit from this type of approach as part of a comprehensive package of cognitive rehabilitation
Wykes et al (1999) recently reported the initial outcome of an important cognitive remediation study. They conducted a randomised controlled trial of intensive cognitive remediation (targeting cognitive flexibility, working memory and planning) involving individual daily sessions for up to three months v. a control condition of intensive occupational therapy. The cognitive remediation also included procedural and errorless learning, targeted reinforcement and massed practice. Cognitive remediation significantly improved performance on selected tests of cognitive flexibility and memory relative to the control condition. Interestingly, those patients who were receiving atypical antipsychotic medication appeared to benefit most from cognitive remediation, suggesting that such a combination treatment may be optimal. It is noteworthy that improvement occurred on cognitive tasks that were dissimilar to those used in the remediation package, that is, generalisation was evident. In addition, improvements in cognitive flexibility were related to improvements in self-esteem. This is a promising finding, but further work is required to determine: (a) the specific effective ingredients; and (b) the long-term outcome. This was an extremely time-intensive individualised intervention, which is likely to prove expensive in clinical practice. However, if the results are robust and replicable, they may well be worth the investment of time and money. As Wykes et al (1999) concluded, costs of such treatment may well be offset by improvements in functioning and reduced dependence on more expensive (e.g. in-patient) services.
Multiple choice questions
-
1. Cognitive impairment in schizophrenia is:
-
a consistent with the neurodevelopmental theory of schizophrenia
-
b present in drug-naïve patients
-
c present in the majority of patients with schizophrenia
-
d clearly related to specific symptoms
-
e is only found in chronic elderly patients.
-
-
2. Studies of people before they developed schizophrenia generally show:
-
a significant impairment of intelligence
-
b cognitive deficits present by the age of eight years
-
c no obvious cognitive abnormalities
-
d that the cognitive impairment clearly causes schizophrenia
-
e a reliable cognitive marker for subsequent development of schizophrenia.
-
-
3. Measures of cognitive impairment in schizophrenia:
-
a show no relationship with functional outcome
-
b have been shown to be clearly related to brain dopamine levels
-
c always improve in tandem with symptomatic improvement
-
d are related to interpersonal problem-solving deficits
-
e are consistently and reliably improved with treatment with atypical neuroleptics.
-
-
4. Cognitive rehabilitation in schizophrenia:
-
a is always effective
-
b has been conclusively shown to produce lasting, generalised improvements that impact on day-to-day functioning
-
c has not been thoroughly evaluated in a sufficient number of well-controlled studies
-
d has been shown to produce some promising results
-
e requires further evaluation using appropriate control conditions and dependent measures that differ sufficiently from the training materials.
-
-
5. Patients with schizophrenia:
-
a may show no cognitive decline following illness onset
-
b often have marked impairment covering a variety of cognitive domains
-
c often have particular verbal memory impairment
-
d always have a characteristic neuropsycho- logical ‘signature’
-
e often show a pre-existing cognitive impairment which can be exacerbated with anticholinergic medication.
-
Table 1 Summary results of meta-analysis of 204 studies of cognitive impairment in schizophrenia (7420 patients with schizophrenia and 5865 controls; adapted from Heinrichs & Zakzanis, 1998, with permission)
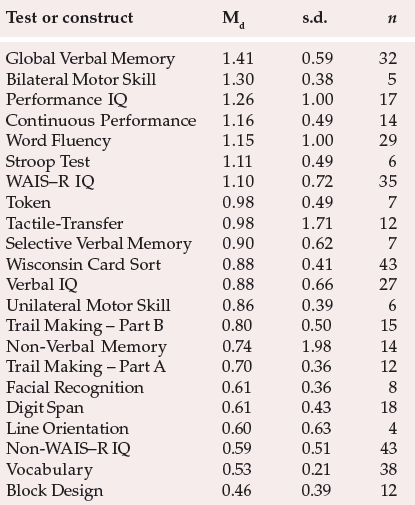
| Test or construct | Md | s.d. | n |
|---|---|---|---|
| Global Verbal Memory | 1.41 | 0.59 | 32 |
| Bilateral Motor Skill | 1.30 | 0.38 | 5 |
| Performance IQ | 1.26 | 1.00 | 17 |
| Continuous Performance | 1.16 | 0.49 | 14 |
| Word Fluency | 1.15 | 1.00 | 29 |
| Stroop Test | 1.11 | 0.49 | 6 |
| WAIS-R IQ | 1.10 | 0.72 | 35 |
| Token | 0.98 | 0.49 | 7 |
| Tactile-Transfer | 0.98 | 1.71 | 12 |
| Selective Verbal Memory | 0.90 | 0.62 | 7 |
| Wisconsin Card Sort | 0.88 | 0.41 | 43 |
| Verbal IQ | 0.88 | 0.66 | 27 |
| Unilateral Motor Skill | 0.86 | 0.39 | 6 |
| Trail Making - Part B | 0.80 | 0.50 | 15 |
| Non-Verbal Memory | 0.74 | 1.98 | 14 |
| Trail Making - Part A | 0.70 | 0.36 | 12 |
| Facial Recognition | 0.61 | 0.36 | 8 |
| Digit Span | 0.61 | 0.43 | 18 |
| Line Orientation | 0.60 | 0.63 | 4 |
| Non-WAIS-RIQ | 0.59 | 0.51 | 43 |
| Vocabulary | 0.53 | 0.21 | 38 |
| Block Design | 0.46 | 0.39 | 12 |
Table 2 Premorbid cognitive impairment in people assessed as children who subsequently developed schizophrenia (from Jones et al, 1994, with permission)
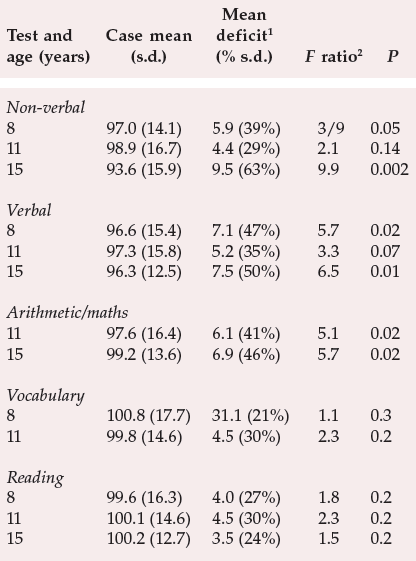
| Test and age (years) | Case mean (s.d.) | Mean deficit 1(% s.d.) | Fratio 2 | P |
|---|---|---|---|---|
| Non-verbal | ||||
| 8 | 97.0(14.1) | 5.9(39%) | 3/9 | 0.05 |
| 11 | 98.9(16.7) | 4.4(29%) | 2.1 | 0.14 |
| 15 | 93.6(15.9) | 9.5(63%) | 9.9 | 0.002 |
| Verbal | ||||
| 8 | 96.6(15.4) | 7.1(47%) | 5.7 | 0.02 |
| 11 | 97.3(15.8) | 5.2(35%) | 3.3 | 0.07 |
| 15 | 96.3(12.5) | 7.5(50%) | 6.5 | 0.01 |
| Arithmetic/maths | ||||
| 11 | 97.6(16.4) | 6.1(41%) | 5.1 | 0.02 |
| 15 | 99.2(13.6) | 6.9(46%) | 5.7 | 0.02 |
| Vocabulary | ||||
| 8 | 100.8(17.7) | 31.1(21%) | 1.1 | 0.3 |
| 11 | 99.8(14.6) | 4.5(30%) | 2.3 | 0.2 |
| Reading | ||||
| 8 | 99.6(16.3) | 4.0(27%) | 1.8 | 0.2 |
| 11 | 100.1(14.6) | 4.5(30%) | 2.3 | 0.2 |
| 15 | 100.2(12.7) | 3.5(24%) | 1.5 | 0.2 |
MCQ answers
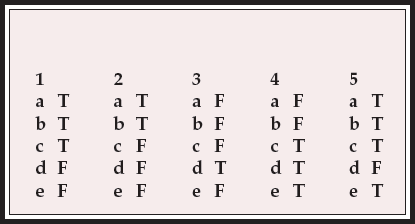
| 1 | 2 | 3 | 4 | 5 | |||||
|---|---|---|---|---|---|---|---|---|---|
| a | T | a | T | a | F | a | F | a | T |
| b | T | b | T | b | F | b | F | b | T |
| c | T | c | F | c | F | c | T | c | T |
| d | F | d | F | d | T | d | T | d | F |
| e | F | e | F | e | F | e | T | e | T |





eLetters
No eLetters have been published for this article.