Introduction
Arterial switch operation is the preferred surgical option in children with transposition of the great arteries: with intact ventricular septum. The risk of arterial switch operation is increased in these subset of patients who present late due to the inability of the left ventricle to support the systemic afterload following surgery, and this significantly affects early survival. Conventionally, left ventricle regression is assumed after three weeks of age. Reference Nicholas, Blackstone, Doty, Hanley and Karp1 The ideal surgical options for patients presenting late with transposition of the great arteries with intact ventricular septum and regressed left vetricle is still unclear. Late primary switch, rapid two-stage switch Reference Kramer, Scheewe and Fischer2,Reference Sharma, Bhan and Choudhary3 and atrial switch operations (e.g., Senning’s/Mustard) are some of the options.
Transcatheter stenting of the patent ductus arteriosus has emerged as a nonsurgical alternative option to retrain the left ventricle in this subset of patients. This procedure has the advantage since it provides pressure and volume overload to the regressed left ventricle. Reference Wernovsky, Giglia, Jonas, Mone, Colan and Wessel4–Reference Soo, Leong and Khalid6 It is also less invasive, thereby making it an alternative option in comparison to the conventional surgical procedures like Pulmonary artery banding with Blalock-Taussig shunt.
We studied the efficacy and safety of ductal stenting in retraining of the regressed left ventricle in patients with transposition of the great arteries-intact ventricular septum. Herein, we describe our experience of eight patients who underwent ductal stenting followed by an arterial switch operation.
Materials and methods
Study setting
This was a single-centre, retrospective study in a tertiary referral centre in Western India investigating all consecutive patients with transposition of the great arteries-intact ventricular septum with regressed left ventricle who underwent ductal stenting for left ventricle retraining from January 2017 to June 2023.
Infants included in this study had presented beyond three weeks of age with a regressed left ventricle on the echocardiogram. Stenting of the ductus arteriosus was done for left ventricle retraining. Follow-up echocardiograms were done, and arterial switch operation was performed after left ventricle was prepared. Written informed consent was obtained from all patients and/ or relatives enrolled in the study. The study was approved by institutional ethics committee.
Echocardiographic evaluation
All babies with transposition of the great arteries-intact ventriuclar septum were evaluated by transthoracic echocardiography before undergoing ductal stenting as well as before arterial switch operation to assess the degree of left ventricle retraining. Left ventricle indices which were taken into consideration included left ventricle end-diastolic diameter in the parasternal long-axis view, left ventricle end-diastolic dimensions and ejection fraction measured on M mode. The left ventricle posterior wall thickness was measured at end-diastole at the mid-cavity level. Left ventricle mass was derived using the dimensions obtained from the parasternal long-axis view in end diastole where left ventricle mass = 0.8 [1.04 x (LVID + IVSd + PWd) Reference Sharma, Bhan and Choudhary3 -LVID Reference Sharma, Bhan and Choudhary3 ] + 0.6 g, where LVID is the left ventricle internal diameter in diastole, IVSd is the interventricular septal thickness in diastole, PWd is the left ventricle posterior wall thickness in diastole, Reference Lang, Badano and Mor-Avi7 1.04 is the specific gravity of the myocardium, and 0.8 is the correction factor. Left ventricle mass was also indexed to body surface area using the Mosteller formula. Reference Gopalakrishnan, Sasidharan and Krishnamoorthy8
The left ventricle geometry was subjectively classified as “satisfactory,” “D-shape,” and “ellipsoid” to describe the degree of regression of the left ventricle, based on the position of the interventricular septum in end systole (Figure 1). Reference Leong, Ahmed Alhassan, Sivalingam and Alwi9

Figure 1. Left ventricular (LV) geometry was assessed on parasternal short-axis view and categorised according to deviation of the intraventricular septum (IVS) as ( a ) “satisfactory” if the IVS bulged to the right. ( b ) “D-shape” if the IVS was central. ( c ) “Ellipsoid” if the IVS bulged to the left (RV = right ventricle).
Patients were taken up for transcatheter stenting of the duct for retraining of the left ventricle if they fulfilled at least two of the following criteria: (1) an indexed left ventricle mass of less than 35 g/m2, (2) left ventrilce posterior wall thickness during diastole of less than 3.5 mm, or (3) an unfavourable left ventricle geometry. Reference Leong, Ahmed Alhassan, Sivalingam and Alwi9
Left ventricle was considered retrained when it fulfilled the following criteria: (1) an indexed left ventricle mass greater than 35 g/m2, (2) left ventricle posterior wall thickness during diastole of more than 3.5 mm, and (3) visually favourable left ventricle geometry.
The size of the atrial septal defect was also determined by echocardiography. The largest diameter of the atrial septal defect in all views was recorded.
The procedure
Aspirin 5 mg/kg loading dose was given 12 hours prior to procedure. Transvenous controlled anaesthesia without mechanical ventilation was used in all the patients. A 5 Fr introducer short sheath was introduced into the femoral artery. Heparinization was done with single dose of 100 units/kg (keeping an activated clotting time >200 s). A check angiogram was performed as a roadmap to identify the position and the course of the patent ductus arteriosus. A 0.014-inch balance middleweight guidewire was advanced through a 5 Fr IMA guiding catheter (Medtronic, Inc.) and negotiated through the ductus arteriosus and parked deep in the main pulmonary artery. Repeat check angiograms were performed through the guiding catheter with the coronary wire straightening the PDA to assess the aortic and pulmonary ends of the duct, and measurements were made. A coronary stent (bare metal) with diameter of 4 mm (for babies <3.5 kg) or 4.5 mm (for babies >3.5 kg) with adequate length to cover the entire duct was deployed. An angiogram was performed to confirm the position of the stent as well as to profile the branch pulmonary arteries after stent placement and before removing the coronary wire. If there was no ductal patency, the ductal ampulla was probed with a 0.014-inch Pilot 50 guidewire to gain entry into the Pulmonary Artery through the ductus arteriosus, and then stenting of the duct was performed. Continuous heparin infusion of 15–20 U/kg/hour was started following the procedure for a duration of 24 hours. Aspirin 5 mg/kg once a day and Clopidogrel 0.5 mg/kg twice a day were continued till the left ventricle was prepared.
Follow-up after patent ductus arteriosus stenting
The infants were monitored in the intensive care unit (ICU) for features of pulmonary overcirculation and heart failure for 48 hours after the procedure. Heparin infusion was continued for 24 hours, and aspirin (5 mg /kg/day) was started immediately after the procedure. Echocardiographic left ventricle assessment was repeated at 72-hour intervals. Once the left ventricle was found to be prepared by echocardiography, arterial switch operation was performed after discontinuing antiplatelet drugs (Figure 2).
Arterial switch operation: surgical procedure and stent removal
Arterial switch operation was proceeded in usual fashion with transaction of the aorta and harvesting of coronary buttons. While the main pulmonary artery was being transacted, selective antegrade cerebral perfusion was maintained by clamping the aorta distal to the left subclavian artery with reduced flows. The ductal stent was removed, and the ductus was divided between ligatures. Cardiopulmonary bypass time and aortic cross clamp time were noted.
Post-operative course
Each infant’s course in the post-operative ICU was monitored for duration of mechanical ventilation, duration of intensive care unit stay, the need for extracorporeal life support, mortality, and serial echocardiographic assessment of left ventricle function.
Statistical analysis
All statistical studies were carried out using SPSS v 26.0 (IBM® SPSS Statistics®, Chicago, IL, USA). Categorical data are described as number with frequency and continuous data as median with interquartile range or mean with standard deviation, as appropriate. Categorical data were compared using the chi-square test and Student’s t-test; quantitative variables were compared using an analysis of variance. Group differences associated with a p value <0.05 were considered statistically significant.
Results
There were eight consecutive babies who presented during a span of five years (January 2017 to June 2023) with a diagnosis of transposition of great arteries with intact interventricular septum who presented late and had evidence of regressed left ventricle on echocardiogram. All these babies underwent ductal stenting for retraining the left ventricle. These babies were divided into two groups depending on the age at ductal stenting group 1 (n = 5) – age less than 90 days and group 2 (n = 3) - age more than 90 days and were evaluated for the degree of successful left ventricle retraining as evidenced by echocardiographic parameters as well as by the post-operative variables.
Table 1 shows the baseline characteristics of the two groups. Of the eight babies, six were male, and all of them had unrestrictive interatrial communication (mean size: 6.5 ± 1.9 mm); hence, none of them needed balloon atrial septostomy. Baseline weight and length of the babies were fairly similar with no statistical difference. The mean age of ductal stenting in these patients was 96.5 ± 56.8 days. There was a significant difference in the age of ductal stenting among the two groups (61 ± 18.1 days as compared to 155.6 ± 47 days). There was an increase in oxygen saturation post ductal stenting as compared to baseline (89.2 ± 3 vs. 74.6 ± 7.3 percent); however, there was no statistical significance among the two groups. The duration of retraining the left ventricle was also not statistically significant (11.6 ± 3.2 vs. 22 ± 19.4 days) with a mean duration of 15.5 ± 12 days, although the second group required longer duration to retrain. However, the age at arterial switch operation differed significantly among the two groups (72.6 ± 17.5 vs. 177.6 ± 58.6 days). The mean cardiopulmonary bypass time (196.1 minutes) and aortic cross clamp time (108.4 minutes) were fairly similar between the two groups.
Table 1. Baseline characteristics of patients

ASD = atrial septal defect, ASO = Arterial switch operation, *p value <0.05 shows statistically significant.
Post ductal stenting, all babies except one baby had uneventful course while waiting for arterial switch operation. One baby developed features of pulmonary oedema with respiratory distress on next day of procedure, for which he was mechanically ventilated and inotropic support was started. After eight days, left ventricle was retrained and underwent successful arterial switch operation on the 10th day of ductal stenting.
Table 2 shows the change in echocardiographic variables pre and post ductal stenting, respectively, between the two groups. There was a statistically significant change in the left ventricle mass and index of the left ventricle after ductal stenting in both the groups.
Table 2. Comparison of echocardiographic parameters before and after ductal stenting
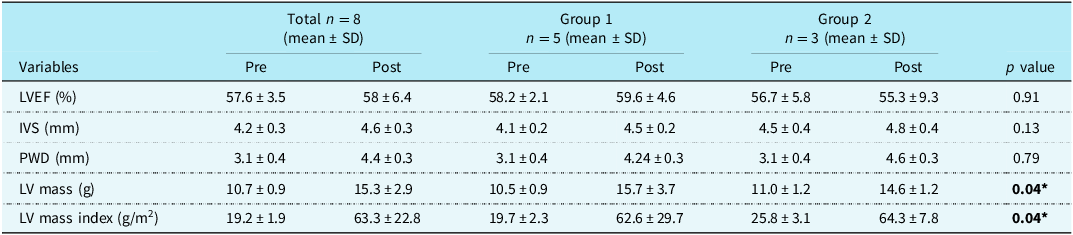
LVEF = left ventricle ejection fraction, IVS = interventricular septum, PWD = posterior wall diameter, LV = left ventricular, *p value <0.05 shows statistically significant.
Table 3 shows the comparison of hemodynamic parameters pre and post ductal stenting between the two groups. There was an increase in pulmonary arterial pressure post ductal stenting; however, it was not statistically significant. But there was a statistically significant reduction in the systolic aortic pressures following ductal stenting in both the groups.
Table 3. Comparison of haemodynamic parameters pre and post ductal stenting
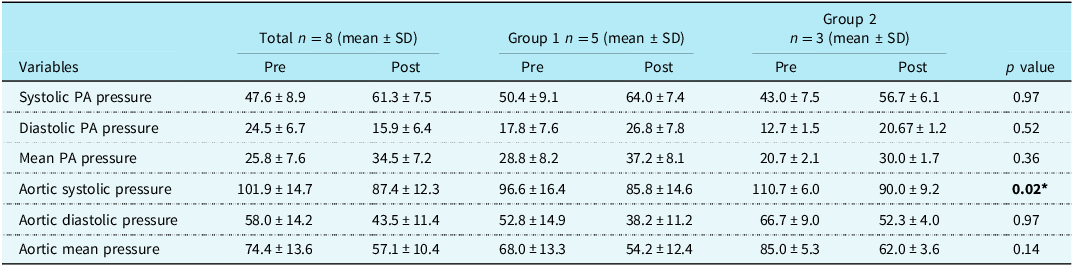
PA = pulmonary artery, *p value <0.05 shows statistically significant.
At baseline, six had ellipsoid and two had D-shaped left ventricle geometry. Post ductal stenting before ASO, six had satisfactory and two had D-shaped left ventricle geometry. Post operatively, one baby from group one expired after seven days due to severe left ventricle dysfunction. Ductal stenting was done for that baby at the age of 190 days and required left ventricle retraining for 44 days. Arterial switch operation was done at 234 days, and the baby required extracorporeal life support for seven days post operatively, but could not be weaned off extracorporeal life support and ultimately succumbed due to severe left ventricle dysfunction. However, the other babies had an uneventful post-operative ICU stay with no statistical difference in the duration of invasive mechanical ventilation (5.6 ± 2.6 vs. 9.6 ± 3.7 days) or total duration of ICU stay (13.2 ± 4.7 vs. 15.6 ± 9 days). Two babies developed moderate left ventricle dysfunction post arterial switch operation. They were managed conservatively with longer ventilatory support and medications and improved during ICU stay. All babies except one had favourable outcomes and were discharged post arterial switch operation in hemodynamically stable condition. On follow-up after six months, all surviving babies were doing well with normal left ventricle function.
Discussion
The ideal management of a patient presenting with transposition of great arteries with intact ventricular septum beyond the initial few weeks of life is controversial because the risk of arterial switch operation is significantly increased beyond three weeks of age due to regression of left ventricle. Reference Nicholas, Blackstone, Hanley and Kirklin10,Reference Ma, Hua and Yang11 Primary arterial switch operation with extracorporeal life support, left ventricle retraining, and atrial switch operations are some of the available alternatives. Reference Bisoi, Ahmed and Malankar12–Reference Cain, Cao and Ghanayem15 Although primary arterial switch is possible in these babies who present late, post-operative morbidities like low cardiac output state, prolonged invasive and non-invasive ventilation, and the need for extracorporeal life support precludes it from being the standard treatment which can be adopted universally by all centres. The rapid two-stage arterial switch was thought to be an effective alternative solution to patients who presented late for surgery. Reference Yacoub, Radley-Smith and Maclaurin16,Reference Jonas, Giglia and Sanders17 The time interval between the stages in rapid two-stage arterial switch operation is characterised by prolonged ventilatory support, severe acidosis, low cardiac output state, extended inotropic support, and prolonged duration of hospitalisation. Reference Wernovsky, Giglia, Jonas, Mone, Colan and Wessel4 In many developing nations where diagnosis of congenital heart disease occurs fairly late and 95% of infants remain untreated, increased resource utilisation makes the two-stage approach less viable due to economic constraints. Reference Iyer, Sharma and Kumar18 Unfortunately, low- and middle-income countries often face the challenges in managing these late presenters due to limited resources in terms of funds, infrastructure, and manpower.
Ductal stenting provides pressure and volume overload to the left ventricle and thus appears to be a `physiological` way for retraining the left ventricle. It was first described by Sivakumar et al and has been sparingly reported. Reference Sivakumar, Francis and Krishnan19 Our study extends the utility of the procedure and provide data in support of the procedure in late presenters with transposition of great arteries. In our study, although the pulmonary arterial pressure increased following ductal stenting, however, it was not found to be statistically significant. The adequacy of left ventricle for ASO has been conventionally judged by echocardiographic measurements of left ventricle mass index, left ventricle posterior wall thickness, and interventricular septal thickness in diastole as well as left ventricle geometry as described by Leong et al. 9 It avoids the distortion of branch PAs and would reduce the neoaortic regurgitation seen with two-stage surgical retraining. Reference Kramer, Scheewe and Fischer2,Reference Sharma, Bhan and Choudhary3 Further, an occluded patent ductus arteriosus in the early infancy might also be recanalised, as reported by Kothari et al. Reference Kothari, Ramakrishnan, Senguttuvan, Gupta and Bisoi5 The procedure was successful in all of our patients, and we did not encounter stent thrombosis as reported by Mumtaz et al Reference Mumtaz and Sivakumar20 and Alwi et al. Reference Alwi21 It is important to recognise that large atrial septal defect might reduce the efficacy of the procedure since large atrial septal defect would reduce the left ventricle preload. An early left ventricle regression with large atrial septal defect has been documented previously. Reference Gopalakrishnan, Sasidharan and Krishnamoorthy8 The course of children with ductal stenting was reasonably smooth without worsening of failure, needing intubation, or other clinical events. These patients were on continuous heparin infusion for 24 hours and aspirin following ductal stenting.
Thus, patent ductus arteriosus stent is a cost-effective procedure for left ventricle retraining that might obviate the need for extracorporeal life support in late presenters undergoing arterial switch operation and should be utilised more often.
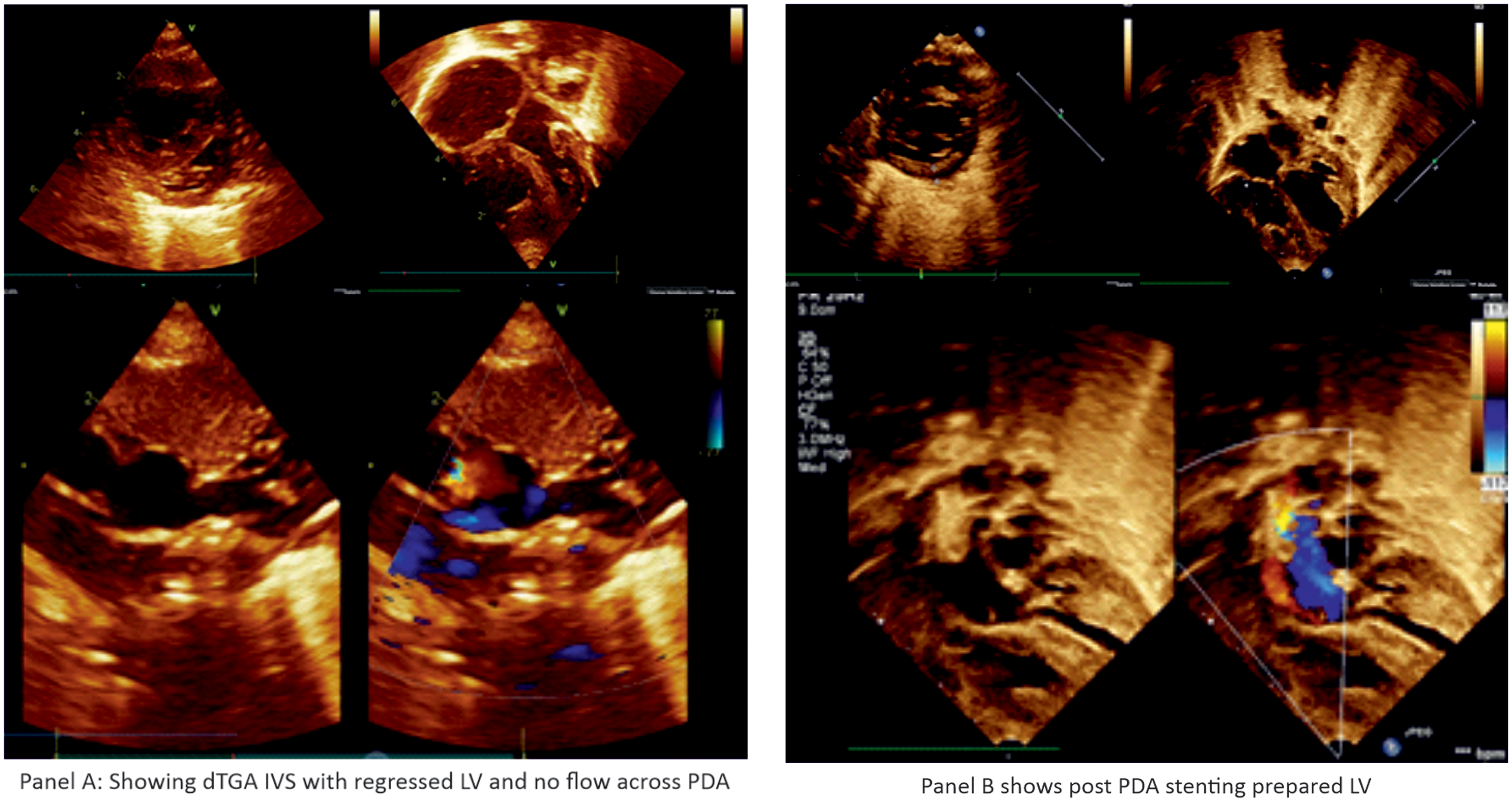
Figure 2. Panel ( a ) dTGA IVS with regressed LV and no flow across PDA. Panel ( b ) post PDA stenting prepared LV.
Limitations
It was a single-centre retrospective observational study with relatively limited sample size. Also, the duration of retraining the left ventricle was not uniform in all the patients.
Conclusion
Ductal stenting is a safe and effective option for left ventricle retraining in late presenters with transposition of great arteries and intact ventricular septum. In the low- and middle-income countries that lack infrastructure for ventricular assist devices and extracorporeal membrane oxygenator support, ductal stenting to retrain the left ventricle followed by arterial switch operation could well be one of the preferred options in this subset of the patients.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S1047951124025964.
Acknowledgement
I thank Dr Shyam Sunder Kothari for guiding in the preparation of manuscript.
Financial support
The author(s) received no financial support for the research, authorship, and/or publication of this article.
Competing interests
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical standard
The authors assert that all procedures contributing to this work comply with the ethical standards, and the study has been approved by the institutional committees (U. N. Mehta Institute of Cardiology and Research Centre).








