In the past decade and a half, there has been universal recognition that increased amounts of protein and energy are necessary to address the nutrition and growth deficits that accrue postnatally in preterm infants. In attempts to address this, parenteral amino acids and lipid are delivered earlier and more aggressively after preterm birth, and also the protein and energy contents of breast milk are increased through the routine addition of human breast milk fortifier, using an assumed milk composition. Despite these strategies, preterm infants often receive less protein and energy than they need( Reference Arslanoglu, Moro and Ziegler 1 ), which is concerning, as growth and composition of weight gain can be influenced by the macronutrient composition of the diet( Reference Kashyap, Forsyth and Zucker 2 , Reference Kashyap, Schulze and Forsyth 3 ), and recent studies suggest that at term-equivalent age, preterm infants have altered and increased adiposity and increased amounts of ectopic lipids, compared with their peers( Reference Uthaya, Thomas and Hamilton 4 , Reference Roggero, Gianni and Amato 5 ).
Although international consensus guidelines suggest that energy intakes should be based on birth weight criteria (<1000 g: 545–629 kJ/kg per d; 1000 to <1500 g: 461–545 kJ) and recommend reducing protein with increasing postmenstrual age (PMA)( Reference Tsang, Uauy and Koletzko 6 ), European guidelines base protein recommendations on body weight and suggest that energy intakes exceeding 565 kJ/kg per d (135 kcal/kg per d) may promote fat deposition rather than accretion of lean tissue( Reference Agostoni, Buonocore and Carnielli 7 ) (Table 1).
Table 1 Protein and energy guidelinesFootnote *
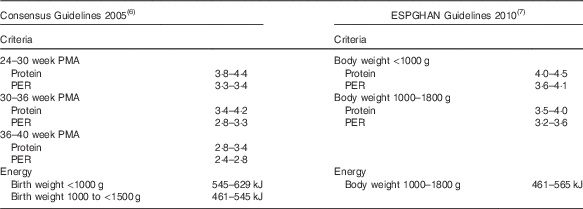
ESPGHAN, European Society for Paediatric Gastroenterology, Hepatology and Nutrition; PMA, postmenstrual age; PER, protein:energy.
* All values are expressed in kg/d except PER (g protein/419 kJ).
Attempts to improve nutritional and growth outcomes by trialling different fortification regimens have provided mixed results( Reference Polberger, Raiha and Juvonen 8 – Reference Arslanoglu, Moro and Ziegler 10 ). Polberger et al.( Reference Polberger, Raiha and Juvonen 8 ) fortified milk with human milk (HM) protein (n 16) or bovine fortifier (n 16) to provide a targeted protein intake of 3·5 g kg/kg per d, based on infant weight, volume intake (150–170 ml/kg per d), feed tolerance and measured protein content of the milk. They found no significant differences in growth or biochemical outcomes between groups. de Halleux et al.( Reference de Halleux, Close and Stalport 9 , Reference de Halleux and Rigo 11 ) measured milk composition and fortified the milk of twenty-four very low birth weight infants each for at least 3 weeks duration from 2006 through to 2011 using a bovine whey protein fortifier and a fat supplement and demonstrated less variation in protein intake than would be achieved theoretically with routine fortification. Arslanoglu et al. ( Reference Arslanoglu, Moro and Ziegler 10 ) did not measure milk composition but demonstrated that in adjusting the fortifier based on plasma urea using a commercially prepared, bovine-based fortifier, infants gained more weight than those who received routine fortification. However, protein intakes for all infants were significantly and consistently lower than the recommended intakes( Reference Arslanoglu, Moro and Ziegler 1 ). Some of these studies have been of short duration, a variety of products and methods have been used to fortify the milk, differences and improvements in growth outcomes have not been consistently shown and body composition, which has emerged as a necessary measure of nutrition adequacy, has not been assessed.
We conducted a randomised pragmatic study to test the hypothesis that growth and body composition of preterm infants more closely matches intra-uterine growth( Reference Ziegler, O’Donnell and Nelson 12 , Reference Fomon, Haschke and Ziegler 13 ) if fortification based on measured milk composition is used to target consensus protein intakes according to PMA and energy intakes according to birth weight (Table 1) rather than using an assumed milk composition to target upper limits of consensus protein (3·8–4·4 g/kg per d) and energy (545–629 kJ/kg per d) intakes( Reference Tsang, Uauy and Koletzko 6 ).
Methods
Recruitment criteria
In total, forty infants born below 30 weeks of gestation admitted to the neonatal intensive care unit (NICU) at King Edward Memorial Hospital (KEMH) in Western Australia were recruited for the study period (January 2009–June 2009) from birth to near-term PMA if they were born without congenital abnormalities, if maternal intention was to feed HM and if living remotely would not prevent participation at all assessments. This age criterion was implemented because all infants born below 30 weeks of gestation admitted to the KEMH NICU are given parenteral nutrition (PN) as first fluids. At this gestational age, a weight of 1000 g corresponds to the 10th percentile on Fenton’s growth chart and our routine practice targets the consensus energy recommendation for infants weighing <1000 g at birth. The primary outcomes for this trial were weight, length, head circumference, weight gain velocity( Reference Patel, Engstrom and Meier 14 , Reference Patel, Engstrom and Meier 15 ) and percentage fat mass (%FM) measured using air displacement plethysmography (PEA POD; COSMED) at discharge/corrected term. The secondary outcomes reported elsewhere( Reference McLeod, Geddes and Nathan 16 ) were sub-cutaneous adipose and muscle tissue measurements taken by ultrasound. Transfer of infants (n 21) during the study from tertiary to Level 2 outlying nurseries within a 30-km radius of the KEMH did not interrupt the study protocol; infants continued to participate in the trial to completion. Comparisons between ‘transferred’ v. ‘not transferred’ infants showed no baseline differences and no growth outcome differences within each treatment group. The Ethics Committees at both KEMH and The University of Western Australia reviewed and approved the study protocol. Written informed consent was obtained from the infants’ mothers before commencing the study. The trial was registered with the Australian New Zealand Clinical Registry (http://www.anzctr.org.au) as ACTRN12610000443099; UTNU1111-1115-4183.
Randomisation and blinding
Infants were randomised to the intervention (individualised fortification based on measured composition according to birth weight criteria and PMA) or routine practice (routinely optimised fortification based on assumed composition to target 3·8–4·4 g protein/kg per d; 545–629 kJ/kg per d (130–150kcal/kg per d)), and the allocation ratio was 1:1. Twins were randomised as individuals. The randomisation sequence was achieved by random draw, without replacement (G. M.). Group allocation was concealed in sequentially numbered, opaque, sealed envelopes, which remained unopened until parental consent had been obtained and infant demographics had been recorded (research nurse or G. M.). Parents and the clinical teams managing the care of infants (including making the clinical decisions, prescribing each infant’s daily fluid intake and fortification status and maintaining clinical records) were blinded to group allocations. Allocated nursing staff in the centralised milk room at KEMH, and in the outlying hospitals, were responsible for collecting and freezing the milk samples before fortification and for adding fortifier to the milk as per the study protocol. The chief investigator (G. M.) was responsible for the following: (i) conducting the milk analysis; (ii) calculating the weekly mean composition of each infant’s milk and the amount of fortifier to be added to the milk of intervention infants; (iii) providing fortification instructions to nursing staff for all study participants, (iv) data collection; and (v) ensuring the study protocol was understood and maintained.
Milk sampling and analysis
Mother’s milk was fed in the order in which it was expressed for at least the first 14 d of enteral feeding, and extending up to the first 28 d if available for infants born below 26 weeks of gestation. Any residual samples of early frozen milk not used before an infant was receiving fresh milk were added to feeds over time, as needed. Therefore, each infant’s daily native milk feed was made up from their own mothers’ individual and pooled collections of expressed milk and may have included milk expressions from different days. A well-mixed sample (3–6 ml) of each infant’s daily milk feed (mothers own milk (MOM) or donor human milk (DHM)) was collected before fortification by nursing staff and labelled and frozen at −20°C (−5°C for outlying Level 2 nurseries). Each batch of weekly samples was gathered and analysed in the Human Milk Bank laboratory at the end of each week by G. M. The samples were defrosted, warmed in a water bath to 40°C, homogenised (1·5 s/ml of sample; Sonics Vibra-Cell, Model VCX-130; Sonics and Materials Inc.) and the protein, fat and lactose concentrations were determined using the MIRIS® (human milk analyser (HMA): processed milk setting). The method has been described and evaluated elsewhere( Reference Menjo, Mizuno and Murase 17 , Reference Casadio, Williams and Lai 18 ). For each week that fortified feeds were prescribed for an infant, the weekly mean content of the infant’s milk samples from the previous week was used to fortify the infant’s milk feeds for the following week.
Fortification methods
A commercial multi-component human milk fortifier (HMF) (Wyeth Nutritionals), a protein powder (Beneprotein; Novartis) and an energy supplement (Duocal; SHS International Limited) were used to fortify milk feeds.
Milk for the intervention infants (Igp) was fortified with variable amounts of these fortifiers (HMF: maximum 4 g/100 ml; Beneprotein: maximum 0·5 g/100 ml; Duocal: maximum 3·0 g/100 ml), depending upon measured milk composition and fluid status (fluid-restricted 130–150 ml/kg per d; non-fluid-restricted 160–180 ml/kg per d). Protein was first adjusted and then, if necessary, energy supplemented to best achieve targets.
Milk for the infants fed according to routine practice (RPgp) was fortified in fixed dose amounts (HMF 4 g/100 ml for non-fluid-restricted infants; HMF 4 g/100 ml+Beneprotein 0·5 g/100 ml+Duocal 2·5 g/100 ml for fluid-restricted infants) using an assumed composition derived from published( Reference Lai 19 ) and unpublished macronutrient milk analysis of preterm milk conducted in our Unit (protein 1·4 %; fat 4·4 %; lactose 6·8 %). Our assumed composition falls within the published range of data describing preterm macronutrient composition of milk expressed over the first 1–2 months of lactation( Reference Anderson, Atkinson and Bryan 20 – Reference Gross, Geller and Tomarelli 23 ).
Nutrition
Until the initiation of fortified milk feeds, all infants were fed according to the Unit’s standard feeding regimen.
Parenteral nutrition
On day 1 of life, 5 or 7·5 % glucose and 1·5 % amino acids were infused until day 2, when individualised PN including lipids (20 %, 1·0 g/kg per d), electrolytes and micronutrients was commenced. Subsequently, concentrations and rates were increased, targeting recommended intakes( Reference Tsang, Uauy and Koletzko 6 ).
Enteral nutrition
Minimal enteral feeds, using frozen MOM in the sequence in which it was expressed, were initiated as early as possible after birth and increased following a standardised regimen. PN was simultaneously reduced. If MOM was unavailable, pasteurised DHM was available for the infants until at least a postmenstrual age of 34 weeks. As per unit guidelines and using clinical discretion, the medical teams prescribed fortification once enteral volumes ≥100 ml/kg per d were achieved, and ceased fortification just prior to discharge.
Nutrition intake during hospital stay
All fluid and intakes consumed during hospital stay were recorded retrospectively from the observation charts from midnight on day 1 of life until the discharge measurements. The milk volume consumed during a breast-feeding session was estimated to be equivalent to that amount normally given at a scheduled feeding session unless a top-up was also given, in which case the estimated volume taken during the breast-feeding session was calculated by subtracting the volume of the top-up from the scheduled feed volume. Macronutrient intakes were calculated using composition data of daily milk feeds obtained for both groups with the HMA, and energy intakes were derived using the Atwater values (kJ/g (kcal/g)) for protein 16 (4), fat 37 (9) and lactose 16 (4), adopted by the National Health and Medical Research Council( 24 ).
Body composition
%FM measurements were obtained at discharge (n 32) or on transfer to outlying Level 2 nurseries (n 8) using air displacement plethysmography (PEA POD). The technical design and the methodology underpinning a PEA POD measurement have been described elsewhere( Reference Urlando, Dempster and Aitkens 25 , Reference Sainz and Urlando 26 ). In brief, the PEA POD utilises the classic two-compartment BC model to measure infants weighing between 1 and 8 kg. Total body density is calculated from the direct measurements of body mass (electronic scale) and volume (air displacement). The software provided by COSMED incorporates algorithms to derive percent body fat and fat-free mass (FFM). These algorithms use the constant fat mass (FM) density value of 0·9007 g/ml and predetermined FFM density values modelled using the data of Fomon et al. ( Reference Fomon, Haschke and Ziegler 13 ).
Gestational age and postmenstrual age
Gestational age was calculated as the time elapsed from the date of the first day of the last menstrual period to delivery. If the date of the last menstrual period was unknown, the modified Ballard method was used. PMA was calculated according to the following formula: gestational age+postnatal age (time elapsed after birth).
Anthropometry
In accordance with the Neonatal Clinical Care Unit’s (NCCU) measurement policy, weight (g), measured in the infant’s incubator or with digital scales (SECA, 10/20 kg; d=5/10 g or PEA POD), crown–heel length and occipital–frontal head circumference were measured at birth, discharge and at term PMA. Fenton’s data were used to convert measurements to z-scores( Reference Fenton and Sauve 27 ). Infants requiring intensive care (NICU) were also weighed daily, and those in special care were weighed twice weekly, with daily weight derived by interpolation between each of the time points. Weight gain velocity was calculated using an exponential model that has been validated in preterm infants( Reference Patel, Engstrom and Meier 14 , Reference Patel, Engstrom and Meier 15 ).
Statistical analysis
Descriptive statistics for continuous data were summarised using means and standard deviations or medians, interquartile ranges and ranges. Categorical data were summarised using frequency distributions. Univariate comparisons of continuous clinical data, nutritional intakes and anthropometric measures were conducted using one-sample t tests, independent t tests or Mann–Whitney tests according to normality, and χ 2 or Fisher’s exact tests were used for categorical comparisons. Reliability of %FM measurements for PEA POD was determined by calculating the standard deviation, CV and technical error. The technical error was defined as √Σd 2/2n, where d is the difference between two repeated tests for the paired observations. Comparisons of BC, measured as percentage body FM, were assessed at discharge using linear regression modelling after adjustment for PMA, weight z-score at measurement and residuals from a linear regression of length on weight at time of measurement. Linear mixed models analysis was conducted to produce growth curve models for weight gain velocity to discharge. A natural logarithm transformation was applied to the outcome to achieve normality, as indicated by residual diagnostics. Protein, carbohydrate and fat intakes and clinical variables were assessed for their effects on the rate of growth. Adjustment was made for birth weight z-score, PMA at the time of measurement and chronological age. SAS 9.1 of the SAS System for Windows, Copyright© 2002–2010 SAS Institute Inc. and PASW® 17 statistical software (SPSS Inc.), was used for data analysis. All tests were two-tailed, and P values <0·05 were considered statistically significant.
Power
Estimation of sample size was based on data from a previous audit( Reference McLeod, Sherriff and Nathan 28 ) using a mean growth rate of 12·8 (sd 5) g/kg per d. A sample size of 20 in each group was sufficient to achieve 80 % power to detect a difference of 3·4 g/kg per d in a repeated measures design with an alpha level of 0·05 (Power Analysis and Sample Size Statistical Software 2008).
Results
Subject demographics
Ninety-one infants admitted to the NCCU between 26 January and 9 June 2009 were born below 30 weeks of gestation. Fifty-one infants were excluded from the study for reasons including congenital renal abnormality (n 1), withheld consent (n 8), living remotely (n 13), paused recruitment (due to an investigator’s absence) (n 19) and death (n 10; Fig. 1). Forty infants (Caucasian n 36, Australian Aboriginal: Igp n 1, RPgp n 1; Asian: RPgp n 1, Other: RPgp n 1) born from either singleton (n 24) or twin (n 16) births at a median age of 27 (range 23, 29) weeks and a birth weight of 1022 (range 480–1475) g were randomly assigned to the intervention (n 20) or routine practice (n 20). Siblings from two sets of twin births were each randomly allocated to the same group (n 1=both twins randomised to Igp; n 2=both twins randomised to RPgp), otherwise siblings were randomly allocated to different groups. The clinical characteristics of the infants did not significantly differ between groups (Table 2). All infants were appropriate for gestational age (10th percentile to 90th percentile), with the exception of two infants fed routinely, whose birth weights were on the 6th weight percentile. The corrected PMA ranges of infants in the intervention and routine practice groups measured at discharge were 33–43 and 33–42 weeks, respectively.
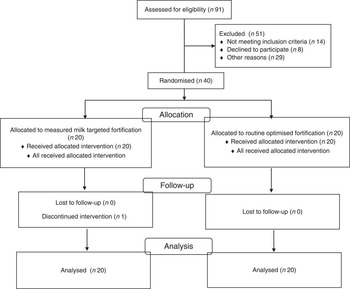
Fig. 1 Enrolment and participation.
Table 2 Clinical data (Numbers and percentages; mean values and standard deviations; medians and ranges)

Igp, intervention group; RPgp, routine practice group; CPAP, continuous positive airway pressure.
Composition of milk feeds
The mean values and standard deviations of measured protein, fat and lactose concentrations and the derived energy content and protein:energy (PER) of milk feeds (n 1870 samples) were similar for both groups (Table 3).
Table 3 Macronutrient composition of milk feedsFootnote * and nutritional intakes (Mean values and standard deviations)

Igp, intervention group; RPgp, routine practice group; PER, protein:energy; CHO, carbohydrate; IV, intravenous.
* Mean macronutrient composition of milk from 14 d of an infant commencing MEF to discharge; Atwater conversion factors: protein 16 kJ/g; fat 37 kJ/g; lactose 16 kJ/g.
Nutritional intakes and growth outcomes
On average, 17 % by volume of the total fluids received by infants while in hospital were given intravenously and human milk constituted 93 % of the enteral intakes (84 % MOM: Igp n 18, RPgp n 19; 16 % DHM: Igp n 5, RPgp n 4), and an estimated 7 % of MOM was breast-fed. Unfortified milk feeds were suspended for one intervention infant for a period of 30 d when PN was provided due to suspected necrotising enterocolitis (Stage 2 NEC)( Reference Bell, Ternberg and Feigin 29 ). Fortified feeds were prematurely ceased and not re-instituted for two intervention infants (feed intolerance n 1=day 15; n 2=day 47) and for two infants fed according to routine practice (n 1=Stage 2 NEC( Reference Bell, Ternberg and Feigin 29 ) day 14; n 2=cows’ milk protein intolerance day 32). One intervention infant did not receive fortifier and transitioned directly from unfortified milk to preterm formula, due to the unavailability of DHM. In total, eleven infants (Igp: n 6, RPgp: n 5) received some infant formula during their hospital stay. The number of days infants consumed fortified milk did not differ between groups (Igp: n 19: 44 (sd 24) v. RPgp: n 20: 42 (sd 23) d; P=0·801), and per study protocol protein (3·2 (sd 0·4) v. 3·9 (sd 0·3) g/kg per d; P<0·001) and energy (510 (sd 39) v. 559 (sd 34) kJ/kg per d; P<0·001) intakes from fortified milk were lower in the intervention infants than in those fed according to routine practice (Table 3). Intention-to-treat analysis showed that after fortification (i.e. once infants made the initial transition from unfortified milk) total protein and energy intakes and the PER did not significantly differ between groups (Table 3). At discharge, weight (g), length (cm), head circumference (cm) and weight gain velocity were similar between groups (Table 4); eighteen infants had weights below the 10th percentile (Igp: n 11, RPgp: n 7; P=0·204).
Table 4 Growth data of infants at discharge (Mean values and standard deviations)
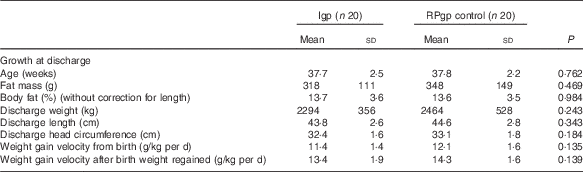
Igp, intervention group; RPgp, routine practice group.
When adjusted for corrected PMA, chronological age and birth weight z-score, no significant difference in weight gain velocity was found between groups (P=0·140; Table 5). There was an average 9 % increase in weight gain velocity (g/kg per d) for every additional g/kg per d of enteral protein (95 % CI 1, 18; P=0·024).
Table 5 Modelling of weight gain and body composition with macronutrient intake data (Mean effects and 95 % confidence intervals)

* Weight gain velocity was analysed using linear mixed models regression and transformed to the natural logarithm for analysis. Estimates and CI have been back-transformed for this outcome in the table, with the result that each estimate now represents the proportion change in weight gain velocity. For example, an additional g/kg per d of enteral protein was associated with an average 109 times increase or 9 % increase in weight gain velocity (95 % CI 1, 18).
At discharge, rates of weight gain achieved by each group were significantly slower than the fetal rate (birth to discharge: Igp: P<0·001; RPgp: P<0·001; and after recovery of birth weight to discharge: Igp: P<0·001; RPgp P=0·051).
Body composition
At discharge (Igp: 38 (sd 2) weeks; RPgp: 38 (sd 2) weeks), both groups had similar %FM, both univariately and after adjusting for PMA and length at measurement (P=0·269; Table 5). Female infants had an average 3 % greater FM than males (95 % CI 1, 5; P<0·001). After inclusion of carbohydrate in the regression model, a protein intake >3·4 g/kg per d (from all nutrition sources) reduced FM by 2 % (P=0·042). The energy intakes of the ten infants (Igp: n 4, RPgp: n 6; 25 %) who consumed these higher protein intakes ranged between 389 and 537 kJ/kg per d, and their mean rate of weight gain, calculated after recovery of birth weight, was similar to the fetal rate (high protein: 14·7 g/kg per d v. fetal: 15 g/kg per d; P=0·514).
At discharge, preterm infants had a significantly greater mean %FM for age than the reference fetus values (Igp: 13·7 (sd 3·6) %; P<0·001, RPgp: 13·6 (sd 3·5) %; P<0·001 v. reference fetus: 9·5 %)( Reference Ziegler, O’Donnell and Nelson 12 ).
Discussion
The fortification design used in this pragmatic clinical trial did not improve growth or body composition outcomes of infants born below 30 weeks of gestation compared with those fed according to routine practice. The method of fortifying human breast milk on measured composition, targeting consensus protein and energy intakes for corrected PMA and birth weight criteria was labour intensive and time consuming and did not prove a superior method over fortifying milk on assumed composition in fixed dose amounts to target upper consensus limits for the extremely low birth weight infant. There are mitigating reasons for these outcomes. First, the milk protein content measured using MIRIS was higher (1·6 g) compared with the assumed value (1·4 g), and as a result lower amounts of fortifier were often added to the milk of intervention infants. Two studies designed to evaluate the accuracy and precision of the MIRIS measurement have shown that the HMA overestimates milk protein by a small( Reference Menjo, Mizuno and Murase 17 , Reference Casadio, Williams and Lai 18 ), but significant( Reference Casadio, Williams and Lai 18 ), amount, whereas a more recent study suggests that it systematically underestimates protein content by a similar amount( Reference Fusch, Rochow and Choi 30 ). As the analytical methods and milk sampling designs differed in these studies, it is difficult to assess whether there was need to make an adjustment to the measurement before calculating the amount of fortifier required to correct the protein deficit in the milk feeds of each of the infants in this trial. Menjo et al.( Reference Menjo, Mizuno and Murase 17 ) suggest that this is a necessary strategy for clinicians, but it was not one adopted in this trial.
Another confounder in this study is that fortification was not adjusted to compensate for the dilutionary effect of breast-feeding (although contributing only an estimated 7 % to milk intake), making continued titration of protein potentially superfluous after breast-feeding replaced one or more scheduled feeds. Furthermore, the maximum amounts of fortifier we arbitrarily permitted to be added to feeds sometimes proved limiting in achieving the desired level of fortification thought necessary to achieve the protein and energy and PER targets for an infant.
Since the publication of the international consensus guidelines on which milk fortification in this study was designed( Reference Tsang, Uauy and Koletzko 6 ), the Europeans have suggested that energy intakes should be maintained between 460 and 565 kJ/kg per d (110 and 135 kcal/kg per d), and protein intakes and PER should be based on a body weight range (<1000 g: PER 3·6–4·1 and 1000–1800 g: PER 3·2–3·6)( Reference Agostoni, Buonocore and Carnielli 7 ). These PER ranges, which are higher than the consensus targets( Reference Tsang, Uauy and Koletzko 6 ) (2·8–3·3), are well above the PER (Igp: 2·6, RPgp: 2·7) that were achieved with milk fortification in this study. They are also higher than the PER achieved by Arslanoglu et al. ( Reference Arslanoglu, Moro and Ziegler 10 ), who demonstrated that an extra 0·8 g of protein powder could be added to fortified milk feeds (HMF) without adverse clinical outcomes. However, using plasma urea to guide fortification still resulted in a range of protein and energy intakes (2·9–3·4 g/kg per d and 535 kJ/kg per d)( Reference Arslanoglu, Moro and Ziegler 1 ) below the consensus guidelines( Reference Tsang, Uauy and Koletzko 6 ), and for protein at least values were below the latest European Society for Paediatric Gastroenterology, Hepatology and Nutrition (ESPGHAN) targets( Reference Agostoni, Buonocore and Carnielli 7 ). If the ESPGHAN recommendations( Reference Agostoni, Buonocore and Carnielli 7 ) are to be achieved in practice, more studies are required to determine a safe, maximum level of fortification in the context of prescribed fluid intakes, variable milk composition, fortification practices, osmolality, feeding tolerance, risk of NEC and other clinical and metabolic outcomes.
Modelled fetal chemical data suggest that the fetus more than doubles its %FM between 33 and 42 weeks (approximately 7–16 %FM)( Reference Ziegler, O’Donnell and Nelson 12 , Reference Widdowson 31 ). After achieving mean protein intakes between 3·2 and 3·4 g/kg per d and energy intakes between 460 and 480 kJ/kg per d (PER 3·0), the mean %FM of the preterm infants in this study at the mean corrected PMA of 38 weeks was 13·7 %. These BC data for preterm infants accord well with %FM estimates by Widdowson et al. ( Reference Widdowson 31 ) of the 38-week-old fetus (12 %) and estimates by Fomon et al. ( Reference Fomon, Haschke and Ziegler 13 ) of the reference male (13·7 %) and female (14·9 %) term infants. Body composition and nutritional outcomes are difficult to measure in very preterm infants due to both the lack of suitable measuring methods in the neonatal setting and the unpredictability of an infant’s clinical condition. Measuring the true effect of different fortification regimens on growth outcomes is also logistically challenging because, despite randomisation, adjusting adequately for variations in metabolic and biological responses by individuals to feeding and clinical treatments is difficult. A larger sample size, routine measurement of breast milk transfer by test-weighing and non-intrusive and accurate bedside measurement methods for measuring growth and changes in BC from birth would assist in addressing these difficulties and were the limitations of this study.
In this trial, the positive relationship between an achieved protein intake and %FM suggests that fortification regimens that target higher protein intakes may improve composition of growth – a concept that should form the basis of any future study design. Current HMF are lacking sufficient protein to correct the deficit in breast milk that would facilitate the attainment of preterm nutrition and growth targets. Guidelines around safe upper limits of fortification are necessary to guide clinicians in their fortification practices. Fortifying breast milk using measured milk composition is time consuming and labour intensive, requiring precision equipment and the method may not be superior to routinely fortifying based on assumed milk composition.
Acknowledgements
The authors express their thanks and appreciation to the following people and organisations for their support: the families and infants who participated in the study; Chooi-Heen Kok, who assisted with measuring infants in the PEA POD; medical, nursing, HM banking and milk room staff at King Edward Memorial Hospital; the Women and Infants Research Foundation.
G. M. gratefully acknowledges Australian Rotary Health, The Rotary Club of Thornlie and The University of Western Australia for providing the PhD Funding Partnership Scholarship, King Edward Memorial Hospital for providing travel funding through The Post Graduate Medical Research Fund, The Stan Perron Charitable Trust that provided funds to King Edward Memorial Hospital for procurement of the PEA POD and Medela AG, which provided laboratory materials through an unrestricted research grant to The University of Western Australia.
Authors’ contributions are as follows: contributions to conception and design: G. M., J. S., K. S., P. E. H.; acquisition of data: G. M., D. G.; analysis and interpretation of data: G. M., J. S., K. S., P. E. H., E. N., D. G.; drafting the article: G. M.; revising it critically for intellectual content: J. S., K. S., P. E. H., E. N., D. G.; final approval of the version to be published: G. M., J. S., K. S., P. E. H., E. N., D. G.
The unrestricted research grant provided to The University of Western Australia by Medela AG provides salary and associated maintenance for D. G. and supports research conducted by both D. G. and P. E. H. P. E. H has received associated honoraria from Medela AG. K. S. has presented at Nestle’s Scientific Meetings and has received associated honoraria. Sponsors and those who have been acknowledged had no role in study design, data collection, analysis or interpretation of the data, writing of the manuscript or in the decision to submit the paper for publication.
Authors have not submitted this manuscript elsewhere and have no conflicts of interest to declare.









