Introduction
According to the latest report by the World Health Organization [1], an estimated 10.4 million people developed tuberculosis (TB) in 2015, with about 17.3% TB-related deaths in that year. In 2015, there were about 480 000 new cases of multidrug-resistant TB cases, turning the fight against the disease highly problematic. Success in the efforts to fight the disease is evident in the continued drop in TB incidence at an average rate of about 1.5% per year since 2000; however, efforts should persist with the aim of achieving the targeted 4–5% annual decline by 2020. TB, caused by members of the Mycobacterium (M.) tuberculosis complex (MTC), which comprises a small number of very closely related species of the genus Mycobacterium, occurs in a wide range of mammalian species [Reference Lécu and Ball2], including humans.
Elephants are one such species and have been known for a long time to be susceptible to the disease [Reference Evans3–Reference Montali, Schwammer, Foose, Fouraker and Olson6]. Based on its prevalence, the disease seems to primarily infect Asian elephants. According to one report, 46 Asian elephants were diagnosed with M. tuberculosis in North America between 1994 and 2010. During the same period, the disease was diagnosed in only three African elephants. In addition, one African elephant was diagnosed with M. bovis [Reference Mikota and Maslow7]. Based on these figures, the prevalence of TB among Asian elephants in North America was 18%, while it was only 2% in African elephants [Reference Mikota and Maslow7]. Prevalence in range countries of Asian elephants seems to be high too, reaching, according to estimates, 15.0–15.9% in India [Reference Abraham, Cheeran, Mikota and Kirk-Baer8, Reference Verma-Kumar9], 13–22% in Nepal [Reference Fagen, Acharya and Kaufman10, Reference Mikota and Kirk-Baer11], 20.4–23.3% in peninsular Malaysia in captive Asian elephants and 24.8% among their handlers [Reference Ong12, Reference Yakubu13], and as much as 36% in Lao PDR [Reference Lassausaie14]. In Asia, M. tuberculosis, thus far, has been reported only in captive Asian elephants except one case of a wild Asian elephant that was previously in close human contact [Reference Obanda15]. In Africa, free-ranging elephants at the Kruger National Park exposed to excessively M. bovis-infected lion and buffalo populations (up to 40%) never developed a manifestation of this endemic disease [Reference De Vos16–Reference Renwick, White and Bengis18].
The vast majority of clinically relevant TB cases in elephants were caused by M. tuberculosis. Other members of the MTC, e.g. M. bovis, may also cause clinical infection and death [Reference Lyashchenko19]. Non-tuberculous mycobacteria (NTM) including M. avium [Reference Yong20], M. szulgai [Reference Lacasse21] and M. elephantis [Reference Shojaei22] may, under certain conditions, also cause severe clinical infection and death. Infection transmission from elephants to humans was reported or suspected [Reference Holt23–Reference Zlot29] and so was possible infection from humans to elephants [Reference Obanda15, Reference Angkawanish30]. Who transmits to whom, however, might, at times, be difficult to discern as in elephants often no clinical symptoms are observed for many years. As some of the working elephants in Southeast Asia have frequent contact with the wild population, it is highly plausible that the disease has been transmitted and is prevalent among wild Asian elephants as well, as a recent report from Sri Lanka suggests [Reference Perera, Szentiks and Schumann31]. This high prevalence in South East Asia may be the result of the mingling of elephants, domestic livestock and humans in this region, a situation vastly different from that in Africa.
Despite its potential to infect humans and its high prevalence in elephants, diagnosis and treatment of the disease in these species are still unsatisfactory. Following several TB cases in elephants during the mid-1990s, TB working groups were set up in North America and Europe, respectively, to formulate diagnostic, therapeutic and monitoring guidelines [32, 33]. Elephant taxon advisory groups in North America and Europe now recommend annual testing of all elephants older than 8 years by trunk wash or tracheal wash, followed by bacterial culture and PCR. How to best perform a tracheal wash in elephants, however, has not been described to date.
The basis of TB diagnosis is the ‘triple sample’ trunk wash, to be performed annually in all captive elephants on three different days, preferably within a 1-week period. In case of death or euthanasia, post-mortem examination is mandatory. Other possible diagnostic tests such as sero-diagnostic assays (multiantigen print immunoassay, ElephantTB STAT-PAK or Dual Path Platform (DPP) VetTB) [Reference Lyashchenko19, Reference Greenwald34, Reference Vogelnest35], interferon γ assay [Reference Angkawanish36], intradermal tuberculin test [Reference Sternberg Lewerin37], multiple antigen ELISA [Reference Lyashchenko19] or Ziehl–Neelsen smears staining to detect microscopically acid-fast bacteria are, at present, not recommended as sole diagnostic test or not at all due to low sensitivity and specificity [Reference Mikota, Larsen and Montali5].
Being the current standard diagnostic test in live elephants and the only officially approved diagnostic method, trunk wash [Reference Isaza, Ketz and Hofmann38] is often performed but its sensitivity is very low. In one report [Reference Angkawanish30], mycobacteria-positive cultures were found in only two out of 60 trunk wash procedures performed in four elephants, three of which were later confirmed M. tuberculosis-infected at necropsy. In another study on infected elephants in Sweden, only seven of 189 trunk wash samples (63 triple trunk washes) detected M. tuberculosis even though all five tested elephants were infected [Reference Moller39]. In yet another study on the prevalence of TB in peninsular Malaysia, of 151 trunk wash samples cultured none was positive [Reference Ong12]. In the same study, there were 10 seropositive elephants and three of these were also positive in TB PCR. Trunk wash has many additional serious limitations [Reference Lyashchenko19, Reference Mikota40]. Training animals to perform trunk wash is an extended process and not all elephants can be trained [Reference Fagen, Acharya and Kaufman10]. Furthermore, animals shed the bacteria only periodically and may happen not to shed on the 3 days of sampling. Even if they do shed, bacteria may not reach the distal trunk compartment, over 1.5 m away from the lungs. Duration of bacterial culture of 8–12 weeks is very long, leaving the animal free to infect others in the meantime. Furthermore, housing and animal management do not allow for quarantine measures in most elephant facilities. In addition, the trunk is constantly exposed to environmental contaminants and consequently cultures may be affected by overgrowth of other bacteria or fungi that inhibit the growth of M. tuberculosis. Laboratories do not always report such overgrowth so the rate of these occurrences is not known. Trunk wash also cannot identify animals infected in body systems other than the respiratory system (e.g. in the semen or uterus [Reference Lyashchenko19, Reference Kik, Szentiks and Schumann24, Reference Holund41, Reference Ladehoff42]), or infected animals that have confined tubercles that do not shed bacteria. Finally, and not least important, trunk wash puts the operator at risk of being infected since elephants forcefully exhale the injected fluids out of their trunk, spraying small droplets on those surrounding them.
At present no alternative diagnostic method is available. Elephants normally do not show clinical signs, so relying on these would be highly insensitive [Reference Mikota43]. The intradermal tuberculin test, extensively used in cattle, has very low sensitivity and specificity in elephants [Reference Mikota40]. Gastric or lung lavage, a testing technique extensively used in humans [Reference Kalawat44] as well as in other wildlife species has not been used in elephants thus far due to technical and anatomical limitations. Serological testing has seen vast advances in recent years but huge differences between tests [Reference Mikota and Kirk-Baer11] and the fact that animals may test positive after treatment make reliance on these as the method of choice problematic. Best example is the previously, widely used STAT PAK serological test. Due to high rate of false-positive results, the test is no longer available. This is despite reported sensitivity of 100% and specificity of 95% [Reference Lyashchenko19], the advantage of identifying infection years before TB is diagnosed by bacterial culture from trunk wash, and the possibility to identify decline in antigen reactivity following treatment [Reference Lyashchenko19, Reference Moller39]. The DPP VetTB, a new specific serological assay for M. tuberculosis in elephants, or non-validated ELISA's detecting selected antibodies against M. tuberculosis are presently the only recommended diagnostic tools in addition to trunk wash [32, 33]. Yet these new sero-diagnostic tools are still subject of debates because of the high number of – probably – false-positive results [32].
The present study aimed at developing a more effective, direct technique for TB testing in elephants as an alternative to trunk wash the unreliable standard for TB diagnosis. With the lungs being the primarily infected and most common excretory organ, animals shedding the bacteria can thus be detected at higher rates of probability using the bronchoalveolar lavage. In addition, aspiration of lung fluids deep from the respiratory tract into a closed collection system reduces the risk of environmental contamination of the sample, bacterial culture overgrowth and potential exposure of staff to pathogenic mycobacteria from a TB-infected animal.
Materials and methods
Animals
The procedure was performed on 14 elephants – eight African (Loxodonta Africana) and six Asian (Elephans maximus), in free contact but mostly protected management system. A restraint chute provided easier access to the animal but was not a mandatory prerequisite for the procedure (Table 1). Eight of the elephants were females and six were males and their age ranged between 9 and 42 years.
Table 1. Bacterial culture, MTC and mycobacterial genus-specific PCR results from bronchoalveolar, trunk and mouth fluids collected from elephants
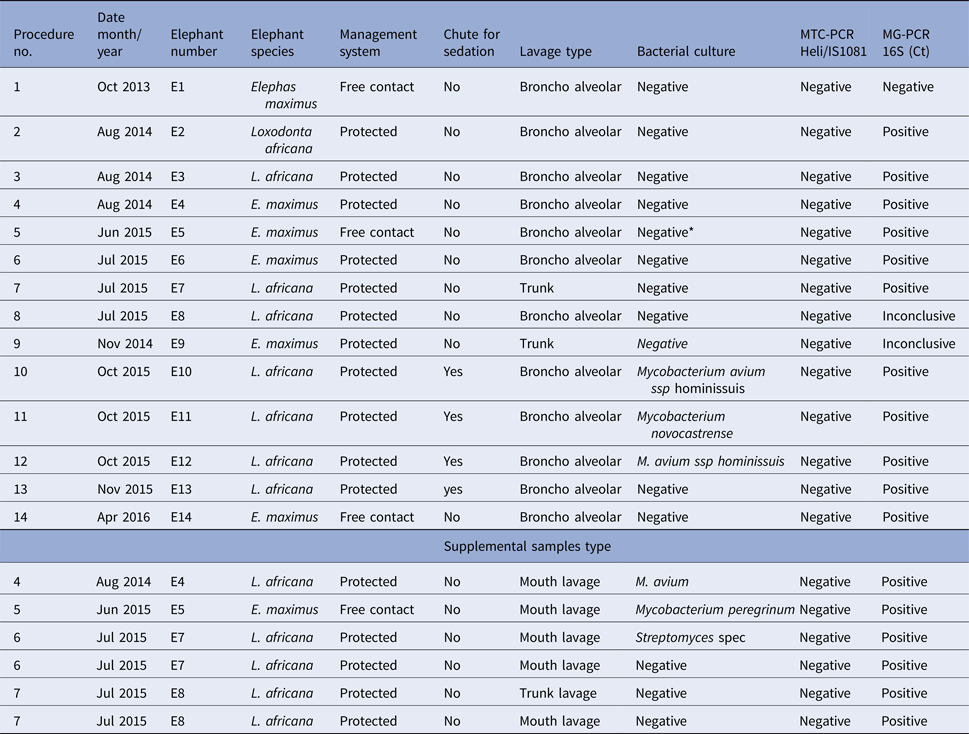
* Culture contaminated with mould. Culture untimely terminated but negative at that time!
Sedation
To allow introduction of the endoscope through the trunk and the larynx and into the trachea, all elephants were subjected to standing sedation [Reference Neiffer45, Goeritz, unpublished data] In brief, sedation was administered by intramuscular injection of 80 mg detomidine hydrochloride (Domidine® 10 mg/ml, Eurovet Animal Health B.V., Bladel, The Netherlands) and 80 mg butorphanol tartrate (Torbugesic® Vet 10 mg/ml, Zoetis B.V., Capelle a/d IJssel, The Netherlands). Adequate sedation for the procedure was achieved after approximately 20 min. When required, sedation was maintained by supplemental injections of 5 mg of each drug into the ear vein. Once sedation became effective, local anaesthesia was administered to the nerve rami at the base of the trunk [Reference Boas and Paulli46], leading to nerve block and relaxation of the area. For this, 10 ml of 2% lidocaine hydrochloride (bela-pharm GmbH, Vechta, Germany) were administered intramuscularly in the vicinity of each ramus (Fig. 1a, b).
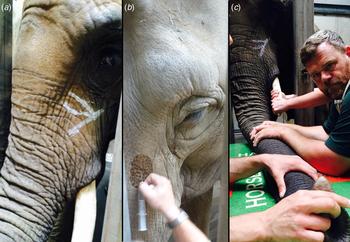
Fig. 1. Nerve block for the trunk. (a) Chalk drawings of the approximate location of the nerve rami on one side at the base of the elephant's trunk. (b) Injection of local anaesthetics at these locations results in local anaesthesia of the trunk base. (c) Placement of the relaxed trunk on a waist height flat surface after introduction of sedation and local nerve block.
Sedation was reversed by intravenous administration of 250 mg naltrexone hydrochloride (Trexonil™, Wildlife Pharmaceuticals (PTY) Ltd., White River, South Africa) and 100 mg atipamezole hydrochloride (Atipam 5 mg/ml, Eurovet Animal Health B.V.) resulting in fast and uneventful recovery.
Bronchoalveolar lavage
Following sedation and local nerve block, the trunk was placed horizontally at about waist height (Fig. 1c). A flexible 3.5 m-long customised video chip endoscope (ESO Jürgen Ohle Endoskopie Technik und Chirurgiemechanik, Wedel, Germany) connected to a mobile video processor and monitor (ESO Jürgen Ohle Endoskopie Technik und Chirurgiemechanik) was introduced into one of the trunk's openings. The endoscope was first guided over the sigmoid stricture caused by the cartilage plate at the base of the trunk (Fig. 2, see also [Reference Boas and Paulli46]). It was then further fed forward through the larynx and into the trachea (Fig. 3). For the lavage, a 6 m long, 3.5 mm wide, sterile disposable catheter (Gynetics Medical Products N.V., Lommel, Belgium) was introduced into the endoscope's working channel and advanced into different bronchi. To facilitate easier recovery of instilled fluids from a larger surface, the lavage catheter had six ellipsoid openings positioned in two parallel rows at the sides of the catheter's end. With this specific design, the tip of the catheter remains round, smooth and closed, while its side ellipsoid openings allow suction of fluids from a larger surface area. The lavage catheter was connected to a sterile, closed suction system (Fig. 4). One hundred to 150 ml sterile 0.9% NaCl (saline) solution was injected into the bronchi at different depths. Using a suction pump (DC15 Endo, Asskea Medical, Greussen, Germany), a mixture of injected saline solution and turbid lung fluids was aspirated into a sterile 50 ml falcon tube sealed with a silicon plug. This procedure was repeated 2–3 times after repositioning the catheter in different bronchi. After collection of bronchoalveolar fluids, the endoscope and catheter were retracted. Upon retraction further fluids were aspirated from the trunk and mouth. Aspirated lung fluids were sealed in sterile zip-lock bags for additional protection and shipped within 48 h at 4 °C, without any further handling, to the TB reference laboratory for testing.

Fig. 2. Placement of the endoscope. A sagittal sliced skull of an elephant is used here to show the location of the endoscope before it is advanced further into the trachea.

Fig. 3. Endoscopic image of the epiglottis and rima vocalis in the elephant with the suction catheter running into the trachea.
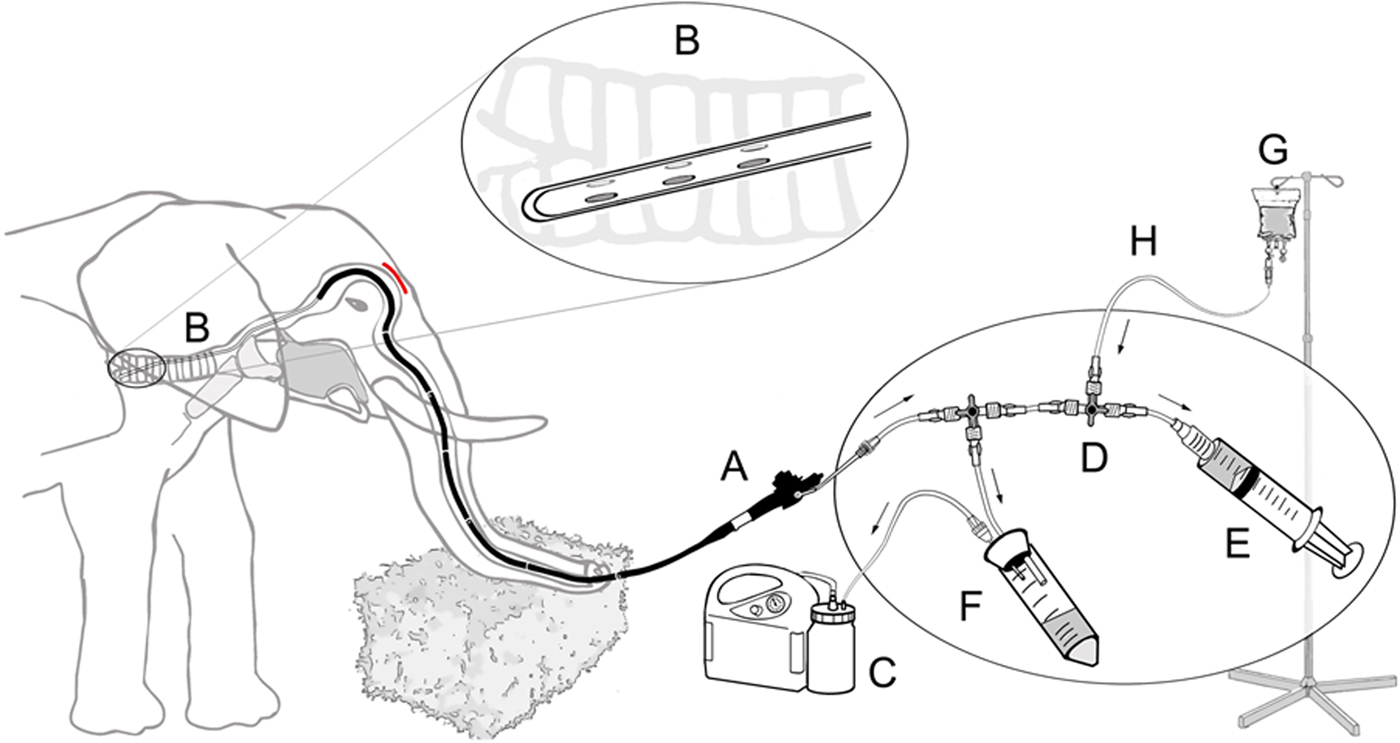
Fig. 4. Schematic diagram of the closed suction system for bronchoalveolar lavage in elephants including endoscope (A), catheter (B) with its specially designed tip (magnified in circle), vacuum pump (C), three way directional control valve (D), syringe filled with sterile 50 ml saline (E), sterile tube serving as collection container (F) and physiological saline solution (G) with connecting silicon tubing (H).
Sample processing and bacterial culture
All samples were tested for the presence of mycobacteria by bacterial culture and MTC-specific real-time PCR. When mycobacteria were found or suspected, further tests were conducted to identify the species and subspecies. Briefly, liquid samples collected from the elephants’ respiratory system were centrifuged at 3800 g for 20 min at 10 °C. The sediments were re-suspended with equal volumes (10 ml) of phosphate-buffered saline (PBS) and N-acetyl-L-cysteine-NaOH (final NaOH concentration: 1%) and incubated at room temperature with gentle shaking for 25 min. After adding 20 ml of PBS to neutralise the suspension, it was mixed thoroughly and centrifuged at 3800 g for 20 min. The sediment was washed again with PBS. The final sediment (if not visible: the suspected sediment) was re-suspended with 1.5 ml PBS, wherefrom 500 µl were removed for direct DNA extraction. Antibiotics mixture (100 µl) containing polymyxin B, amphotericin B, nalidixic acid, trimethoprim, and azlocillin (Becton Dickinson, Heidelberg, Germany) was added to the remaining 1.0 ml of suspension. Two solid growth media (i) Stonebrink medium with pyruvate and antibiotics mixture containing polymyxin B, amphotericin B, carbenicillin and trimethoprim (PACT) (Bioservice Waldenburg, Waldenburg, Germany), and (ii) Löwenstein–Jensen medium with glycerol and PACT (Artelt-Enclit, Borna, Germany) were then inoculated with 150 µl of the suspension. Incubation was performed at 37 °C for at least 12 weeks.
Molecular detection of DNA
For extraction of DNA directly from wash samples, the separated 500 µl portions (see above) were centrifuged at 13 000 g for 10 min and the sediments were processed using DNeasy Blood and Tissue kit (Qiagen, Hilden, Germany) following the manufacturer's instructions. DNA was then subjected to a MTC-specific qPCR performed as duplex qPCR, targeting insertion sequence (IS) 1081 and a hypothetical helicase (HELI) [Reference Toussaint47] and qPCR targeting the 16S rRNA gene to detect all members of the genus Mycobacterium[48]. The following sequences for primers and probe were designed using Geneious 5.6.2 (Biomatters, New Zealand): forward primer Mycobac16S_F TGC GGG CGA TAC GGG CAG RCT, reversed primer Mycobac16S_R CCA CAC CTA GTW CCC ACC GTT TAC, probe Mycobac16S_S FAM-TGG CGA AGG CGG GTC TCT GGG CAG TA-BHQ1. Co-amplifying a fragment of the β-actin DNA was used as internal control for the mycobacteria-specific assays [Reference Toussaint47, 48]. The forward primer sequence was AGC GCA AGT ACT CCG TGT G, the reversed primer was CGG ACT CAT CGT ACT CCT GCT T, probe was YakimaYellow-TCG CTG TCC ACC TTC CAG CAG ATG T-BHQ1.
For the qPCR targeting the 16s rRNA gene, a 25 µl reaction mixture composed of TaqMan Gene Expression Master Mix 12.5 µl, forward primer Mycobac16S_F, 50 nM, reversed primer Mycobac16S_R, 300 nM, probe Mycobac16S_S, 200 nM, RNase-free water 3.75 µl, primer-probe mix Actin 2 µl (primer each 200 nmM, probe 100 nM) and DNA extract was added at a volume of 5 µl. Thermal cycling was set as follows: 50 °C for 2 min, 15 min at 95 °C, 45 cycles of 95 °C for 15 s, 60 °C for 60 s and 72 °C for 30 s. Signals were measured in the FAM and HEX channels during the annealing phase. PCRs were performed using the Applied Biosystems 7500 Real-Time PCR System (Applied Biosystems, Darmstadt, Germany). With respect to the susceptibility of sample contamination, the cut-off threshold cycle Cq value was set at 36.
Molecular identification of bacterial isolates
From bacterial isolates, DNA was extracted by suspending colony material in 100 µl sterile water, heat-inactivating the bacteria at 80 °C for 20 min, ultra-sonicating (35 kHz) and boiling the suspension for 10 min each and then centrifuging at 13 000 g for 5 min. The supernatant was harvested and centrifuged again. The final supernatant containing DNA extract was then subjected to conventional PCRs for identification of the genus Mycobacterium [Reference Kirschner, Böttger, Parish and Stoker49], IS 1245 and IS 901 for identification of M. avium [Reference Guerrero50, Reference Kunze, Portaels and McFadden51], and a HELI [Reference Moser52, Reference Rodriguez53] for identification of members of the MTC. Mycobacterial species not identifiable by these PCRs were identified by DNA sequence analysis of the 16S rRNA gene (GATC, Konstanz, Germany).
Ethics statement
This study was conducted on captive elephants. The study was carried out in strict accordance with the German National Protection of Animals Act from 24 July 1972 and its last revision from 15 July 2009. Under this Act, an examination directed towards diagnosing an animal's disease is not defined as an animal experiment (§7) but as a mandatory act of animal welfare. Although not mandated by law, the study on bronchoalveolar lavage for diagnosis of TB infection in elephants was also approved by IACUC animal ethics committee of the Leibniz Institute for Zoo and Wildlife Research (Permit number: 2013-09-01).
Results
Bronchoalveolar fluids were collected in 86% of the elephants (n = 12/14). Two elephants awoke from the sedation during endoscopy. The procedure was aborted prematurely in these two animals before completion of bronchoalveolar lavage yet trunk fluids were still obtained. On average, about 78% of the administered saline volume was recovered on each lavage. The collected fluids were turbid and rich in mucous from the respiratory system. In addition to bronchoalveolar fluids, additional lavage samples were also harvested from the trunk and mouth. Samples from all animals were investigated by bacterial culture and MTC-specific real time qPCR (n = 19) for the presence of mycobacteria and mycobacterial DNA, respectively. Neither bacteria of the MTC nor their DNA was identified in any of the elephants. Yet, one bacterial culture had mold overgrowth and culture was untimely terminated (1/20). At the time of culture termination, the sample was MTC negative but positive for NTM (Table 1).
Despite these negative results for MTC, 25% (5/20) of the bacterial cultures from five elephants showed growth of NTM or closely related bacterial species. Isolates of M. avium, M. peregrinum, and M. novocastrense, three NTM species, were detected in samples from the lung or mouth. One isolate of Streptomyces spec., a closely related bacterial genus, was identified in the mouth of one of those four elephants.
Even more surprising, DNA of NTM or closely related bacterial species was detected in 85% of the samples (17/20) from all elephants (Table 1).
Thanks to the endoscope, the procedure also allowed visualisation of the mucosa lining of the lower and upper respiratory system (Fig. 3). It appeared normal and healthy in all but two elephants. In one elephant, the trunk mucosa was slightly oedematous and in the other it contained many ulcers partially covered with puss. Yet, the bacterial species identified by culture was M. avium ssp. hominissuis.
Discussion
Elephants are known to be susceptible to bacteria of the MTC and may be infected also by other Mycobacterium species. As such they potentially pose a public health concern. Because of their close association with zoo personnel, the public and animals in nearby enclosures, the infection circle can easily expand [Reference Michalak25–Reference Stephens27, Reference Oh54]. With their long trunk and their ability to spray large volumes far and wide, one can say that elephants were specifically ‘built’ to transmit infections of the respiratory tract. To prevent this from happening, elephants, and staff members frequently associated with them, should be tested regularly for TB [32]. Like in many wild animals, elephants normally do not show discernible signs of disease till late stages [Reference Maslow and Mikota55]. At such point, they may have already infected their enclosure mates and/or handlers. Treatment at this stage may also be problematic, especially for bacteria such as mycobacteria that are known to stay intracellularly and get encapsulated by the host's immune reaction inside well-defined granulomas inaccessible to antibiotics.
Despite decades of research, the only trusted and proven diagnostic tool used today in elephants is the same one used by Robert Koch more than a century ago – bacterial culture [Reference Cambau and Drancourt56]. Culturing mycobacteria, however, takes 12 weeks or more and is prone to interference by contaminants in the sample. Furthermore, trunk wash has very low sensitivity as was demonstrated in several reports in the past [Reference Angkawanish30, Reference Moller39]. Notwithstanding all these shortcomings, trunk wash is still the standard diagnostic tool for TB in elephants. Other diagnostic tools either cannot be performed in adult elephants (chest radiographs) or have poor correlation with bacterial culture (intra-dermal skin test). A number of serological tests are also available. Because elephants have a marked immune response to mycobacterial infection, these tests show high sensitivity and specificity [Reference Greenwald34]. Still, none of them has thus far gained the official diagnostic tool status like bacterial culture. These tests, however, are in use, at least for screening purposes. Noting the possibility of false-positive diagnosis and the consequential treatment or even euthanasia, one needs to be extremely cautious when using these yet-to-be-validated and approved tests. The currently practiced trunk wash procedure has several further disadvantages. First and foremost, it puts despite all work safety precautions the examiners at risk. Once the fluids have been injected into the trunk's lumen, the elephant forcefully sprays them out, along with the bacteria, into a container as well as on the people around it. The second disadvantage of the technique is that it relies on the excretion of the bacteria in large enough numbers from the respiratory tract into the far distant trunk. Even when shedding does occur, the level of trunk contamination varies from case to case and trunk wash may not always harvest sufficient numbers of the bacteria. If this happens, or if the elephant does not shed bacteria at the time, infected elephants may go undetected, risking the health of other elephants, vertebrates and humans (care givers, general public) in the facility; and finally, the third issue is that various bacteria and fungi can easily contaminate the trunk, including NTM collected from the soil or browse, leading at times to culture contamination or overgrowth. The bronchoalveolar lavage reported here is thought to counter many of the shortcomings characterising the current practice. The sterile and closed suction system does not rely on the elephant to expel possibly hazardous fluids, thus protecting the surrounding people and animals from risk of getting contaminated by an infected animal. By sedating the elephant and blocking the innervations at the base of the trunk, this is actively prevented from happening. Furthermore, by working in a closed suction system, we make sure that the examiners, elephant handlers and the surroundings remain unaffected by the procedure. In addition, the sample is protected from environmental contamination. Trunk–laryngeal–tracheal endoscopy allows visualisation of the mucosal lining of the respiratory system as far as the trachea for identification of local lesions, inflammation or tumours and, when indicated, local or systemic treatment. Furthermore, suspicious findings could be subject to endoscopy-guided biopsy.
qPCR of elephant lung lavage fluids in this study detected mycobacterial DNA in the majority of the samples examined. DNA of TB causing mycobacteria (MTC) was not found in any of them.
Since the mycobacterial genus-specific qPCR is a bit less than one order of magnitude less sensitive than the MTC qPCR used in this study, it can be argued that, in samples with positive genus-specific results, MTC DNA would have been detected if present [48]. Culturable NTM were found only in samples of two animals, possibly suggesting the higher sensitivity of qPCR compared with culture. Mouth and saliva are unsterile environments. Therefore, it was not surprising to detect environmental bacteria including M. avium and M. peregrium in these washings. The finding of M. avium and M. novocastrense, both of them NTM species with unclear clinical relevance in a tracheal wash, was more surprising since the deeper respiratory tract would be expected to be sterile. However, despite the closed suction system used, environmental contamination of samples cannot be absolutely excluded. The high prevalence of NTM, mycobacterial DNA in lung fluids collected in this study suggests that immune response to non-MTC in elephants might be behind the frequent false-positive results in non-validated immunological tests like STAT PAK or, more recently, DPP. From about 150 mycobacterial species identified to date, some, or even many, may possess cell wall compounds that cross-react with immunologically important cell wall components of members of the MTC. Given long and intensive contact with these bacteria may result in immune response comparable to the one MTC members may induce. While in humans antibiotic treatment is started without waiting for final MTC diagnosis, false-positive or true-positive immunological tests in elephants bear substantial consequences for the animal health and possibly survival and the finances of its owner. Based on false-positive immunological results animals might be subjected to expensive daily antibiotic treatment for over 12 months or even be euthanised. Based on our findings we now strongly recommend performing bronchoalveolar lavage in TB-suspected elephants to confirm results from immunological tests before taking further, more drastic, measures into consideration. Therefore, regardless of how a diagnostic TB sample in elephants is obtained, by trunk wash or bronchoscopy, this dual molecular approach of testing for NTM or bacteria closely related and MTC in parallel provides an improvement in elephant TB diagnostics putting results from immune-related, serological tests better into perspective.
Overall, the bronchoalveolar lavage in elephants is a new and technically advanced TB diagnostic. In contrast to the trunk wash performed by the elephant care takers, it additionally requires veterinary expertise, animal sedation and standard equine endoscopy equipment, all of which is commonly available in North American and European Zoos. The study demonstrates that bronchoscopy of elephants is feasible under varying management and housing conditions. Involvement of national TB reference laboratories using bacterial culture and PCR if additionally requested is recommended to become standard in elephant TB testing regardless of the collection method, trunk wash or bronchoalveolar lavage. Further comparative studies on sensitivity and specificity of the trunk wash and bronchoalveolar lavage are warranted. Yet, under field conditions in range countries, bronchoalveolar lavage might be still be challenging but feasible [Reference Lueders57]. In conclusion, we have described here bronchoalveolar lavage for the diagnosis of TB infection of the respiratory tract in elephants. We demonstrated that DNA of NTM is well represented in the bronchi deep inside the respiratory tract of the tested elephants raising great concerns over the elephant's immune response to mycobacteria in general and current limited ability to distinguish between immune response to MTC and NTM. Different from trunk wash, bronchoalveolar lavage is rather safe for the testing personnel, and has higher probability of harvesting the bacteria in active MTC-infected and mycobacteria shedding animals, while, at the same time, exclude or substantially minimise possible environmental sample contamination. If the lavage technique reported here for the first time for elephants is frequently and repeatedly used, it may help overcome the low sensitivity of the commonly used trunk wash for TB diagnosis in elephants.
Acknowledgements
The authors thank the staff of all institutions involved for their dedicated work to make this study happen.
Declaration of Interest
None.







