INTRODUCTION: BRIEF HISTORY OF CONCUSSION
That concussion occurs and is commonplace is not in dispute. The United States Government's Center for Disease Control (CDC) estimates that there are more than one million concussions that occur annually in the United States, using their definitional statement of concussion being a condition “of temporarily altered mental status as a result of head trauma (www.cdc.gov, see Rutland-Brown et al., 2006).”
What is controversial is whether one fully recovers without symptoms from having sustained a concussion. Given the commonness of concussions along with the adaptive nature of brain function combined with neural plasticity (Duffau, 2006; Giza & Prins, 2006; Moucha & Kilgard, 2006; Priestley, 2007), it might be assumed that any transient impairment as a result of concussion would not result in any neurological sequelae. Indeed, historically the original Latin term “commotio cerebri” was used to describe concussion, thought to occur because of “traceless disturbances” that produced momentary functional impairment without any damage to brain tissue (see reviews by McCrory & Berkovic, 2001; Vos et al., 2002) . Hence, for decades, one of the venerable definitions in standard neurology textbooks, exemplified by the following quote from Grinker's Neurology was as follows: “the usual patient loses consciousness briefly, soon recovers and thereafter is without symptoms” (Vick, 1976; p. 651). In that concussion was thought to be mostly benign, the non-biological and psychodynamic theories that dominated the beginnings of clinical psychology and psychiatry minimized the effects head injury could have on behavior. This is captured by the 1947 quote by Page (1947) in an abnormal psychology textbook that “Head injuries and gunshot wounds involving damage to the brain occasionally produce mental disturbances, but such injuries are not an important cause of mental disease (p. 330)”. Persistent maladaptive symptoms in this time frame were believed to be more an expression of a “neurosis” than anything possibly “organic.” So, “persistent” problems following concussion were interpreted within a psychogenic framework. In fact, one of the most cited publications in the clinical literature on concussion is that of Miller (1961) whose series of articles centered on the theme of concussion being nothing more than “Accident Neurosis”, which others have labeled as “Compensation Neurosis” (Levy, 1992) because of the prevalence of lawsuits involving mild head injury (Hall & Chapman, 2005; Mooney et al., 2005). No doubt “psychological” factors play an important role in the residuals from concussion (Meares et al., 2006; Whittaker et al., 2007; Wood, 2007), but they and other “functional” factors are also the source of intense debate and controversy over the existence of post-concussive symptomology (Cantu, 2007; Evans, 1994; King, 2003). These controversies will be discussed in greater detail later in this review.
Part of this controversy has to do with nomenclature and definition. Years ago, Vick (1976) also stated that terms like concussion are “… of little value” because of “such wide and indefinite connotations” (p. 650). Much has been written about the definition of concussion (Blostein & Jones, 2003; Boake et al., 2005; Ruff & Jurica, 1999), including the definitional statements by major organizations and consensus panels as presented in Table 1, wherein the terms concussion and mild traumatic brain injury (mTBI) are used interchangeably. For this review, in referring to studies, if the authors used the term concussion then that term will be used in referring to the study and likewise, if the term mTBI is used by the authors, that term will be used; otherwise, mTBI and concussion will be used interchangeably in the current review. However, this review focuses on the persistence of symptoms following concussion or what has been referred to as post-concussive syndrome (PCS), but this term then brings up additional controversies. In the majority of those concussed, symptoms abate within minutes to hours to days post-injury. Thus, some refer to PCS if the symptoms persist for more than a few days and in particular, if the symptoms persist for more than a week (Anderson et al., 2006; Sheedy et al., 2006). If the symptoms last more than 3 months then the term persistent post-concussive syndrome or PPCS has been used (Begaz et al., 2006; Chamelian et al., 2004; Iverson, 2006; Rees, 2003; Satz et al., 1999; Stalnacke et al., 2005; Willer & Leddy, 2006). Whereas there is a relationship between severity of concussion and who develops PPCS (Hessen et al., 2006), concussion severity by itself is a poor predictor of who develops PPCS (Guskiewicz et al., 2004).
The Multiple Definitions and Grading Systems of Concussion

DSM-IV lists PCS as a disorder under its “research” classification and some have referred to it as a syndrome (King, 2003; Rees, 2003; Ryan & Warden, 2003) and there are differences in symptom criteria between DSM-IV and the International Classification of Disorders (ICD-10) that further cloud this taxonomy issue (Kashluba et al., 2006; McCauley et al., 2005). Whether PCS is a disorder or syndrome is another ongoing debate (Hall & Chapman, 2005; Smith, 2006). Neither DSM-IV or ICD use the PPCS label. Nosological issues are not the focus of this review and there are several excellent recent reviews on this topic ((Hall & Chapman, 2005; Silver et al., 2005; Smith, 2006; Zasler et al., 2007). Thus, for the current review PPCS is operationally defined as symptoms that persist beyond three months following a concussion (having met at least one of the definitions as listed in Table 1), implicating chronic sequelae.
As demonstrated by Table 1, there are many definitional statements about what constitutes a concussion or mTBI. Neuropsychological research on this topic would be aided to have a universally accepted definition as the standard (see Tagliaferri et al., 2006). Nonetheless, all definitions in Table 1 have general agreement that “mTBI is defined as the consequence of blunt (non-penetrating) impact with sudden acceleration, deceleration or rotation of the head with a Glasgow Coma Score (GCS) of 13–15 … (Vos et al., 2002, p. 207).” Thus, concussion occurs because of impact physical forces affecting the brain and if, physical forces are insufficient to injure the brain, no injury has occurred.
Regardless of the etiology, recovery from concussion is typically rapid and ostensibly complete in most individuals. Clearly, the best-controlled studies examining outcome following concussion, demonstrate good to complete recovery in the majority of individuals (Iverson et al., 2007). Additionally, at least with sports concussion, major consensus statements of the past five years have resulted in statements like “concussion typically results from the rapid onset of short lived impairment of neurological function that resolves spontaneously” and that “concussion may result in neuropathological changes but the clinical symptoms largely reflect a functional disturbance rather than structural injury” as reviewed by (Cantu, 2007, p. 963). So this review focuses on the minority of subjects who sustain a concussion, who remain symptomatic after three months. Large-scale studies demonstrate approximately 70% of all head injury cases seen in the emergency room (ER) are in the mTBI category (Udekwu et al., 2004). However, as pointed out by the CDC and other studies (Delaney et al., 2005), a substantial number of concussions is never evaluated in the ER, making it difficult to obtain precise numbers as to the true annual incidence rate. Bazarian et al. (2005) estimate that the annual mTBI incidence rate is 503.1/100,000, of which PPCS rates have been conservatively estimated at 10% (Ruff et al., 1996; Wood, 2004). Thus, despite the overall good to complete recovery rates from concussion, this remains a major public health concern (Langlois et al., 2005) and the field of neuropsychology should better understand the disorder (Kelly, 1999; Langlois et al., 2005).
From a neuropsychological standpoint, symptoms of impaired attention, memory, and executive function along with changes in emotional regulation dominate the clinical picture of PPCS (Lundin et al., 2006). An objective of this review is to understand these features in terms of a common pathological basis. To accomplish this, how evolutionary factors may have shaped recovery from concussion, followed by an up-to-date review of important new studies on the biomechanics of concussion and a thorough discussion of what is now currently known about the neuropathological and pathophysiological basis of concussion will be offered. The last part of the review will focus on more traditional neuropsychological concepts as they relate to concussion and conclude with suggestions on improved research tactics on this topic. There may be nothing more controversial in contemporary clinical neuropsychology, than the issues to be discussed in this review. At the outset, these controversies are acknowledged and the approach of this review is to first overview the contemporary neuroscience of concussion and deal with the most controversial issues at the end of the review.
EVOLUTIONARY ASPECTS OF INJURY
Undoubtedly concussions have been part of mammalian life since the beginning. The universality of concussions is that the stunned, motorically wobbly appearance commonly observed in an athlete, particularly a boxer who has been concussed, is replicated with animal models (Shaw, 2002). Survivability across mammalian species following concussion is testament to the fact that most concussions are but transient disruptions in normal brain function allowing the animal (including humans) to recover quickly and fully return to pre-injury abilities and activities. Because of the commonness of concussions, it is likely that genes that promoted certain brain morphologies and/or positive recovery characteristics have been passed down. However, concussions prior to the modern era would have occurred only from falls, falling or thrown objects, fisticuffs, combat, and the like. All of these remain major sources of concussions but with the modern era, concussions also occur from high-speed impacts that simply were never the source of injury in earlier times. So, whatever evolutionary advantages occurred they did so prior to the modern era. Likewise, genes selective for their ability to promote survivability of a brain injury were most likely only associated with simple concussion and not more severe brain injury, because prior to modern medical treatment the majority of moderate-to-severe injuries would not have been survivable or lead to disability that could not be sustained.
From a structural standpoint, the position of the irregular skull base to the dural surface of the frontal and temporal lobes, housed with in the anterior and middle cranial fossa provides a means for holding the brain in position, in response to movement and/or mild trauma to the head (Bigler, 2007). Likewise, the position of the ventricles dissipates some of the strain effects with movement, including that of concussion (Ivarsson et al., 2002). Both of these have significant evolutionary advantage. It is also very likely that a selective bias occurred that favored rapid brain reparative mechanisms once a concussion occurred (Diaz-Arrastia & Baxter, 2006). In fact the most common of injuries for a particular organ system are the very ones most likely shaped by evolution (Martin & Leibovich, 2005). So key to recovery from concussion is a fast acting reparative system and this would emphasize a transient cellular response that immediately re-establishes neural homeostasis. In addition to reparative metabolic and cellular responses, redundancy and back-up neural circuitry activated once a primary system were injured would be critical to recovery (Bach-y-Rita, 2004; Desmurget et al., 2007; Duff, 2001; Guigon et al., 2007; Kercel et al., 2005). These redundant systems can either share in or take over function for injured neurons and networks. A neural systems reserve capacity probably directly relates to how rapid recovery from brain injury occurs, including concussion (Berker, 1996; Stern, 2007) and the role of genes in this recovery process is being examined (Alexander et al., 2007; McAllister et al., 2006)
One final evolutionary speculation will be made and that is based on the appearance and “design” of the fornix (see Fig. 1), the major white matter output from the hippocampus. At least half of the fornix is suspended beneath the corpus callosum and loosely connected with the septum pellucidum as it dives toward its connection with the mammillary body and septum (Andersen et al., 2006). One look at this delicate anatomical structure and it is obvious that it was not selected for its ability to withstand brain trauma. Evolutionarily, in lower mammals the fornix is clearly imbedded in brain parenchyma, but moving up the evolutionary tree with the expansion of the cerebral hemispheres, and the ventricular system, the fornix becomes more progressively suspended (see Crosby & Schnitzlein, 1982). The importance of the hippocampus and fornix in understanding mTBI is a major part of this review.
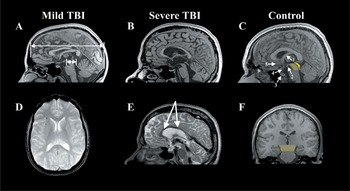
The top row presents mid-sagittal T1 MRIs comparing the fornix and corpus callosum from a control (C) to that observed in mTBI (A) and severe TBI (B). The control figure is labeled where the following structures are identified: (1) fornix, (2) mammillary body (3) pituitary, where it is situated in the sella turcica, (4) hypophysis of pituitary stalk, and (5) region of the basal forebrain. The light yellow depicts the region of the tegmentum of the midbrain (shown in coronal view in F), whereas the darker yellow represents the tectal region of the midbrain. The coloration of the midbrain is also done to highlight the relative smallness of the mibrain compared to the size of the cerebrum, and how the cerebrum ‘rests’ atop the midbrain. As shown in (A) from the patient with mTBI, the length of the cerebrum (long arrow) is approximately 10 times the length of the midbrain (short arrow). (D) depicts an axial gradient recall echo (GRE) depicting multiple deposits (black dots) of hemosiderin in the frontal region, implicating shear injury, note how they are mostly located at the gray-white matter junction. This patient had sustained an mTBI in a MVA. Note that there is thinning of the corpus callosum and a shear lesion in the isthmus region. (E) is a T2 mid-sagittal MRI depicting extensive hemosiderin deposition along the body of the corpus callosum (arrows) and also note the generalized atrophy of all structures in the severe TBI case compared to the mTBI and control.
Physics of TBI
Given that concussions are so commonplace it must be easy to at least transiently impair the brain through mechanical deformation and there must be common neurological structures affected [see Figs. 1 and 2; (Ropper & Gorson, 2007)]. In a most innovative experiment by Bayly et al. (2005) human volunteers were studied using MRI to determine momentary brain parenchymal deformation when the head falls just 2 cm. MRIs of the brain were obtained before and immediately after the drop, comparing the degree of brain deformation or warping by measuring changes in fixed points between the two scans. These movements were far below the threshold for concussion and the authors liken this to the type of head (and brain) acceleration when jumping vertically a few inches and landing flat-footed. The authors estimated that it was 10% to 15% of the acceleration of “heading” a soccer ball. However, even with this mild impact the brain deforms. Bayley et al.'s conclusions were as follow: “When the skull decelerates, the brains center of mass continues to move, but the motion of the base of the brain appears constrained near the sellar and supra-sellar space. Tethering loads may be borne by the vascular; neural; and dural elements, which bind the brain to the base of the skull. Such anatomic structures might include the distal internal carotid arteries, the optic nerves, the olfactory tracts, the oculomotor nerves, and the pituitary stalk. All these structures pass through fixed bony or dural rings, which restrict their movement. These features attach to or penetrate the more mobile brain parenchyma. As a result, the brain begins to rotate about this region, while material anterior is compressed and material posterior is stretched by initial effects. As the brain rotates backward and upward relative to the skull, the superior-frontal surface of the brain appears to compress against the top of the cranial vault. Normal forces, tangential forces, and possibly tension in the bridging veins on the superior surface of the brain eventually arrest the rotation of the brain in front of the superior contact region is compressed and pushed forward. Behind the superior points of contact, the brain is elongated as the brains inertia pulls it backward and clockwise. Finally, behind the basal tethering region, material in the brainstem experiences shortening and shear as the posterior and inferior parts of the brain continue rotating downward and forward (p. 852).” Bayly et al. (2006) have also performed this type of modeling on the rat pup brain with similar findings of significant transient mechanical deformation of the brain.
Viano et al. (2005b) used a different approach by simulating movement within the cranium by a “finite element analysis using a detailed anatomic model of the brain and head accelerations from laboratory reconstructions of game impacts (p. 891)” based on National Football League (NFL) players who experienced on the field concussions that were videotaped. The exceptional innovativeness of this study was the ability to model the brain, including white and gray matter, the ventricular system, meninges, and in particular the falx cerebri and tentorium cerebelli along with the skull (most of these anatomical regions are shown in Figs. 1 and 2). In a number of those concussed, the initial strain occurred in the temporal lobe adjacent to the impact and then migrated though the temporal lobe to other brain regions. This is depicted in Figure 3. In all subjects concussed the largest strains that occurred in the migration of the brain deformation occurred in the fornix, midbrain and corpus callosum. Dizziness correlated with early strain in the orbital-frontal cortex and temporal lobe.
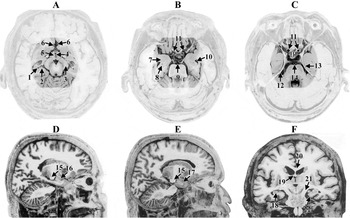
All views are post-mortem, adapted from Mai et al. (2004) (and used with permission from Elsevier). A, B, and C represent axial views where the highlighted area represents some of the common regions where the greatest strain effects were demonstrated in the Bayly et al. (2005) and Viano et al. (2005b) studies. (A) 1—hippocampus, 2-subiculum, 3-cerebral peduncle, 4-III ventricle, 5—hypothalamus, 6—anterior cerebral artery, (B) 7—amygdala, 8—hippocampus, 9—basilar artery, 10—temporal horn of the lateral ventricle, 11—internal carotid arteries, (C) 12—free-edge of the tentorium, 13—entorhinal cortex, 14—basilar artery, P = pituitary in the position of the sella turcica. D and E are sagittal views: 15—cerebral peduncle, 16—amygdala, 17 temporal pole and F is a coronal view: 18—hippocampus, 19—fornix, 20—corpus callosum, (21) cerebral peduncle, and 22—entorhinal cortex adjacent to the free-edge of the tentorium. Note the closeness of all of these regions and any movement, lifting or twisting of the brain at its base would simultaneously affect all of these structures.

From Viano et al. (2005b) published with permission from Lippincott Williams & Wilkins. The model on the left represents a coronal (top) and axial (bottom) view of the tagged brain model with the ventricle in pink and the skull encasing in yellow. The left hand column represents the baseline, where no movement occurred; notice the midline is vertical in the coronal plane and straight in the axial plane. Time in msec is shown on the x-axis. By 25 msec the model indicates that this player's brain had a maximal shift, where it is evident that there is particular distortion in the medial temporal and hypothalamic region. This was from a player concussed on a kick return who had brief LOC, and PCS symptoms of headache, fatigue, dizziness, and photophobia and sleep disorder as physical symptoms. Note that the modeling of this subjects brain would involve all of the structures identified in Figure 1, and indeed, the high strain findings modeled in this subject supported such a locus of injury (see Appendix 1B of the Viano et al., 2005b paper that detail individual characteristics of the subjects).
The Viano et al. (2005b) modeling study found, in general, excursions of the concussed brain to be between 3–6 mm at 24–26 ms post-impact. Of particular importance to neuropsychology is that this modeling shows 4–5 mm displacements of the hippocampus, caudate, amygdala, anterior commissure, and midbrain (again refer to Fig. 2). In addition to these brain regions showing significant displacement, they also related to various cognitive and physical symptoms from concussion in this group of NFL players. Also, increased strain at the level of the hypothalamus was associated with at least transient cranial nerve symptoms.
The models described earlier occur in well-controlled experimental conditions. Obviously, high speed impact head injuries are not a controlled experiment and likely involve more significant pressure and shear-strain forces than what are seen in sports concussion (Bradshaw et al., 2001; Zhang et al., 2006a). Regardless of these factors, the same brain regions as described earlier and as shown in Figures 1 and 2 are likely involved in all concussions, just a matter of degree. Similarly, much of the cognitive and neurobehavioral symptoms of concussion can be explained by the involvement of the brain regions highlighted in Figures 1 and 2. In these Figures, note the proximity of the medial temporal lobe to the midbrain, the fact that the free-edge of the tentorium makes contact with the medial temporal lobe and midbrain as well as the nearness of the basal forebrain to these regions along with the hypothalamus, hypophysis and pituitary stalk, and the arterial vasculature. So, within a few centimeters are critical brain structures that, if affected, could represent the structural basis to many symptoms associated with concussion.
Pathophysiology of Concussion
Iverson (2005) and Hovda (2004) provide an excellent and detailed reviews of the pathophysiology of concussion, which need not be re-elaborated here. Whereas initiated by immediate biomechanical forces, as describe above, much of the pathology of acute concussion is believed to be transient biochemical induced neurotransmitter disruptions initiated within 25–50 msec of impact. Tensil forces also disrupt the cytoskeletal status of the axon and its ability to function, including disrupted axonal permeability and transport (Povlishock & Katz, 2005). Disrupted cytoskeletal architecture, renders cells less functional and may have widespread effects on the injured brain (Hall et al., 2005), albeit transient in concussion.
Since the Iverson (2005) and Hovda (2004) publications there are several important studies that add to our understanding of the potential microscopic pathology that can occur from concussion. Zetterberg et al. (2006) examined cerebrospinal fluid (CSF) taken by from lumbar puncture in 14 amateur boxers 7 to 10 days and 3 months after a bout compared to matched controls without any contact. They used several markers of neuronal and astroglial injury that can be readily detected in the CSF, finding significant indicators of neuronal injury byproducts in CSF that were positively related to the actual number of hits during a bout, most apparent in the initial samples taken after the amateur boxing contest. None of the boxers received a knock-out punch and likely did not meet any of the behavioral criteria for concussion. This study confirms the presence of acute pathological changes in the brain that can occur from boxing, in blows to the head that are below the threshold for producing what behaviorally would be classified as a concussion in these conditioned athletes.
Using a different approach, Zhang et al. (2006b) examined conventional MRI along with diffusion tensor imaging (DTI) in a group of professional boxers. While the majority had normal clinical imaging, 7 of the 42 examined had abnormal white matter findings, which should not be evident in an otherwise young, healthy subjects (Hopkins et al., 2006). More importantly, even those without clinical abnormalities as a group exhibited DTI differences from their matched controls, suggesting subtle white matter abnormalities, particularly at the level of the corpus callosum. Recall that Viano et al. (2005b) showed that the corpus callosum was one of the brain regions receiving the biggest strain effect in concussion. Similarly, Chappell et al. (2006) using DTI methods demonstrated similar white matter pathology in a group of 81 professional boxers. These studies focused on professional boxers without known neurological impairment, otherwise they would not be boxing, and show that sensitive MRI methods do detect with a higher frequency abnormalities of white matter. Along these lines Cohen et al. (2007) have shown MR spectroscopic and subtle brain volume loss in mild TBI. Such imaging findings demonstrate that pathological changes in brain parenchyma can be detected in mild TBI using contemporary neuroimaging methods.
Bigler (2004) demonstrated hemosiderin and residual inflammatory reaction in the post-mortem brain of an individual with PPCS, where the autopsy was performed seven months post-injury. Similar findings were observed in a post-mortem of a professional football player who had developed cognitive decline later in life (Omalu et al., 2005). Combining the imaging and neuronal injury biomarker studies discussed earlier, with the Bigler (2004) and Omalu et al. (2005) post-mortem studies provide indisputable evidence that structural pathology can be present in mTBI. Additionally, these type of hemorrhagic lesions can be observed with specialized high-field MRI studies (see Ashwal et al., 2006; Scheid et al., 2006) as shown in Figure 4. As such, some aspects of the so-called “traceless injury” of concussion are being revealed with newer techniques.

This 12 year-old male had sustained a concussion in a skate-boarding accident. Eyewitness accounts estimate LOC to be approximately 7 minutes, but in the ER the patient was alert and not amnesic. However, because of the positive LOC a CT scan was performed (A), followed by the more routine GRE sequence (B) which revealed only a hint of hemosiderin deposition, however, the susceptibility-weighted sequence (C) clearly demonstrated multiple foci of hemosiderin deposition (see arrows). (Reproduced by permission from Jill Hunter, M.D., Texas Children's Hospital, Houston, Texas).
What is the significance of these pathological residua in those concussed, even when ostensibly reparative and restorative mechanisms return function to apparent baseline? Are there still potential sequelae that can be elicited and are these expressed overtime? Do these lesions relate to neuropsychological function? What Gronwall and Wrightson (1975) demonstrated years ago suggested that concussion may not be as benign as Miller (1961) had implied, but may be very dependent on the cognitive demands placed on a patient. Routine cognitive tasks may be unaffected, whereas more complex functions affected. This has been revisited more recently by Chen et al. (2003) using functional brain imaging in a small group of subjects (N = 5) who had sustained concussion, only two of whom had brief LOC (less than 2 minutes). In this study the concussed patients, none of whom were in litigation, all had neurobehavioral symptoms of PPCS, but their resting PET metabolism did not differ from controls. However, when given a spatial working memory task to perform, differences in regional cerebral blood flow were detected in prefrontal cortex in PPCS subjects. In other words, unless a significant cognitive demand was placed on the subject that required more than typical cognitive effort, no differences could be determined. Similarly, Bernstein (2002) demonstrated that by increasing the complexity of a dual task involving auditory and visual discrimination and measuring evoked responses that those with a history of concussion but ostensibly no residual complaints could be differentiated from controls (Dockree et al., 2006a).
Moreover, confirmation of the likely residual pathology from concussion is clearly demonstrated in the second-injury circumstance, where a prior concussion increases the likelihood of a second concussion and greater morbidity of the second concussion in both human and animal studies (Huh et al., 2007; Longhi et al., 2005; Manville et al., 2007; Moser et al., 2005; Omalu et al., 2005; Pellman et al., 2004; Wall et al., 2006). The most straight forward explanation of the pathology of the second injury concussion is that the first concussion is simply not benign, but that the brain adapts quickly to the injury in most cases. It should be noted that there is some controversy over the second injury hypothesis (Iverson et al., 2006b; Schnadower et al., 2007) and much more animal and human research is needed to fully understand this phenomena (Laurer et al., 2001). From a clinical management standpoint, repeated concussions are the basis for recommendations to retire from sports (Cantu, 2003) and reported to be related to the presence of neuropsychiatric symptoms in professional North American Football players (Guskiewicz et al., 2007).
While petechial hemorrhage associated with concussion has been well documented neuropathologically for decades (Ashwal et al., 2006), the shearing phenomena may only be part of the pathological story of vascular injury in concussion. A most intriguing animal study by Ueda et al. (2006), inducing what would be at least a moderate brain injury, has shown that the perivascular nerve network is injured in TBI as well. It is often overlooked that there is a neural regulation of blood vessels and blood vessels can contract and expand under neurogenic control. In fact, it is the dispersion of blood in response to autoregulation and localized activation that is at the basis of functional neuroimaging. If a blood vessel has a subtle abnormality in its ability to regulate regional flow, this may contribute to the neuropsychological sequelae expressed in a concussed individual. This remains to be investigated and represents speculation at this time. Thus, in TBI the same mechanisms that stretch the neuron can stretch the blood vessel and this may impair the neurogenic response of the blood vessel. Thus, the functional neuroimaging findings in concussion may not just be a consequence of brain parenchymal injury, but vascular and blood-brain barrier disruptions (Korn et al., 2005).
Along these same lines, is how the peri-vascular spaces that house cerebral vasculature are affected by injury, because much of the surrounding tissue is white matter. Numerous studies have shown the vulnerability of white matter damage in TBI (de la Plata et al., 2007; Inglese et al., 2005a; MacKenzie et al., 2002) have all shown that in mTBI increased frequency of dilated perivascular spaces, changes in white matter volumetry and chemical composition occur and relate to persistence of symptoms. Significant inflammatory reactions and hemosiderin deposits occur in the perivascular space in response to injury and their presence is considered a marker of white matter injury (Beschorner et al., 2002; Konsman et al., 2007). What is potentially so important about these observations is that inflammatory reactions that may originally injure white matter parenchyma, at least experimentally, have been shown to disrupt dopaminergic function (Roy et al., 2007), which heuristically, could be the basis for some of the neuropsychiatric symptoms associated with damage to white matter.
Another neuropathological complexity that is only beginning to be understood is the individual differences and heterogeneity of injury to individual cells (Buki & Povlishock, 2006; Reeves et al., 2005; Singleton & Povlishock, 2004). This too may be under genetic control where individual differences to injury susceptibility relates to outcome. It just may be that certain neurons are more susceptible to injury and certain injury forces or dynamics than others (Park et al., 2006).
There are other biomarkers of injury that have also been examined in human mTBI. For example, Stalnacke et al. (2005), using a blood biomarker of brain injury, serum concentrations of S-100B and neuron-specific enolase, found that S-100B levels during the acute phase of mTBI related to long-terms sequelae. S-100B findings have not been universal in mTBI (see also Bazarian et al., 2006a; Bazarian et al., 2006b; De Kruijk et al., 2002; Savola & Hillbom, 2003) and these observations are but some of the first. The level of initial CSF tau, a microtubular binding protein, believed to be a marker of axonal injury, correlates with outcome in severe TBI (Ost et al., 2006), but it has not been systematically studied in mTBI.
Functional Neuroanatomy of Concussion and PPCS
In concussion, regardless of the definitional criteria used as outlined in Table 1 and the variability in clinical presentation, it is clear that 4 features dominate concussive symptoms—(1) brief alteration in consciousness or neurological function with at least acute changes in mentation and speed of processing; (2) physical symptoms of headache, dizziness and/or vertigo along with increased fatigability; (3) impairments in short-term memory, attention and concentration (particularly for multi-tasking); and (4) increased likelihood for changes in mood and emotional function. Where and how can these symptoms be integrated in understanding the functional neuroanatomy of concussion? The assumption is that there must be a common origin to these symptoms.
Figure 1 is a sagittal MRI view of the brain. The average adult brain weighs somewhere between 1150 and 1450 grams (2.5–3 pounds), with most of that weight located in the cerebrum, above the cerebellum in the figure. The anterior aspect of midbrain region of the upper brainstem is comprised of the cerebral peduncles which house all of the major ascending and descending white matter pathways connecting the cerebrum with the periphery of the body and the connections between the cerebrum and the cerebellum. In an earlier review, these anatomic regions, pathway and structures have been outlined in detail (Bigler, 2007). As can be clearly visualized in the Figure 1, the midbrain at the level of the cerebral peduncle is small, opposed to frontal-occipital linear dimension, and in the vertical position the tegmental aspect of the upper midbrain “rests” on or is adjacent to the dorsum sellae and the anterior clinoid, partially shown in Figures 1 and 2. Just in front of the tegmentum is the hypophyseal fossa that house the infundibulum (or pituitary stalk), the neural connection between the ventral hypothalamus and the pituitary, situated in the sella turcica. Immediately lateral to the cerebral peduncle is the carotid groove of the sphenoid bone wherein the internal carotid artery ascends into the brain to form the anterior and medial cerebral arteries. Next, moving laterally just past the carotid groove is the inner edge of the greater wing of the sphenoid and the beginning medial surface of the temporal lobe (see Fig. 2). The entrance of internal carotid into the cranium through the carotid canal occurs just adjacent to the midbrain. What is particularly interesting about this region of the brain is that the tentorium cerebelli extends from a covering of the cerebellum to attach at the junction of the clinoid and lesser wing of the sphenoid. As the tenortium projects to its clinoid-lesser wing of the sphenoid connection, the lateral surface of the upper brainstem touches the “free edge” of the tentorium cerebelli, and just on the other side of the tentorium at this level is the medial surface of the temporal lobe, where the peririhinal and entorhinal surfaces also touch the “free edge” of the tentorium (Bigler, 2007; Van Hoesen et al., 1999). What is also of particular interest with regards to consciousness is that arterial branches of the posterior circulation of the brain actually cross the free edge of the tentorium and these arterial branches supply blood to the brainstem (Blinkov et al., 1992).
Biomechanics of concussion inform us that concussions are more likely to occur if there is some rotational force present (Fijalkowski et al., 2006; Viano et al., 2005a; Vorst et al., 2007). Returning to this midbrain region of the brain, if there is any stretching and/or rotational force at this level, note what occurs: the upper brainstem stretches across the clinoid and lesser wing of the sphenoid, with its lateral margins potentially striking the free-edge of the tentorium, the pituitary stalk stretches disrupting hypothalamic-pituitary connections, the internal carotid stretches against the carotid canal and posterior circulation to the brainstem is also disrupted, the medial temporal lobe strikes the lateral surface of the free-edge of the tentorium as well as the medial wall of the sphenoid. Just in from this medial surface of the temporal lobe are the amygdala and hippocampus, with the hippocampus giving rise to the fornix that not only connects with the anterior thalamus via the mammillary body but also to the septum and pituitary (McDonald et al., 2006). Just anterior to the hypophysis-hypothalamic region is the basal forebrain; just posterior are the mammillary bodies. So at one level, if there is slight mechanical deformation either in terms of compression or uplift and particularly if rotation occurs, there are putative functional neuroanatomical connections disrupted for consciousness (upper brainstem, reticular activating system), memory (mechanical compression of perirhinal and entorhinal cortices disrupting input to the hippocampus or hippocampal output via the fornix and its connection with the anterior thalamus and cingulate), emotional regulation (medial temporal lobe and basal forebrain), post-traumatic migraine (stretching the internal carotid and all vasculature that forms the circle of Willis as well as stretching/irritation of the dura and other vessels) and fatigue as well as hormonal changes secondary to hypothalamic-pituitary disruption.
Indisputably, clearly demonstrated immediately after concussion (Barrow et al., 2006a; Barrow et al., 2006b), even in those who go on to fully recover, is slow speed of processing (Crawford et al., 2007; De Monte et al., 2005). Speed of processing is dependent on the integrity of white matter pathways maintaining their optimal inter-connectiveness. Returning to the biomechanical deformation effects reviewed above, long-coursing axons are going to be more vulnerable, particularly interhemispheric connections, especially the corpus callosum and anterior commissure (Cecil et al., 1998; Holshouser et al., 2006; Inglese et al., 2005b; Mathias et al., 2004; Wilde et al., 2006a; Wilde et al., 2006c). Thus, neuropsychological tasks that require interhemispheric integration and/or multiple intracortical connections often show differences in the form of slowed responding, even in those with mTBI (Mathias et al., 2004)
So, the hypothesis put forth in this section is that the biomechanics of brain injury simultaneously disrupt neurological function in the upper brainstem, pituitary-hypothalamic axis, medial temporal lobe, and basal forebrain concomitant with irritative injury to the vasculature and meninges, which gives rise to the symptoms observed in the post-concussive state and the neuropsychological sequela associated with such an injury. How rapidly these neural, dural, and vascular areas return to homeostasis or recovery from some adaptive mechanism or do not recover, provides the biological basis for the symptoms expressed.
Animal Models of Concussion
The advantage of animal models is the controlled environment where the reproducibility of an adverse effect can be tested, in this case a concussive brain injury. There have been numerous animal models of brain injury over the years (Leker et al., 2002), but most focused on what would be moderate-to-severe brain injury with focal cortical impact, producing not only focal brain injury but diffuse injury readily demonstrated by histological analysis. It has been challenging to develop an animal model of concussion that mimics human concussion, because of a host of differences associated with brain morphology, skull-brain interface, and species differences (Leker et al., 2002).
Nonetheless, several excellent animal models of concussive injury have recently been established (Gurkoff et al., 2006; Henninger et al., 2005; Milman et al., 2005; Tang et al., 1997; Tashlykov et al., 2007; Ucar et al., 2006; Yoshiyama et al., 2005; Zohar et al., 2003, 2006). For example, Henninger et al. (2005) modifying methods of Tang et al. (1997) have used a weight drop device to the exposed skull that replicates human concussion. As stated by these researchers, “immediately after impact, all TBI animals lost their muscle tone and righting reflex response (p. 450)” but it shortly returned. This is analogous to what is observed acutely in sports concussion (McCrory & Berkovic, 2000). After reflex recovery the concussed rats behaved “normally” in comparison to sham controls. In this study, memory was assessed using the Morris Water Maze (MWM), where the concussed animals also showed no differences from controls in ability to swim and other species typical behaviors. Thus, in terms of ordinary rat behavior, function returned without discernable abnormality following concussion. However, given time to heal from the minor surgery to expose the skull, the concussed animals exhibited memory deficits on the MWM when assessed nine days post-injury. This study also included high-field MRI which was negative. However, histology demonstrated several pathological changes including a reduction in the number of cortical neurons as well as in the hippocampus. A limitation of this study is that it only examined memory nine days post-injury but Milman et al. (2005) and Zohar et al. (2003, 2006) using somewhat similar methods, but in mice, have demonstrated these type of persistent cognitive differences in concussed animals for longer periods of time post-injury. Gurkoff et al. (2006) have demonstrated this in rats with a fluid percussion injury model and Tashlykov et al. (2007) have shown apoptotic changes in cerebral cortex and hippocampus using this weight drop technique as well.
So animal models of concussion do support the notion that persistent cognitive deficits can occur, although not all studies have found lasting effects (Gaetz, 2004; Leker et al., 2002). The difference between those studies that find persisting symptoms and those that do not is probably the severity of the concussion. For example, in the Tashlykov et al. (2007) study pellets of incremental weight from five to 30 g were dropped on the head of mice under light ether anesthesia. Of particular interest in this study is that none of the weight amounts produced any discernable change in the species typical behavior of the mice once recovered from the ether anesthesia, yet related to the weight amount of the pellet pathological changes were proportional to the impact. The 5 g weight drop was insufficient to produce any detectable pathological changes. A minimum of 10 g was necessary for showing pathological neuronal changes, but 15 g was necessary to initiate apoptotic changes. Thus, the threshold to produce injury varies depending on what pathological changes are under investigation and whether a certain injury threshold has been reached.
IS BRAIN INJURY ON A CONTINUUM: CONCUSSION → SEVERE TBI?
In examining the post-mortem brain of several human subjects who had sustained a “mild concussion,” but died for reasons other than the head injury, Blumbergs et al. (1994) demonstrated presence of axonal injury, particularly in the fornix. Blumbergs et al. (1995) in a follow-up study demonstrated that the microscopic pathology was on a continuum from mild (GCS of 13–15) to severe (GCS of 3–8), again demonstrating the susceptibility of the fornix. As shown by Viano et al. (2005b), the fornix is distinctly vulnerable to the stress/strain effects of concussion and is a common area of damage in moderate-to-severe TBI, as visualized using MRI (Gale et al., 1995; Tate & Bigler, 2000; Tomaiuolo et al., 2004), where the degree of atrophy is related to severity of injury (Bigler et al., 2006; Tate & Bigler, 2000; Tomaiuolo et al., 2004; Wilde et al., 2006b). Because the fornix is a white matter structure containing projecting axons from the hippocampus, disruption in fornix integrity likely relates to the concussive effects of disrupted short-term memory, at least transiently.
At the histopathological level, severity can be graded by the degree of cell loss, cytoskeletal changes, presence of inflammatory cellular reaction, biochemical markers of cell damage or death, etc. and all seem to relate to severity on some continuum (Anderson et al., 2003; Vorst et al., 2007). Taken together, in well controlled animal models there is a continuum associated with severity of impact injury supporting the contention that injury is on a continuum (Gurkoff et al., 2006; Igarashi et al., 2007; Kharatishvili et al., 2006; Maegele et al., 2005; Ucar et al., 2006). Understanding this continuum means that at the mildest level of brain perturbation there may, in fact, be no lasting effect. However, once a threshold is reached, lasting sequelae begin to occur (Zhu et al., 2006).
Human neuroimaging studies also support the concept of continuum of injury. For example, a linear relationship with cerebral atrophy relates to injury severity measures such as GCS, PTA, and duration of LOC (Bigler et al., 2006; Wilde et al., 2006b). Likewise, complicated mTBI is more likely to have positive neuroimaging findings (Levine et al., 2006; McAllister et al., 2001; Vorst et al., 2007) and significant residuals (Kennedy et al., 2006). If boxing is considered a model for detecting “pre-clinical” or asymptomatic brain injury, recent diffusion tensor imaging studies have demonstrated abnormalities in boxers (Chappell et al., 2006; Zhang et al., 2006b). Thus, animal and human studies support the contention of injury on a continuum, implicating that understanding the variables that relate to severity of injury are likely very important in understanding neuropsychological sequelae (see Wilde et al., in press; Lewine et al., 2007).
VULNERABILITY OF THE MEDIAL TEMPORAL LOBE AND IN PARTICULAR, THE HIPPOCAMPUS
Elsewhere, I have reviewed research demonstrating that the brain-skull interface in the anterior and middle cranial fossa is a major factor for the vulnerability of these regions in TBI (Bigler, 2007). Potentially the most critical structure injured for neuropsychological sequelae in TBI is the hippocampus and its afferent and efferent connections (Wilde et al., 2007). The Viano et al. (2005b) study demonstrated that the typical deformation of the hippocampus to be 4–6 mm in concussion associated with professional football. Numerous human and animal studies have demonstrated the vulnerability of the hippocampus (and fornix) to injury in TBI (Bigler et al., 2006; Geddes et al., 2003; Royo et al., 2006; Tashlykov et al., 2007; Tasker et al., 2005; Wilde et al., 2006b) and functional neuroimaging studies using SPECT also demonstrate medial temporal lobe hypoperfusion in mTBI (Gowda et al., 2006). Thus, given the location of the hippocampus in the medial temporal lobe and its connection and location to the fornix, these brain regions are key to understanding PPCS neuropsychology, and should be the focus of intense neuropsychological investigation.
LIMITATIONS OF NEUROPSYCHOLOGICAL RESEARCH TO ADVANCE THE FIELD
The Litigation Conundrum: Forensic Implications for Clinical Neuropsychology
From the anatomical and pathophysiological discussions earlier, it is plainly evident that the brain is at least momentarily and transiently injured in concussion but for the majority of those injured persistent sequelae do not occur. Because animal models have demonstrated that lasting negative effects can occur with concussion (see Tashlykov et al., 2007), it is reasonable to assume that PPCS will exist in some individuals. It is in these individuals that neuropsychological research needs to direct its best and most unbiased research efforts. Unfortunately, as pointed out by the World Health Organization's task force on mTBI, poor research designs and the cross-sectional nature of many of the studies on this topic, restrict generalizations of the findings (Carroll et al., 2004a; Carroll et al., 2004b). What can be done to correct short-comings of research in this area?
More than 40 years after Miller (1961) wrote about concussion and “compensation neurosis”. Kertesz and Gold (2003), reviewing outcome from concussion make the following statement: “the involvement of insurance claims, litigation, and the expense of rehabilitation makes this area very contentious (p. 629).” Belanger et al. (2005) performed a meta-analysis of 39 studies involving 1463 cases of mTBI assessing clinical neuropsychological test findings. Their findings were similar to what has also been described by Binder et al. (1997), Frencham et al. (2005), and Schretlen & Shapiro (2003), implicating short-term, but not necessarily long-term neuropsychological effects, except for those cases who were in litigation, where either “stable or worsening of cognitive functioning over time (p. 215) was observed.” Mooney et al. (2005), in a university based rehabilitation service, examined those with “disappointing recoveries” and observed that “in cases of poor recovery after mTBI where compensation or litigation may be a factor, most of the variance in recovery seems to be explained by depression, pain, and symptom invalidity (p. 975) .” With regards to symptom invalidity, Loring et al. (2007) reported 20% of subjects including those with history of head injury who were evaluated in a University-based clinical assessment laboratory but who were also in litigation did not pass symptom validity testing (SVT). Plainly, presence of litigation is a major confound in research in mTBI and its presence in research studies has likely obscured the true effects of concussion, including PPCS. Also, whenever analyzing group data, if all subjects with concussion are examined at a particular time period, the effects on individual subjects who may be symptomatic get washed out by the total group effects (Iverson et al., 2006a; Kent, 2007; McHugh et al., 2006; Sterr et al., 2006). This is a very important point, because few studies compare symptomatic versus non-symptomatic subjects who have been concussed and those who do, find those who are symptomatic to have greater neuropsychological impairment (Collie et al., 2006; Iverson et al., 2004; Sterr et al., 2006).
The fact that the litigation process is adversarial and that neuropsychological testimony occurs on both sides of the legal argument, raises the specter of potential bias in what has been written about PPCS depending on the type of forensic work an author may participate in. If one is exclusively retained in legal settings for one side or the other in a legal matter, that could have a bearing on what is studied and reported (Racette et al., 2006). The legal side that retains a clinician or researcher may influence directly or indirectly what is published by that individual (Bigler, 2006). For example, it would be difficult for the individual in private practice whose sole income is derived from their forensic work and consistently retained by the defense to publish on the subtle sequelae of PPCS, including its lasting and enduring adverse effects. Oppositely, but just as likely, the clinician who is exclusively retained by the plaintiff's side is unlikely to publish on the “myth” of PPCS.
Neuropsychological research from countries that do not have the kind of litigation and medical care system that the United States has may provide important information about PPCS, if the proper large scale studies are done. There are cultural differences in the expression of whiplash associated disorders (WAD) (Obelieniene et al., 1999), and the same may be expected in PPCS. Incomplete effort is another major factor contaminating any study looking at long-term neuropsychological sequelae of concussion (Ross et al., 2006a; Ross et al., 2006b), which represents a topic of its own for review (Iverson & Binder, 2000).
Ecological Validity of the Clinical Neuropsychological Approach
Ecological validity of neuropsychological assessment remains an ever present concern (Chaytor et al., 2006; Moritz et al., 2004; Odhuba et al., 2005; Wood & Liossi, 2006). As an example, the antemortem clinical neuropsychological testing in the concussed patient previously described who met PPCS criteria and who at autopsy had verified pathology of brain injury, was all normal yet this individual had “real-world” difficulty running his business, problems not evident before his injury (Bigler, 2004). Standardized paper-and-pencil tests typically conducted in the sterile laboratory may simply not tap the cognitive symptom being experienced by the individual with PPCS. This very point has been made by Collie et al. (2006) in determining which kinds of measurements are most sensitive in detecting problems in those who remain symptomatic after concussion. Obviously, cognitive skills, in particular working memory and executive function, can place much higher demands on neural integrity in the real world than what can be assessed by any current clinical neuropsychological technique in the laboratory.
Assessment in sports concussion has recognized the need to move beyond traditional neuropsychological assessment with the development of more tailored assessment tools in the athlete with concussion (Broglio et al., 2007; Parker et al., 2007). Such assessments are also taking advantage of computerized and virtual assessment techniques as well as the ability to automate the assessment (Cernich et al., 2007; Iverson et al., 2005; Schatz & Putz, 2006; Slobounov et al., 2006). Likewise, various cognitive neuroscience measures either by themselves or combined with functional neuroimaging methods hold great promise for more accurate assessment of the effects of TBI on behavior and cognition (Bergemalm & Lyxell, 2005; Casson et al., 2006; Chan, 2001; Chen et al., 2007; Cicerone et al., 2006; Dockree et al., 2006b; Jantzen et al., 2004; Mendez et al., 2005; O'Keeffe et al., 2007a; O'Keeffe et al., 2007b; Scheibel et al., 2007; Suh et al., 2006). These types of studies applied to PPCS will likely advance the field rather than another round of testing with traditional “clinical” neuropsychological measures (Heitger et al., 2004, 2005, 2006).
Confounding Factors That Must be Considered in the Design of PPCS Studies and the Accurate Determination of Neuropsychological Sequelae
The fact that the eight symptoms of PCS [i.e., (1) becoming fatigued easily, (2) disordered sleep, (3) headache, (4) vertigo or dizziness, (5) irritability, (6) anxiety, depression or affective lability, (7) changes in personality, and (8) apathy or lack of spontaneity] as outlined by DSM-IV (pp. 704–705) all overlap such that all coexist with a myriad of other medical and psychiatric diagnoses, underscores how complicated the design of the ideal study has to be to truly assess PPCS. For example, Iverson (2006) points out the commonness of misdiagnosing PPCS when the symptoms are really driven by depression and how depression can be misattributed to concussion (Chamelian & Feinstein, 2006; Meares et al., in press). In fact every PPCS symptom can occur independent of a head injury (Iverson et al., 2007). Also, a threshold issue exists where symptoms have to rise beyond a baseline before PPCS can be diagnosed (Chan, 2005). Post-traumatic pain correlates with presence of PPCS (Sheedy et al., 2006); and pain has its own set of correlates, by itself, potentially affecting cognitive performance and emotional status (Alfano, 2006; Karp et al., 2006). None of this even addresses the complexity of WAD, as already mentioned, and WAD pain-related problems (Holm et al., 2006; Johansson, 2006; Zumsteg et al., 2006), nor post-traumatic headaches (Lew et al., 2006; Weiss et al., 1991) which are commonplace in concussion, especially those caused by MVAs.
Not only is the brain concussed, but also other organs such as the eye, inner ear, and soft internal organs (Frater & Haindl, 2003; Keane & Baloh, 1992; Nolle et al., 2004); and injury to these organs can be a source of symptoms. With regards to organs of the head, vertigo, dizziness, tinnitus, and ocular disturbance are commonplace; and they relate to cognitive sequela associated with mTBI (Suh et al., 2006). Presence of any of these symptoms may confound the neuropsychological presentation and sequela of the mTBI patient but are rarely controlled. What is particularly important about pain, regardless of its source, is that pain changes the functioning of the brain, demonstrated by both structural as well as functional imaging (Schweinhardt et al., 2006). Also, the nature and extent of early medication treatment in those who sustain mTBI, may also relate to who develops PPCS (Meares et al., 2006).
Fatigue is a common and persistent problem in PCSS (Stulemeijer et al., 2006; Ziino & Ponsford, 2006), and it too has its own set of neurochemical, neuroimaging, and neuropsychological differences (de Lange et al., 2004; Kozora et al., 2006). The same can be said about the co-occurrence of PTSD in those involved in accidental injury or assault as the source of their concussion (Bryant, 2001; Creamer et al., 2005; McCauley et al., 2001) and the role of stress hormones in the behavioral response to injury (Sojka et al., 2006). PTSD alone has its own unique effect on neuropsychological performance (Vasterling & Bremner, 2006; Vasterling et al., 2006; Veltmeyer et al., 2005). Even for those who do not develop PTSD, being in an accident (Mayou & Bryant, 2002) or an assault (Johansen et al., 2006) or just sustaining a brain injury (Prigatano et al., 2005) is stress producing.
Mooney and colleagues have documented that many with persistent symptoms following concussion meet criteria for conversion disorder (CD (Mooney & Speed, 2001). However, the neurobiology of CD, including neuroticism, is starting to emerge and it may not be as “functional” as believed (Allet & Allet, 2006; Atmaca et al., 2006; Ghaffar et al., 2006; Schonfeldt-Lecuona et al., 2006; Stonnington et al., 2006; Ward et al., 2003; Wright et al., 2006). Theories and functional neuroimaging studies of CD imply the involvement of limbic regions, inferior frontal and medial temporal lobe structures, the very regions most likely injured in TBI. Is there an increased prevalence of conversion disorder in individuals concussed because these areas are injured? Neuropsychology should be exploring the potential neurobiology of this observation, not merely writing this off as a mere functional manifestation of concussion (Ashman et al., 2006). Recently, Wood (2005) has put forth a diathesis-stress model as a beginning attempt to describe these relationships.
It has long been known that pre-morbid factors predispose those with history of neuropsychiatric disorder to be more likely to experience PPCS once concussed (Karzmark et al., 1995; Ponsford, 2005). As such, any study that examines PPCS that does not take into consideration pre-morbid factors likely overlooks important and relevant information that may contribute to the disorder.
It is interesting that only recently has research begun to examine the role of pituitary injury in TBI to functional outcome, even in concussion (Acerini et al., 2006; Kelestimur, 2005; Kelly et al., 2006; Tanriverdi et al., 2007). As shown by the Bayly et al. (2005) and Viano et al. (2005b) studies, the hypothalamic-pituitary axis is particularly vulnerable to physical strain in concussion. Pituitary dysfunction can be associated with many of the same symptoms as seen by PPCS (Casanueva et al., 2006; Powner et al., 2006), yet this has not been systematically investigated in PPCS. This is particularly important because of some pituitary associated physical and neuropsychiatric symptoms are treatable.
Similarly, the basal forebrain resides just anterior to the hypothalamus housing important nuclei and pathways for cholinergic innervation of the brain. The basal forebrain is another region that sustains significant strain effects in biomechanical modeling of concussion and in moderate or greater injury, is consistently damaged (Bigler, 2005; Conner et al., 2005). However, this region has never been systematically examined in PPCS.
The elegant reconstruction of concussion by Viano et al. (2005b) clearly demonstrates that each concussion places unique stress and strain on the brain. Just as clear from this research is that no two concussions are identical in terms of how the brain is impacted. So if one does not take into consideration the impact and physical dynamics of the injury and subject characteristics (including genetics), neuropsychological sequelae could vary widely with regards to the brain regions most likely injured even though all subjects had sustained a “concussion”. Unfortunately, for most research on PPCS such information has never been obtained and this has never been systematically investigated other than in sports concussion. For example, concussion in MVA victims may be different depending on whether it was a front, side or rear impact, whether the car spun, rolled or flipped, etc., the type of car driven, and how and what seat belts were worn, etc. (Elliott et al., 2006). To date this heterogeneity in injury dynamics has simply been overlooked and all such subjects are merely lumped into a single category yet these injury dynamics may make a significant difference in how and where the brain is injured, differences that may be critical in the expression of PPCS.
The prospective design used by the McCrea et al. (2003) study is the proper prototype and standard that should be sought in studying PPCS in non-sports related concussion. To date this has not been done and therefore there are no large-scale, long-term prospective studies of non-sports related concussion that have been conducted. However, the study by Jackson et al. (2007) is a first attempt to accomplish such, where they examined their university-based Trauma I center registry for a single year and identified 97 adult trauma patient survivors from their ICU who had negative CT scan for intracranial hemorrhage, ostensibly eliminating those with obvious severe head injury. Within 12 to 24 months post-discharge, they were able to follow-up with a comprehensive neuropsychological battery of tests on 58 of these subjects and presence of concussion was associated with poorer neuropsychological outcome.
CONCLUSION
From a neuropathological standpoint, this review demonstrates that concussion can lead to structural damage. From the biomechanics of concussion, the vulnerability of the upper brainstem, hypothalamic-pituitary axis, medial temporal lobe and basal forebrain and long-coursing white matter fibers, particularly involving the corpus callosum and fornix are the brain regions most likely to give rise to post-concussive symptoms.
Confusion in the literature on this topic comes from differences in terminology and definitional standards as well as poor research designs where small sample sizes, samples of convenience, selected clinical sub-samples and research that may have an agenda behind it has created serious interpretative problems with regards to the neuropsychology of concussion and its sequelae. Prospective studies of concussion where large trauma centers assess, follow and tract patients with concussion and follow such a cohort prospectively using uniform and more ecologically valid cognitive assessment protocols simply have not been done. In such a group it would be reasonable that additional data could be obtained that would provide more information about the biomechanics of injury and a host of other medical and demographic factors, including attempts to be establish pre-injury level of function. In one of the largest reviews of mTBI, the WHO task force that reviewed mTBI literature up to 2004 concluded that mTBI research is “of varying quality and causal inferences are often mistakenly drawn from cross-sectional studies (p. 84,” (Carroll et al., 2004a), see also (Ragnarsson, 2006) The only correction for this gaffe in the neuropsychology of concussion, and potential long-term sequelae of PPCS, will be large, unbiased prospective studies that address the issues raised in this review. The importance of understanding this more accurately and completely is the fact that concussion is reportedly the most common of all neurological injuries and this is also true of the Iraq and Afghanistan war (Das & Moorthi, 2005; Okie, 2005; Warden, 2006; Warden & French, 2005), where unofficial estimates place the numbers in the tens of thousands (Bob Woodruff Reports. February 27, 2007: www.abc.com), potentially as high as, “1 out of every 10 returning service men and women” [p. 16, American Academy of Neurology News, 20(6), 2007]. Neuropsychology needs to better understand PPCS and this review offers a number of very testable hypotheses for future research.
ACKNOWLEDGMENTS
The information in this manuscript and the manuscript itself is new and original and has never been published whether electronically or in print. The author also acknowledges the support in part by the National Institutes of Health federal grant 1 R01 HD048946-01A2 and the Ira Fulton Foundation. The technical assistance of Tracy Abildskov and Craig Vickers and the editorial assistance of Jo Ann Petrie are also gratefully acknowledged.







