INTRODUCTION
Nosocomial multidrug-resistant (MDR) bacterial infections are becoming more common in veterinary hospitals and the incidence of these infections is expected to increase [Reference Ogeer-Gyles, Mathews and Boerlin1]. Pathogens that have been reported to cause nosocomial infections in dogs and cats include Acinetobacter spp., Escherichia coli, Enterobacter spp., Enterococcus, Klebsiella spp., Serratia marcescens and Staphylococcus spp. including methicillin-resistant S. aureus [Reference Glickman2–Reference Scott Weese8]. The majority of these pathogens are derived from endogenous microbiota of the skin, respiratory and/or gastrointestinal tracts of hospitalized animals or from exogenous sources in the hospital environment, although it is now recognized that veterinary personnel may also play a role in human to animal transmission of nosocomial pathogens [Reference Johnson9].
The gastrointestinal tract is the most important reservoir for nosocomial Gram-negative organisms such as MDR E. coli and Enterobacter spp. and, in humans, intestinal carriage often precedes clinical extraintestinal infection [Reference Cookson10]. In humans, the risk of acquiring intestinal colonization or carriage during hospitalization was increased by time spent in intensive-care units [Reference Filius11], disease conditions [Reference Harris12], age [Reference Harris12], urinary and arterial catheterization (probably reflecting manipulation by healthcare personnel) [Reference Lucet13, Reference Pena14], and antimicrobial drug use [Reference Filius11, Reference Harris12, Reference Yagci15]. In animals, such risk factors remain undefined.
MDR E. coli were isolated from a cluster of nosocomial extraintestinal infections in dogs occurring at a veterinary teaching hospital in Australia [Reference Sidjabat6]. To limit further occurrence of extraintestinal infections, an infection control programme was initiated in which rectal swabs were obtained from dogs on admission for hospitalization and throughout their hospitalization period to isolate MDR coliforms on MacConkey agar containing enoxacin and gentamicin (MCAEG) [Reference Sidjabat16]. Preliminary characterization (pulsed-field gel electrophoresis) of a subset of rectal swab isolates revealed the same two distinct clonal groups of MDR E. coli that had been identified from extraintestinal infections, with clonal group 1 corresponding to E. coli phylogenetic group A and clonal group 2 to phylogenetic group D [Reference Sidjabat17]. Furthermore, resistance phenotyping and a multiplex PCR test were used to rapidly identify and distinguish between both clonal groups [Reference Sidjabat16].
The infection control programme data from this study provides a unique opportunity to identify specific risk factors that influence gastrointestinal colonization of hospitalized dogs with MDR E. coli. In previous work, we identified risk factors for dogs returning a positive rectal swab for MDR E. coli on the day of admission to the veterinary teaching hospital [Reference Gibson18]. Hospitalization, treatment with a fluoroquinolone, and diagnostic imaging within the 42 days prior to admission increased the risk of carriage of MDR E. coli at admission [Reference Gibson18]. While identifying dogs which are more likely to introduce MDR pathogens into the hospital environment is an important control strategy, identifying dogs which are at increased risk of developing carriage with MDR E. coli during hospitalization is just as important. Therefore, we performed a case-control study using the same infection control programme data to identify risk factors for dogs becoming rectal carriers of MDR E. coli while hospitalized. A secondary objective was to compare the clonal group status for rectal isolates obtained during the study period to that of E. coli isolates which were causing extraintestinal infections during the same time period [Reference Sidjabat6, Reference Sidjabat16], using resistance phenotyping and multiplex PCR.
METHODS
Study overview
This study was a retrospective risk-based case-control study using information collected during an infection control programme at The University of Queensland Veterinary Teaching Hospital (UQVTH), a first opinion and referral hospital in Brisbane, Australia, between 7 August 2000 and 15 November 2002. As part of the programme, rectal swabs were collected from dogs on admission to hospital, during hospitalization, and at discharge and were screened for MDR E. coli [Reference Sidjabat16]. The unit of interest for this study was the individual admission (where an admission is housing of a dog in the hospital for one or more consecutive nights), and cases and controls were selected at the admission level. Frequencies of exposures to potential risk factors were compared between admissions which became rectal carriers of MDR E. coli during hospitalization (cases) and admissions which did not become carriers during hospitalization (controls).
Case and control selection
Study admissions were selected from all admissions to the UQVTH between 1 March 2001 and 30 October 2002. This date range was chosen as compliance with rectal swabbing at admission was the highest during this phase of the infection control programme [Reference Gibson18]. Compliance with rectal swabbing procedures during hospitalization was assessed for 1 week of each month. During the 21 weeks from this period that were assessed, 74% of admitted animals were swabbed both during hospitalization and at discharge. During this period, hospitalized dogs were typically swabbed every second day (i.e. median 2 days, range 1–10 days). For the selection of MDR coliforms, rectal swabs were cultured on MacConkey agar containing enoxacin (5 mg/ml) and gentamicin (5 mg/ml) (MCAEG) as previously described [Reference Sidjabat16]. Isolates were stored in Luria–Bertani broth with 15% (v/v) glycerol at −80°C.
Admissions were eligible for selection if: (a) the dog was swabbed on either the day of admission and/or the following day with negative results (no growth on MCAEG), (b) at least one more rectal swab was collected on a subsequent day of the same admission and (c) the dog was privately owned. Both primary-care and referral admissions were eligible for selection. Most study dogs were swabbed on only one of the first 2 days of hospitalization. All admissions that had at least one positive rectal swab (growth on MCAEG) while hospitalized at any stage after their negative admission swab were selected as cases. Control admissions were matched to the distribution of dates of admission of cases. For each case admission, one control admission was randomly selected using computer-generated random numbers (generated by the randbetween function in Microsoft Excel) from admissions commencing on the same date where the dog had only negative rectal swabs during hospitalization. If there was no admission eligible for selection as a control from admissions commencing on the same date as the case admission, a control was randomly selected from admissions commencing on an adjoining day where available or the next closest day within a week either before or after the case admission. For dogs selected at more than one admission only the first admission within the study period was retained for analysis.
Potential risk-factor data collection
Potential risk factors were selected based on relevant evidence for similar studies conducted in human public hospitals. Data for potential risk factors were collected by examining hospital records as well as the records from referring veterinary practices. We included exposures to potential risk factors from 42 days before admission to start of hospitalization (defined as the pre-admission period) [Reference Gibson18]. This time period was selected based on durations of rectal colonization in dogs following experimental infection with MDR E. coli [Reference Trott19] (Table 1). Exposures during the hospitalization period were assessed for the period when the dog was at risk of acquiring rectal carriage with MDR E. coli; this at-risk period was from admission until either the first positive rectal swab for cases or the last negative rectal swab while hospitalized for control admissions (Table 1). General exposures examined included age, gender, breed, source of admission, and underlying disease or condition (Table 1). Breed was analysed using two categorizing methods: a genetic breed category based on the genetic structure of the purebred domestic dog [Reference Parker20]; and breed group according to the Australian National Kennel Council (ANKC) database [21].
Table 1. Putative risk factors for carriage of multidrug-resistant E. coli in hospitalized dogs that were assessed in a retrospective, risk-based, case-control study. Exposures to time-varying factors were for the 42-day period prior to admission (the ‘pre-admission period’) and the ‘time at risk while hospitalized’ (the number of days from admission to first positive swab for cases, or from admission to last negative swab for controls)

NSAIDs, Non-steroidal anti-inflammatory drugs.
* Variables in the first column apply to hospital admissions preceding the index admission.
† Cumulative days hospitalized or treated.
‡ Study dogs were classified as having been admitted to The University of Queensland Veterinary Teaching Hospital (UQVTH), other veterinary hospitals in the surrounding area or a combination of the UQVTH and another veterinary hospital.
§ Antimicrobials included: amoxicillin/clavulanic acid, ampicillin, first-generation cephalosporin, fluoroquinolones (enrofloxacin, orbifloxacin), aminoglycoside (gentamicin), carbapenem (imipenem), lincosamide (clindamycin), metronidazole, tetracycline (doxycycline), trimethoprim/sulfonamide.
∥ Non-steroidal anti-inflammatory drugs (NSAIDs) include carprofen, meloxicam and piroxicam.
¶ Steroidal anti-inflammatories included dexamethasone and prednisolone.
# Opioids included morphine, fentanyl and buprenorphine.
Other putative risk factors could be grouped as hospitalization-specific, non-antimicrobial and antimicrobial-specific treatments, and diagnostic procedures (Table 1). Diagnostic imaging procedures included radiography, echocardiography, ultrasonography, and computer tomography, and other diagnostic procedures included aspirates (chest, joints, wounds), cerebral spinal fluid tap, endoscopy, ear swabs, and tracheal washes.
Data analyses
Associations between potential risk factors and becoming a rectal carrier of MDR E. coli were assessed by fitting maximum-likelihood logistic regression models using the logistic command in Stata v. 10.1 (Stata Corp., USA). Overall significance of each variable was assessed using likelihood ratio test P values, and significance of individual levels of risk factors (relative to the reference level) was assessed using Wald P values. Potential risk factors with no control admission for one or more levels were assessed using exact logistic regression models, fitted using LogXact 8 (Cytel Inc., USA). For these models, P values for each level of the risk factor (relative to the reference level) were calculated as two times the one-sided exact P values. Exact probability P values were used for hypothesis testing of the overall significance of these risk factors. Odds ratios for these exposure variables were obtained using the median unbiased estimator [Reference Hosmer and Lemeshow22].
Potential risk factors were analysed using both univariable analysis and after adjusting for start date of admission, grouped into 3-month categories. For all factors assessed, the odds ratios changed by <30% after this adjustment. Accordingly, start date of admission was not fitted into multivariable models and univariable results were used.
This study was taking place at the same time as an infection control programme and this may have altered the risk of acquiring MDR E. coli during admissions later in the study period. Therefore, the risk of acquiring MDR E. coli was compared between admissions commencing during the first half (1 March 2001 to 30 November 2001) and the second half (1 December 2001 to 18 October 2002) of the study period.
After univariable analysis, all variables with overall likelihood ratio test P values <0·2, other than those requiring analysis using exact models, were examined after adjusting for time at risk of acquiring rectal carriage with MDR E. coli while hospitalized.
After adjusting for time at risk while hospitalized, all variables with overall likelihood ratio test P values <0·2 (Supplementary Table 1, available online) other than those with no control admission for one or more levels and those described below were assessed using multivariable modelling. Each of these variables was fitted using a forward selection approach with each variable sequentially fitted in ascending order based on likelihood ratio P value. Variables with overall P values <0·05 were sequentially excluded before further variables were fitted. Once excluded, variables were not eligible for re-inclusion. Time at risk while hospitalized was forced into all models. The binary variables ‘treatment with any antimicrobial in the pre-admission period’ and ‘treatment with any antimicrobial during time at risk while hospitalized’ were not included in the multivariable modelling process as we wanted to assess effects of treatment with particular antimicrobials.
Fit of the final maximum-likelihood logistic model was assessed using the Hosmer–Lemeshow goodness-of-fit test and by comparing observed to expected numbers of cases and controls for ten groups based on predicted probabilities. The discriminatory ability of this model was assessed using the area under the receiver-operating characteristics curve (ROC) and by assessing sensitivity and specificity of the model at varying probability cut-points [Reference Dohoo, Martin and Stryhn23].
Treatment with fluoroquinolones and route of administration of cephalosporins (oral or parenteral) in the pre-admission period, and treatment with gentamicin during time at risk while hospitalized had no control admission for one or more levels and so were further assessed using exact logistic regression by fitting each separately with the variables from the final maximum-likelihood model.
Duration of treatment with any antimicrobial, interval between admission and treatment with any antimicrobial, and the number of antimicrobials during time at risk while hospitalized were further assessed with both univariable analysis and adjusted for time at risk, only for those dogs which were given antimicrobials. Similarly, the route of administration of cephalosporins (oral or parenteral) during time at risk while hospitalized was further assessed only for those dogs which received cephalosporins.
Microbiological characterization of isolates
Microbiological characterization of isolates was performed to compare the rectal E. coli isolated during the infection control programme to E. coli which was causing extraintestinal infections during the same time period [Reference Gibson5, Reference Sidjabat6] and to extend the observations made by Sidjabat et al. [Reference Sidjabat16] regarding carriage of CG1 and CG2 MDR E. coli in hospitalized dogs. Case dogs often had more than one positive rectal swab taken, over the duration of their hospitalization. Fifty cases had one positive rectal swab, 21 cases had two positive swabs, 12 cases had three positive swabs, three cases had four positive swabs, and four cases had five or more positive swabs, resulting in a total of 162 E. coli isolates. One hundred and thirty-three MDR E. coli isolates from the 90 cases were recovered from long-term storage. Disc diffusion susceptibility testing for amoxicillin/clavulanic acid, cefotaxime, cefoxitin, chloramphenicol, enrofloxacin, and spectinomycin was performed using methods described in Clinical and Laboratory Standards Institute (CLSI) guidelines [24, 25]. Isolates were confirmed to be AmpC β-lactamase-producing E. coli and were categorized into putative clonal groups based on results of a multiplex PCR for E. coli uspA, bla CMY and a class 1 integron-associated dfra17-aadA5 [Reference Sidjabat16]; isolates positive for all three genes were categorized as MDR E. coli putative clonal group 1 and isolates positive for uspA and bla CMY only were categorized as putative clonal group 2.
RESULTS
Numbers of cases and controls and underlying disease conditions
In total, 112 admissions met the study selection criteria and all were enrolled as cases but 13 (12%) were subsequently excluded because the dog's clinical case file was missing, leaving 99 assessable case subjects. Clinic files were missing for six (6%) of the 99 control admissions initially selected; these were excluded and replacement control admissions selected. This resulted in 99 case admissions and 99 control admissions from 183 dogs. After retaining only the first admissions for dogs with multiple admissions, 90 case and 93 control admissions were analysed. The underlying disease or conditions for case and control admissions are shown in Supplementary Table 2 (available online). For these control and case admissions, there were, respectively, 137 and 180 intervals between swabs while dogs were at risk of acquiring rectal carriage with MDR E. coli. The medians and 90th percentiles of these intervals were 2 and 4 days for both controls and cases. For controls and cases, respectively, 75% and 64% of intervals were 1 or 2 days, 12% and 22% were 3 days, and 13% and 14% were ⩾4 days.
Table 2. Results of final maximum-likelihood logistic model of rectal carriage with multidrug-resistant E. coli in dogs while hospitalized

* Cumulative days, for time at risk, hospitalized or treated.
† Non-steroidal anti-inflammatory drugs (NSAIDs) included carprofen, meloxicam and piroxicam.
Univariable and multivariable analyses
Associations between potential risk factors and becoming a rectal carrier of MDR E. coli were assessed. On univariable analysis, no general risk factors (age, gender, breed, source of admission, underlying disease or condition) had a P value of <0·2 (results not shown). Exposures in the pre-admission period, variables which apply to the hospital admission preceding the index admission, with P values of <0·2 on univariable analysis were: duration of hospitalization (P=0·07), treatment with any antimicrobial (P=0·18), treatment with amoxicillin/clavulanic acid (P=0·17), cephalosporins (P=0·03), and fluoroquinolones (P=0·06), route of administration of cephalosporins (oral or parenteral) (P=0·03), duration of treatment with non-steroidal anti-inflammatory drugs (NSAIDs) (P=0·07), and surgery (P=0·18) (Supplementary Table 1).
The risk of becoming a rectal carrier of MDR E. coli during time at risk while hospitalized increased markedly with time hospitalized (P=0·02). On univariable analysis, the following exposures during time at risk while hospitalized were also associated (P<0·2) with an increased risk of becoming a rectal carrier: housed in intensive-care unit ward (P=0·09); treatment with any antimicrobial (P=0·04); duration of treatment with any antimicrobials (P=0·003); interval between admission and treatment with any antimicrobial (P=0·04); the number of antimicrobials used (P=0·003); use and duration of treatment with cephalosporins (P<0·001); treatment with fluoroquinolones (P=0·02), metronidazole (P=0·07), and gentamicin (P=0·01); route of administration of cephalosporins (oral or parenteral) (P=0.01); duration of intravenous fluids (P=0·04); interval between admission and intravenous fluids (P=0·12); duration of treatment with NSAIDs (P=0·15); interval between admission and NSAID treatment (P=0·14); number of general anaesthetics (P=0·02); and number of diagnostic imaging procedures (P=0·004) (Supplementary Table 1).
After adjusting for time at risk while hospitalized, 22 exposure variables remained associated with an increase risk of being a rectal carrier of MDR E. coli during hospitalization. In the pre-admission period these included: duration of hospitalization (P=0·05) treatment with any antimicrobial (P=0·08); treatment with cephalosporins (P=0·006), and fluoroquinolones (P=0·04); the route of administration of cephalosporins (oral or parenteral) (P=0·001); duration of treatment with NSAIDs (P=0·14); and surgery (P=0·19) (Supplementary Table 1).
During time at risk while hospitalized these included: being housed in the intensive-care unit ward (P=0·1); treatment with any antimicrobial (P=0·004); duration of treatment with any antimicrobials (P=0·02); interval between admission and treatment with any antimicrobial (P=0·03); the number of antimicrobials used (P=0·01); use and duration of treatment with cephalosporins (P=0·001); treatment with fluoroquinolones (P=0·028), metronidazole (P=0·06), and gentamicin (P=0·03); route of administration of cephalosporins (oral or parenteral) (P=0·02); duration of intravenous fluids (P=0·08); interval between admission and intravenous fluids (P=0·2); interval between admission and NSAID treatment (P=0·13); number of general anaesthetics (P=0·04); and number of diagnostic imaging procedures (P=0·008) (Supplementary Table 1).
As infection control procedures at the UQVTH were in place during the study, we compared odds of becoming a rectal carrier for admissions in the first and second halves of the study period. On univariable analysis, the odds of becoming a carrier did not differ significantly between admissions in the first and second halves of the study period (OR 0·94, 95% CI 0·53–1·69, P=0·85).
The final multivariable model consisted of the time at risk while hospitalized; treatment with cephalosporins in the pre-admission period; use and duration of treatment with cephalosporins, treatment with metronidazole, and the number of diagnostic imaging procedures during time at risk while hospitalized; and the interval between admission and treatment with NSAIDs. The result of the final maximum-likelihood logistic model of rectal carriage with MDR E. coli in dogs while hospitalized is shown in Table 2.
Treatment with fluoroquinolones and route of administration (oral or parenteral) of cephalosporins in the pre-admission period, and treatment with gentamicin during time at risk while hospitalized, could not be fitted using exact logistic regression modelling with LogXact, possibly due to excessive numbers of zero cells.
The antimicrobial variables: duration of treatment with any antimicrobial; interval between admission and treatment with any antimicrobial; and the number of antimicrobials during time at risk while hospitalized, were not significantly associated with carriage of MDR E. coli during hospitalization when only those dogs which were given antimicrobials were included. This indicates that the significant associations between these variables and carriage when all dogs were analysed were probably largely due simply to use of antimicrobials and not to particular durations of use, intervals between admission and treatment, or numbers of antimicrobials used. The route of administration of cephalosporins (parenteral or oral) during time at risk while hospitalized was also not associated with increased risk of MDR E. coli carriage during hospitalization when only those dogs which received cephalosporins during time at risk while hospitalized were analysed.
Model fit and discriminatory ability
The final model fitted the data reasonably well with the largest proportional differences between the numbers of observed and expected cases at low and intermediate predicted probabilities. The Hosmer–Lemeshow goodness-of-fit test P value was 0·777, providing no basis for concluding that the fit was poor. The discriminatory ability of the final model was fair with the area under the ROC equal to 0·80. At a probability cut-point of 0·48, the model's sensitivity and specificity were both around 0·75.
Characterization of MDR isolates
The antimicrobial disk susceptibility and putative clonal groups of 133 isolates are shown in Table 3. Sixty-four (48%) isolates were identified by multiplex PCR as putative clonal group 1 strains (positive for uspA, dfrA17-aadA5 and bla CMY), and 64 (48%) isolates as putative clonal group 2 strains (positive for uspA and bla CMY only). Five (4%) isolates could not be assigned to either of the two clonal groups. These isolates were all identified as E. coli; two contained the dfrA17-aadA5 gene and were possibly clonal group 1 strains that had lost bla CMY, whereas the remaining three isolates may have been clonal group 2 strains that had lost bla CMY, clonal group 1 strains that had lost both the integron and bla CMY, or unrelated isolates. The resistance profiles of CG1 and CG2 MDR E. coli isolates are extremely similar and they only differ in their resistance to chloramphenicol. In 93% (n=84) of cases, dogs which become rectal carriers of MDR E. coli, carried the same putative clonal group throughout hospitalization. However, in six cases, dogs were found to carry a different clonal group at subsequent samplings. In five cases, dogs that initially returned a swab that was positive for putative clonal group 1 were shown to carry a putative clonal group 2 strain on a subsequent swab during hospitalization and in one case; there was a change from putative clonal group 1 to a non-classified group.
Table 3. Putative clonal group and resistance profile for multidrug-resistant E. coli isolates from 90 cases (dogs that became carriers) during hospitalization at The University of Queensland Veterinary Teaching Hospital between 1 March 2001 and 30 October 2002
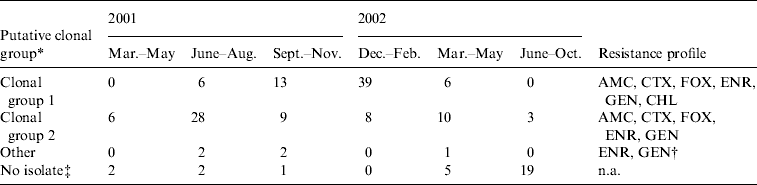
AMC; Amoxicillin/clavulanic acid, CTX; cefotaxime, FOX; cefoxitin, ENR, enrofloxacin; CHL; chloramphenicol, GEN; gentamicin; n.a., not applicable.
* Putative clonal group 1: positive for E. coli uspA, bla CMY and dfra17-aadA5. Putative clonal group 2: positive for uspA and bla CMY only. Other: all contained uspA, 2 contained dfrA17-aadA5 [Reference Sidjabat16, Reference Sidjabat17].
† Two of these isolates were also resistant to chloramphenicol.
‡ Isolate non-viable or not stored after original isolation on MCAEG.
DISCUSSION
This study demonstrated that hospitalization for >6 days is an important risk factor for dogs becoming rectal carriers of MDR E. coli independently of some antimicrobial treatments. As the duration of hospitalization increases, risk and/or number of contacts with contaminated surfaces and fomites, other hospitalized animals (including MDR E. coli carriers), and hospital personnel would also be expected to increase. All are established mechanisms for transmission, gastrointestinal colonization or carriage, and subsequent extraintestinal infection with MDR E. coli in humans [Reference Cookson10].
Antimicrobial-specific risk factors for dogs becoming rectal carriers of MDR E. coli during hospitalization include treatment with cephalosporins in the 42 days prior to admission and treatment with cephalosporins or metronidazole during hospitalization. Treatment with fluoroquinolones prior to hospitalization and treatment with gentamicin during hospitalization were also identified as risk factors for dogs becoming carriers of MDR E. coli during hospitalization on univariable analysis.
Antimicrobials suppress susceptible indigenous microbiota [Reference Sullivan, Edlund and Nord26] and allow other organisms to exploit the vacated ecological niche within the gastrointestinal tract [Reference Donskey27]. These organisms could potentially be spontaneous resistant mutants, but given the results of rectal isolate characterization, they are more likely to be MDR E. coli that were either ingested following exposure to sources within the hospital, or pre-existing subpopulations normally suppressed to below detectable concentrations by resident microbiota [Reference Donskey27]. A range of antimicrobial agents including cephalosporins, fluoroquinolones, aminoglycosides, and trimethoprim/sulfonamides are significant risk factors for colonization or carriage due to MDR Enterobacteriaceae in hospitalized humans [Reference Wiener28–Reference Graffunder30]. Treatment with fluoroquinolones has been identified as a risk factor for the development of multidrug resistance in rectal E. coli in dogs [Reference Gibson18, Reference Ogeer-Gyles31].
The most common cephalosporins administered to hospitalized dogs were first-generation cephalosporins. Cefazolin was administered by the parenteral route and cephalexin orally. Cefazolin was often given as one prophylactic injection at the time of surgery. This may explain the lower risk of one day of treatment with cephalosporin during hospitalization compared to more days. In general, parenterally administered cephalosporins are less likely to select for the emergence of resistant Enterobacteriaceae in the intestinal microbiota compared to orally administered cephalosporins [Reference Sullivan, Edlund and Nord26], even though cephalosporins administered by either route have some suppressive effect on susceptible Enterobacteriaceae and anaerobic bacteria within the gut [Reference Sullivan, Edlund and Nord26]. However, in this study, there was no difference in risk between the routes of administration of cephalosporins (oral or parenteral).
In this study, all but five of the MDR E. coli isolates characterized were confirmed to possess a bla CMY gene. Cephalosporin treatment would certainly provide selection pressure for E. coli strains carrying the CMY β-lactamase to be maintained in the gastrointestinal tract of hospitalized dogs. However, co-amoxyclavulanate, a potentiated β-lactam, was the most commonly administered antimicrobial agent, but unexpectedly, it was not found to be associated with MDR E. coli rectal carriage. CMY AmpC β-lactamases are resistant to clavulanic acid and experimental colonization or carriage studies [Reference Lautenbach32] will be required to explore the reasons for this key difference.
In the current study, metronidazole and gentamicin treatments were both identified as risk factors for becoming carriers of MDR E. coli during hospitalization. However, metronidazole and gentamicin were always administered in combination with other antimicrobials and it is possible that neither treatment increases risk of carriage and the observed associations were due to confounding by other factors, including effects of exposure to other antimicrobials.
Our final multivariable model included interval between admission and treatment with NSAIDs (with lowest risk of carriage in dogs treated from admission start date), and number of diagnostic imaging procedures during hospitalization (with reduced risk in dogs receiving one procedure). We are not aware of biological reasons for such protective effects against the development of MDR E. coli carriage during hospitalization but in a previous study, dogs undergoing diagnostic imaging techniques in the pre-admission period were more likely to be carriers of MDR E. coli at admission [Reference Gibson18]. Unidentified confounding factors probably explain these associations. It is possible that dogs treated with NSAIDs from the date of admission and dogs requiring one diagnostic procedure differed from other dogs. However, underlying disease or condition was not found to be a risk factor for the development of MDR E. coli carriage during hospitalization in this study.
The resistance profile and multiplex PCR results generated from rectal isolates demonstrated that the isolates that the case dogs acquired while in hospital were the same as or similar to those isolated from extraintestinal infections [Reference Sidjabat6, Reference Sidjabat16]. Thus selection pressures are likely to be the same for the emergence of both clonal groups.
The current study had a number of limitations. The selective media (MCAEG) used to isolate may have prevented some MDR E. coli from being detected (e.g. fluoroquinolone-resistant E. coli strains that carried bla CMY but were gentamicin sensitive), resulting in false negatives.
Diagnostic sensitivity of rectal swabbing in our study may not have been 100%. Sensitivity of rectal swabbing in humans in one study was 90% [Reference Lautenbach32] and in experimental dogs [Reference Trott19] swabbed repeatedly over 21 days, 70% of swabs taken when dogs were known to be carriers were positive for MDR E. coli (D. J. Trott, unpublished data). Such errors in admission swabs would have resulted in inappropriate inclusion of dogs who were carriers on admission. If this occurred, the observed odds ratios for each risk factor would reflect the combined effects of that factor on risks of dogs being a carrier and becoming a carrier. False-negative swab results during hospitalization could result in some dogs that became a carrier being incorrectly categorized as controls. For most risk factors, such errors would be expected to be non-differential, i.e. to have occurred with similar frequency in exposed and non-exposed dogs that became carriers. If so, the observed odds ratios for binary exposure variables would be biased towards 1 (i.e. less extreme than actual) and so the true strengths of association would be greater than that indicated by our reported odds ratios.
Rectal swabbing is highly specific for identifying gastrointestinal carriers [Reference Sullivan, Edlund and Nord26], so there was probably no important bias due to false-positive swab results. Some otherwise eligible admissions were ineligible because they were not swabbed after admission. Because this was due to staff not complying with the hospital swabbing protocol, it is unlikely to have been differential by exposure and case/control status. If so, this would not have been a source of selection bias.
In conclusion, duration of hospitalization, treatment with cephalosporins and metronidiazole during hospitalization, and treatment with cephalosporins prior to hospitalization were important risk factors for dogs acquiring MDR E. coli rectal carriage during hospitalization in this study. Partial characterization of rectal isolates confirmed that in almost all cases, the MDR E. coli strains were the same or similar to those isolated from clinical extraintestinal infections occurring during the study period. Identification of hospitalized dogs exposed to these risk factors may lead to improved infection control. In addition, risk of acquiring infection could be reduced through prudent antimicrobial use. Although it has been suggested that risk factors for carriage with MDR Enterobacteriaceae may differ from those associated with extraintestinal infection [Reference Lucet13], most nosocomial clinical infections are preceded by intestinal colonization or carriage [Reference Cookson10, Reference Lucet13]. Therefore, these strategies could reduce the occurrence of MDR clinical infections within large veterinary hospitals when included as part of infection control programmes.
NOTE
Supplementary material accompanies this paper on the Journal's website (http://journals.cambridge.org/hyg).
ACKNOWLEDGEMENTS
We thank staff and students at the University of Queensland Veterinary Teaching Hospital for the collection of isolates. We also acknowledge The University of Queensland Veterinary Diagnostic Laboratory for the collection and storage of isolates, in particular Susan Moss and Dr Kirsty Townsend.
DECLARATION OF INTEREST
None.





