Disruption of the Guillain-Mollaret triangle may result in hypertrophic olivary degeneration (HOD), resulting in palatal myoclonus, dysmetria, oscillopsia, dysarthria, and ataxia. Damage typically occurs within the dentate nucleus, dentatorubral tract, or rubro-olivary tract within the central tegmental tract,Reference Gautier and Blackwood 1 which is thought to result in deafferentation of the inferior olivary nucleus via trans-synaptic degeneration.Reference Jellinger 2 Radiologically, a T2 hyperintense and hypertrophied inferior olivary nucleus is seen. Hypertrophy generally occurs 5-15 months after the pontine insult, while hyperintensity may occur as soon as 3 weeks post-insult.Reference Kitajima, Korogi, Shimomura, Sakamoto, Hirai and Miyayama 3
Here, we describe a case of HOD that presented acutely as a stroke mimic 14 months after a pontine cavernous malformation resection. Hypertrophic olivary degeneration may result from various pontine lesions,Reference Jellinger 2 such as following the resection of a pontine cavernous malformation,Reference Hornyak, Osborn and Couldwell 4 – Reference Yun, Ahn, Park, Kwon, Kwun and Kim 6 which in one case resulted in a delayed, although not as acutely symptomatic, presentation of HOD.Reference Hornyak, Osborn and Couldwell 4 We present here the only known acute presentation of HOD following pontine cavernous malformation resection. We suggest that an acute presentation of HOD should be a rare but recognized complication of such resections to avoid unnecessary investigations, treatments, or procedures.
A 58-year-old male was admitted to the neurology service 5 days after the acute onset of disequilibrium and gait disturbance. He had undergone a left pontine cavernous malformation resection via left temporal craniotomy 14 months earlier. The surgery resulted in persistent dysarthria, right-sided sensory impairment, and the requirement of a four-wheeled walker to ambulate. His deficits were stable until 5 days before his presentation, when he awoke and discovered that he was unable to ambulate with his walker and had a constant sensation of disequilibrium. He also noted a deterioration of his right-hand coordination, exemplified by being unable to pour water from a jug without spilling. He denied visual changes, diplopia, new dysarthria, dysphagia, vertigo, tinnitus, hearing loss, weakness, or new sensory impairment. There was no nausea, vomiting, or headache.
Neurologic examination was significant for a partial left abducens nerve palsy, bilateral gaze-evoked nystagmus, and upbeat nystagmus with upgaze. Saccades were hypometric to the left and hypermetric to the right. Facial sensation was decreased in V1, V2, and V3 of the left trigeminal nerve, but unchanged compared to post-cavernous malformation resection. There was no palatal myoclonus. Speech was dysarthric, but unchanged. There was a spastic catch in the left upper limb, but otherwise motor examination was normal. A rubral tremor was present in the right upper limb. There was 3+ hyperreflexia in the right brachioradialis, right biceps, and bilateral patellar reflexes. Plantar responses were downgoing. Vibration sense was decreased in the right hemibody. There was bilateral dysmetria on finger-nose and heel-shin testing that was more pronounced on the right. Dysdiadochokinesis was present in the right upper limb. Gait was assessed with assistance and was wide-based with irregularly spaced steps and marked ataxia of the right leg. He was unable to perform tandem gait.
Owing to the acute onset of symptoms, computed tomography/computed tomography angiography of the head and neck was performed to rule out a brainstem stroke. The findings showed the earlier resection bed, but no acute hemorrhage or vascular occlusion. An enhanced MRI of the brain demonstrated a hypertrophied T2 hyperintense (Figure 1D) left inferior olive with no diffusion restriction (Figures 1E and 1F). A small area of T1 hyperintensity near the prior resection site suggested subacute microhemorrhage (Figure 1B).
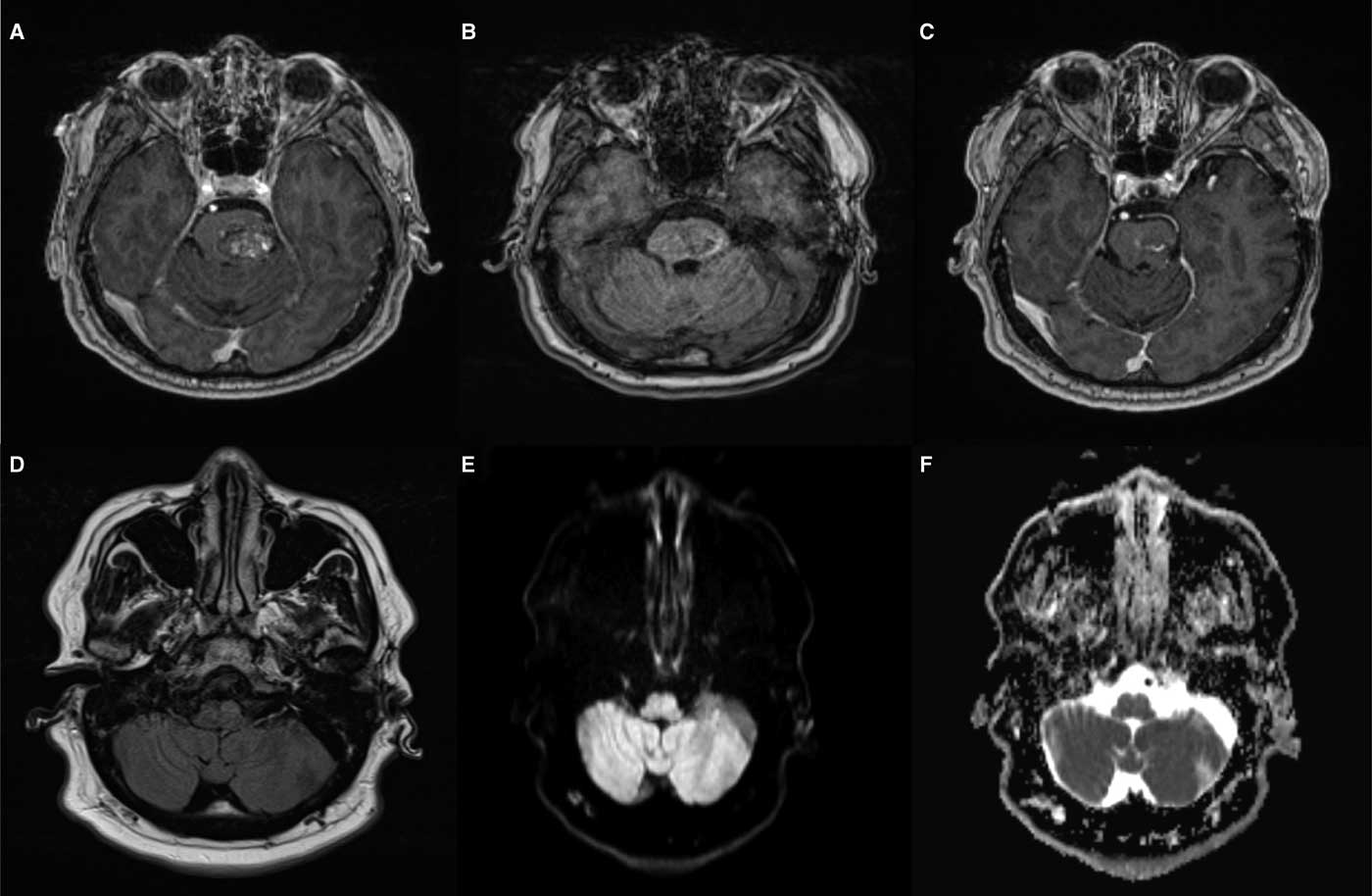
Figure 1 Hypertrophic olivary degeneration. (A) Pre-surgical T1 post-gadolinium imaging shows a sharply demarcated heterogeneously enhancing lesion within the left hemipons consistent with a cavernous malformation. (B) T1 at the time of presentation demonstrates subtle hyperintensity along the inferolateral margin of the resection cavity consistent with subacute microhemorrhage. (C) T1 post-gadolinium at the time of presentation shows enhancing residual lesion. (D) T2 fluid attenuated inversion recovery shows hyperintensity and hypertrophy of the left inferior olivary nucleus. (E) Diffusion weighted imaging demonstrates similar subtle hyperintensity. (F) Apparent diffusion coefficient correlation also shows hyperintensity in keeping with T2 shine-through rather than diffusion restriction.
The MRI findings were specific for hypertrophic degeneration of the left inferior olive. Hypertrophy with degeneration is unique to the inferior olivary nucleus. Therefore, non-enhancing, T1 isointense, T2 hyperintense hypertrophy of the lateral medulla in the region of the inferior olivary nucleus leaves essentially only the diagnosis of HOD.
The finding of HOD on MRI explains our patient’s new right-sided dysmetria, ataxia, rubral tremor, and gait disturbance.Reference Lee, Choi and Son 7 Signs and symptoms typically manifest contralaterally to the lesion. Signs contralateral to the affected olive include dysmetria and limb dyscoordination. Other reported signs and symptoms include paresthesias, hypoesthesia, ataxia, dysarthria, diplopia, hemiparesis, and lower limb spasticity.Reference Hornyak, Osborn and Couldwell 4 – Reference Yun, Ahn, Park, Kwon, Kwun and Kim 6 Many of these findings are likely a product of the inciting pontine lesion. Damage to the central tegmental tract and the encapsulated rubro-olivary tract results in ipsilateral HOD and contralateral symptoms.Reference Jellinger 2 Palatal myoclonus, which was not present in our patient, is found in 7-33% of cases of HOD.Reference Gautier and Blackwood 1 , Reference Jellinger 2
In our patient, the previous cavernous malformation resection likely led to partial damage of the central tegmental tract. The acute onset of symptoms in our patient may have been the result of reaching a critical threshold of trans-synaptic degeneration. Alternatively, a microhemorrhage may have damaged the remaining few axons in the rubro-olivary tract that had been maintaining a functionally complete circuit.
Our patient was discharged from the acute neurology service for intensive inpatient rehabilitation. It is hoped that he will improve with time and physiotherapy, as has been reported in other cases.Reference Bouz, Woods and Woods 8
In summary, we present an acute clinical presentation associated with HOD from prior pontine cavernous malformation resection, with microhemorrhage as a possible explanation for the rapid onset of symptoms. Hypertrophic olivary degeneration is a complication that should be considered in patients presenting with acute symptomatology who have previously undergone brainstem cavernous malformation resection. Symptoms typically manifest contralaterally to the lesion, with variable rates of palatal myoclonus. Radiologic traits follow a well-defined time course, and are fairly specific should lateral medullary non-enhancing T2 hyperintense hypertrophy be seen at the level of the inferior olivary nucleus.
DISCLOSURES
Calvin Howard, Alexander Arnold, and Tyson Brust have nothing to disclose.
STATEMENT OF AUTHORSHIP
CH drafted the initial manuscript and assisted with figure creation. AA provided editing and critical revisions to the intellectual content as well as assisted with figure creation. TB provided editing and critical revisions to the intellectual content.



