Although not an ideal biomarker, serum prostate specific antigen is the only predictive and prognostic prostate cancer (PCa) biomarker widely used in clinical practice. Several proteins have been proposed as candidate biomarkers exhibiting all or some of these predictive or prognostic properties( Reference Madu and Lu 1 – Reference Netto and Epstein 3 ). Among these are androgen-, cell cycle-, proliferation-, apoptosis- and neuroendocrine differentiation-related biomarkers.
The soya isoflavone, genistein, is a promising chemopreventive agent in PCa based on molecular, epidemiological and clinical studies( Reference Banerjee, Li and Wang 4 , Reference Steiner, Arnould and Scalbert 5 ). We have previously reported the clinical endpoints of a phase 2 clinical randomised trial with short-term genistein intervention in patients with localised PCa. Genistein reduced the level of serum prostate specific antigen without any effects on hormones( Reference Lazarevic, Boezelijn and Diep 6 ). The purpose of the present study was to investigate the modulation of potential candidate PCa tissue biomarkers by genistein at a dose that can be obtained from a diet rich in soya-based food.
Experimental methods
Patients and study design
A total of forty-seven patients with localised PCa scheduled to be treated by radical prostatectomy were randomised during April 2007–August 2008. Next, a total of forty-one prostates were analysed and forty were considered as evaluable according to the study protocol. However, one specimen only contained enough tumour material for pathological analysis and could therefore not be analysed for biomarkers. The study was a single-centre, placebo-controlled, randomised and double-blind phase 2 clinical trial with two treatment arms( Reference Lazarevic, Boezelijn and Diep 6 ). One arm was given 30 mg synthetic genistein (geniVida® ( Reference Ullmann, Metzner and Frank 7 )) daily and one arm received placebo. The intervention period was 3–6 weeks (average 33 d) before radical prostatectomy. This study was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures involving human subjects/patients were approved by the Norwegian Medicines Agency, the Regional Ethics Committee, the Privacy Ombudsman and the Prostate Biobank at the Oslo University Hospital, Aker. Written informed consent was obtained from all subjects/patients. The study has been registered in the ClinicalTrials.gov registry (study identifier: NCT00546039).
Tissue processing, laser capture microdissection and RNA isolation
After surgery, the prostates were immediately transferred to the pathology department and macro-dissected by a pathologist. Fresh tissue cubes, approximately 0·5 cm3, were cut from tumour and normal tissue and frozen at − 80°C. Frozen 10 μm sections were stained with haematoxylin and eosin. At least 10 000 cells from histologically benign and cancer glands from each patient were selected and captured using laser capture microdissection (Leica LMD 6000; Leica Microsystems) and frozen at − 80°C( Reference Bonner, Emmert-Buck and Cole 8 ).
Total RNA was isolated using the Arcturus PicoPure RNA Isolation Kit (Molecular Devices) according to the manufacturer's protocol. The concentration of RNA was determined by measurements in the NanoDrop ND-1000 spectrophotometer (NanoDrop Technologies). Reverse transcription of 50 ng total RNA was performed with the iScript cDNA Synthesis Kit (Bio-Rad) according to the manufacturer's protocol. Although fresh prostate tissue was acquired from most patients, tumour was found in only ten study subjects from the genistein arm and twelve from the placebo arm.
Semi-quantitative real-time RT PCR
Primers were designed with Primer3 provided by the Whitehead Institute for Biomedical Research (http://fokker.wi.mit.edu/cgibin/primer3/primer3_www.cgi). Sequences are listed in Table 1. Relative quantification of gene expression by semi-quantitative real-time RT-PCR was performed by analysing the expression of human housekeeping delta-aminolevulinate synthase gene. Real-time PCR was performed on the Bio-Rad Opticon DNA Engine. All amplifications were run as triplicates with standard curves. Here, 1 ng of template was used in each reaction following the manufacturer's protocol (Bio-Rad SYBR Green Master Mix). The same set-up was used for all primers (sixty-six cycles of 55°C annealing, 72°C extension and 90°C denaturing).
Table 1 Primers for PCR
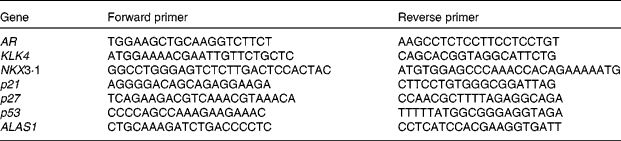
AR, androgen receptor; KLK4, kallikrein-related peptide 4; NKX3·1, NK3 homeobox 1; p21, cyclin-dependent kinase inhibitor 1A; p27, cyclin-dependent kinase inhibitor 1B; p53, tumour protein p53; ALASI, aminolevulinate synthase gene.
Tissue processing and immunohistochemical analysis
The prostates were fixed in 10 % buffered formalin, macro-dissected and paraffin-embedded. Tissue microarrays were assembled using the TMABooster (Alphelys) sampler. Normal, Gleason grade 3 (G3) and Gleason grade 4 (G4), if available, prostate tumours were marked on haematoxylin/eosin-stained sections. From each patient, three 0·6 mm biopsy cores were taken from normal prostatic tissue and each available Gleason grade tumour. The sampling size of three cores has previously been shown to be representative of PCa( Reference Rubin, Dunn and Strawderman 9 ). Next, 10 μm tissue microarray sections on glass slides (SuperFrost®) were treated according to the procedures listed in Table 2. ‘Benchmark XT’ used automated immunohistochemical staining (Benchmark XT; Ventana Medical Systems). The slides were treated in a dry cabinet for 60 min at 60°C, cooled and washed with a detergent before conditioning with cell conditioning 1 (CC1) buffer (Ventana Medical Systems). Detection of the bound primary antibody was visualised with the iView™ DAB Detection kit (Ventana Medical Systems). The cells were counterstained with haematoxylin and Bluing reagent (Ventana Medical Systems) for 4 min. ‘Autoclave’ used citrate buffer pH 6·4 at 120°C for 40 min for antigen retrieval. Immunohistochemistry was performed according to the manufacturer's protocol (BioGenex Detection kit). The slides were visualised by diaminobenzidine staining followed by haematoxylin staining. ‘PT-module’ used different buffers according to Table 2. The slides were treated in 98°C for 25 min in the PT-Module (Lab Vision Corporation) for antigen retrieval. Immunohistochemistry and visualisation were performed according to the manufacturer's protocol (UltraVision ONE Detection System; Thermo Fisher Scientific). The cells were counterstained with haematoxylin. The stained tissues were then independently analysed by two consultant pathologists (A. S. and C. H.), who later made a consensus evaluation on each sample. Samples not containing the designated Gleason grade were not analysed and treated as missing.
Table 2 Antibodies for immunohistochemistry
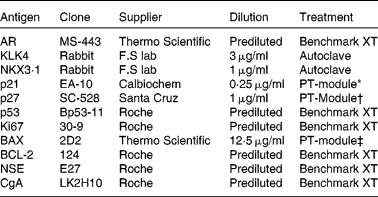
AR, androgen receptor; KLK4, kallikrein-related peptide 4; NKX3·1, NK3 homeobox 1; p21, cyclin-dependent kinase inhibitor 1A; p27, cyclin-dependent kinase inhibitor 1B; p53, tumour protein p53; BAX, B-cell CLL/lymphoma 2-associated X protein; BCL-2, B-cell CLL/lymphoma 2; NSE, neuron-specific enolase; CgA, cytoplasmic chromogranin A.
* Citrate buffer pH 6.
† Tris buffer pH 9.
‡ EDTA buffer pH 8.
The staining intensities of nuclear androgen receptor (AR), cytoplasmic and nuclear NK3 homeobox 1 (NKX3·1), cytoplasmic kallikrein-related peptide 4 (KLK4), cytoplasmic and nuclear cyclin-dependent kinase inhibitor 1B (p27Kip1) and cytoplasmic B-cell CLL/lymphoma 2-associated X protein (BAX) were scored as either weak/moderate (0) or strong (1), whereas the staining intensities of cytoplasmic B-cell CLL/lymphoma 2 (BCL-2), cytoplasmic neuron-specific enolase (NSE: also called ENO2) and cytoplasmic chromogranin A (CgA) were scored as negative (0) or positive cytoplasmic staining (1).
Sample sizes for the placebo arm were: sixteen containing normal glandular tissue, sixteen G3 and six G4 tumours; and for the genistein arm: twenty-three normal glandular tissue, twenty-two G3 and ten G4. In the placebo arm, one G3 disappeared due to technical reasons for AR and p27Kip1. In the genistein arm, one G4 disappeared for NKX3·1 and cyclin-dependent kinase inhibitor 1A (p21Waf1/Cip1).
Statistical analysis
Values are expressed as means with their standard errors for continuous and ordinal data. Differences between two related and independent samples were tested by Fisher–Pitman permutation tests for paired and two-sample interval-scaled data. The P-values were based on 2000 simulations and considered significant at P < 0·050. The analyses were carried out in STATA/IC 11.1 for Windows (32-bit) and Sigmaplot 11.0 for Windows was used to create the figures.
Results
Androgen-related biomarkers
Genistein intervention significantly reduced KLK4 mRNA expression in tumour cells (P = 0·033). The down-regulation of AR protein expression and KLK4 mRNA level in normal cells were not statistically significant (P = 0·123 and P = 0·087; Fig. 1(c) and (d)). There was a general non-significant tendency by genistein intervention to reduce the expression of androgen-related biomarkers (Fig. 1).

Fig. 1 Expression of androgen-related biomarkers. Real-time RT-PCR of the mRNA expression of (a) androgen receptor (AR), (b) NK3 homeobox 1 (NKX3·1) and (c) kallikrein-related peptide 4 (KLK4) in laser micro-dissected cells from normal and tumour areas of prostatectomy specimens from patients treated with either placebo (
![]() ) or genistein (
) or genistein (
![]() ). The data were normalised to aminolevulinate synthase gene and are shown as means, with their standard errors. The protein expression levels of (d) AR, (e) NKX3·1 and (f) KLK4 were determined by immunohistochemical staining of tissue microarrays containing normal and Gleason grade 3 (G3) and/or Gleason grade 4 (G4) spots of prostatectomy specimens. The figures show the mean staining intensity, with their standard errors.
). The data were normalised to aminolevulinate synthase gene and are shown as means, with their standard errors. The protein expression levels of (d) AR, (e) NKX3·1 and (f) KLK4 were determined by immunohistochemical staining of tissue microarrays containing normal and Gleason grade 3 (G3) and/or Gleason grade 4 (G4) spots of prostatectomy specimens. The figures show the mean staining intensity, with their standard errors.
The AR and NKX3·1 nuclear protein expression in both study arms were reduced in higher Gleason grades, whereas both the mRNA and protein expression were increased for KLK4. AR mRNA expression was unchanged. Fig. 2(a) depicts a patient treated with placebo showing strong nuclear AR staining intensity in normal cells and weak intensity in the case of tumour. The cytoplasmic expression of NKX3·1 was equivalently increased (data not shown). Fig. 2(b) shows a patient from the genistein group with strong nuclear/weak cytoplasmic NKX3·1 staining intensity in normal cells and weak nuclear/strong cytoplasmic staining intensity in tumour. Fig. 2(c) depicts a patient treated with genistein showing weak cytoplasmic KLK4 staining intensity in normal cells and strong intensity in tumour.

Fig. 2 Immunohistochemical staining of androgen-related biomarkers. Each row shows tissue microarray spots from a single patient containing normal, Gleason grade 3 (G3) and Gleason grade 4 (G4) prostate tissues. Encircled areas contain designated Gleason grade. Magnification × 400. (a) Androgen receptor, (b) NK3 homeobox 1 and (c) kallikrein-related peptide 4.
Cell cycle-related biomarkers
Genistein intervention had no significant effects on p21Waf1/Cip1, p27Kip1 or tumour protein p53 (p53) mRNA and protein expression (Fig. 3). There was a non-significant reduction in the expression of p21Waf1/Cip1 mRNA expression in tumour (P = 0·184) and a slight reduction in p27Kip1 and p53 mRNA expression, whereas 27kip1 protein nuclear expression was slightly increased in G3 and G4 in the genistein arm compared with placebo.

Fig. 3 Expression of cell cycle-related biomarkers. Real-time RT-PCR of the mRNA expression of (a) cyclin-dependent kinase inhibitor 1A (p21Waf1/Cip1), (b) cyclin-dependent kinase inhibitor 1B (p27Kip1) and (c) tumour protein p53 (p53) in laser micro-dissected cells from normal and tumour areas of prostatectomy specimens from patients treated with either placebo (
![]() ) or genistein (
) or genistein (
![]() ). The data were normalised to aminolevulinate synthase gene and are shown as means, with their standard errors. The protein expression level of (d) p21Waf1/Cip1, (e) p27Kip1 and (f) p53 was determined by immunohistochemical staining of tissue microarrays containing normal and Gleason grade 3 (G3) and/or Gleason grade 4 (G4) spots of prostatectomy specimens. The figures show the mean staining intensity or percentage positive cells, with their standard errors.
). The data were normalised to aminolevulinate synthase gene and are shown as means, with their standard errors. The protein expression level of (d) p21Waf1/Cip1, (e) p27Kip1 and (f) p53 was determined by immunohistochemical staining of tissue microarrays containing normal and Gleason grade 3 (G3) and/or Gleason grade 4 (G4) spots of prostatectomy specimens. The figures show the mean staining intensity or percentage positive cells, with their standard errors.
The mRNA and protein expression results for the cell cycle-related biomarkers were coherent in both study arms, showing increased levels of p21Waf1/Cip1 and p53 and reduced levels of p27Kip1 in tumour tissue compared to normal tissue. The percentage p21Waf1/Cip1 positive cells was less than 1 % in normal prostate tissue and increased with increasing Gleason grade to about 2 % in G3 cells and 4 % in G4 cells. Fig. 4(a) depicts a patient from the genistein group showing an increasing number of p21Waf1/Cip1 positive nuclei from normal tissue to G4. The nuclear expression of p27Kip1 was significantly reduced in G3 (P = 0·016) compared to normal, whereas the mRNA level was non-significantly reduced in tumour compared to normal cells (Fig. 3(b) and (e)). Fig. 4(b) shows a patient from the placebo group with strong nuclear/weak cytoplasmic staining intensity in normal cells and weak nuclear/strong cytoplasmic p27Kip1 staining in G3. Fig. 4(c) shows a patient treated with placebo with no p53 positive cells in normal tissue and with single p53 positive cells in G3 and G4 tissues.

Fig. 4 Immunohistochemical staining of cell cycle-related biomarkers. Each row shows tissue microarray spots from a single patient containing normal, Gleason grade 3 (G3) and/or Gleason grade 4 (G4) prostate tissues. Magnification × 400 for (a) cyclin-dependent kinase inhibitor 1A and (b) cyclin-dependent kinase inhibitor 1B. Magnification × 600 for (c) tumour protein p53.
Proliferation- and apoptosis-related biomarkers
Genistein intervention had no significant effects on the protein expression of Ki67, BAX or BCL-2 (Fig. 5).

Fig. 5 Expression of proliferation- and apoptosis-related biomarkers. The protein expression of (a) Ki67, (b) B-cell CLL/lymphoma 2-associated X protein and (c) B-cell CLL/lymphoma 2 was determined by immunohistochemical staining of tissue microarrays containing normal and Gleason grade 3 (G3) and/or Gleason grade 4 (G4) spots of prostatectomy specimens. The figures show the mean staining intensity or percentage positive cells, with their standard errors.
![]() , Placebo;
, Placebo;
![]() , genistein.
, genistein.
In both study arms, Ki67 and BAX expression increased with increasing Gleason grade. Ki67 was expressed by 1 % of normal epithelial cells and it increased significantly to 3 % in G3 cells (P < 0·001) and further to approximately 5 % in G4 cells (Fig. 5(a)). Fig. 6(a) depicts a patient treated with genistein showing increasing number of Ki67 positive cells from normal tissue to G4. BAX protein expression increased significantly (P = 0·011) in G3 compared to normal cells (Fig. 5(b)). Fig. 6(b) shows a patient treated with genistein with weak cytoplasmic BAX staining in normal tissue and increasing intensity in tumour tissue. BCL-2 was in general not expressed in normal epithelial cytoplasm (Fig. 5(c)). The increased expression in malignant tissue was not statistically significant (P = 0·125). Fig. 6(b) shows a patient treated with genistein with weak BCL-2 staining in normal tissue and stronger staining in tumour tissue.

Fig. 6 Immunohistochemical staining of proliferation- and apoptosis-related biomarkers. Each row shows tissue microarray spots from a single patient containing normal, Gleason grade 3 (G3) and Gleason grade 4 (G4) prostate tissues. Magnification × 400. (a) Ki67, (b) B-cell CLL/lymphoma 2-associated X protein and (c) B-cell CLL/lymphoma.
Neuroendocrine differentiation-related biomarkers
Genistein intervention had no significant effect on NSE or CgA (Fig. 7).

Fig. 7 Expression of neuroendocrine differentiation-related biomarkers. The protein expression of (a) neuron-specific enolase and (b) cytoplasmic chromogranin A was determined by immunohistochemical staining of tissue microarrays containing normal and Gleason grade 3 (G3) and/or Gleason grade 4 (G4) spots of prostatectomy specimens. The figures show the mean positive/negative stain, with their standard errors.
![]() , Placebo;
, Placebo;
![]() , genistein.
, genistein.
In both study arms, the expression of the neuroendocrine differentiation-related biomarkers indicated reduced levels with increasing Gleason grade. Fig. 8(a) shows a patient treated with placebo with decreasing number of NSE-positive cells in G3 tumour. There was a clear presence of CgA-positive cells in normal tissue, which was completely abolished in G4 tissue in both treatment arms (P < 0·001; Fig. 7(b)). Fig. 8(b) shows a patient treated with genistein with decreasing number of CgA-positive cells with higher Gleason grade.

Fig. 8 Immunohistochemical staining of neuroendocrine differentiation-related biomarkers. Both rows show tissue microarray spots from the same patient containing normal and Gleason grade 3 (G3) prostate tissues. Magnification × 400. (a) neuron-specific enolase and (b) cytoplasmic chromogranin A.
Discussion
To our knowledge, this is the first study which exclusively investigates the effects by genistein alone in men with PCa. We showed that genistein intervention significantly down-regulated the mRNA expression levels of KLK4 in cancer cells in men with localised PCa. Genistein has previously been shown to decrease AR nuclear binding to the transcriptional binding site for AR( Reference Davis, Kucuk and Sarkar 10 ). We also report a decrease by genistein on most of the selected androgen-related biomarkers, corroborating the results of our in vitro studies( Reference Lazarevic, Karlsen and Saatcioglu 11 ). However, the average plasma level in the present study was at least 25-fold lower than the concentrations used in cell culture studies, indicating that in vivo nutritional relevant levels of genistein may have comparable effects to high-dose cell studies. This may be related to longer in vivo exposure. The androgen-related biomarkers used in this study have been attributed roles in the development or progression of PCa. NKX3·1 is an androgen-regulated homeobox gene located on chromosome 8p21·2, a region that shows a high degree of loss of heterozygosity in PCa( Reference Bova, Carter and Bussemakers 12 , Reference Bieberich, Fujita and He 13 ). Reports of its function and expression in PCa have been conflicting. However, most reports indicate that NKX3·1 acts as a non-classical tumour suppressor and that its expression is reduced in primary PCa and further reduced in metastatic PCa( Reference Abate-Shen, Shen and Gelmann 14 , Reference Gurel, Ali and Montgomery 15 ). However, our immunohistochemistry results indicated a translocation of NKX3·1 from the nucleus to the cytoplasm and not a total reduction with increasing Gleason grade. Interestingly, increased cytoplasmic stain intensity in primary PCa was briefly mentioned by Gurel et al. ( Reference Gurel, Ali and Montgomery 15 ), although it was not taken into account during scoring. Different splicing variants of NKX3·1 and antibodies have previously been attributed for the conflicting results of NKX3·1 expression. Human kallikrein 4 (KLK4), a serine protease belonging to the prostate specific antigen-related kallikrein family, is overexpressed in PCa and might be a proliferative factor acting directly or indirectly on cell cycle regulators( Reference Klokk, Kilander and Xi 16 ). Increased KLK4 levels in PC-3 cells have also been associated with a transcriptional repression of E-cadherin and increased Matrigel motility( Reference Veveris-Lowe, Lawrence and Collard 17 ). Our results were consistent with previous reports showing an overexpression of KLK4 mRNA and protein in PCa.
There are no previous reports on in vivo studies on the effects of genistein on the cell cycle-related genes p21 Waf1/Cip1 , p27 Kip1 and p53. However, in vitro studies on prostate, breast and lung cancer cells report that genistein up-regulates them, in line with its inhibitory effect on cell cycle progression( Reference Kobayashi, Nakata and Kuzumaki 18 , Reference Pinski, Wang and Quek 19 ). We did not observe any significant effects by genistein, but the mRNA levels of p21Waf1/Cip1, p27Kip1 and p53 were slightly down-regulated. The reason for this discrepancy is not clear. Interestingly, we showed an increased expression of p21Waf1/Cip1 and p53 mRNA and protein expression in G3 and that the expression may further increase with increasing Gleason grade. Navone et al. ( Reference Navone, Troncoso and Pisters 20 ) showed that p53 accumulation is associated with late-stage PCa. With a cutoff value at 5 % of p53 positive cells, they detected no accumulation in nineteen patients with Gleason score 5–7, whereas nineteen of forty-two patients with Gleason score 8–10 showed accumulation. p21Waf1/Cip1 may act both as a cell cycle negative regulator and anti-apoptotic mediator and its protein expression has been shown to correlate strongly with p53 in PCa( Reference Janicke, Sohn and Essmann 21 , Reference Shiraishi, Watanabe and Muneyuki 22 ). The reduction of p27Kip1 mRNA and nuclear protein expression in both study arms and the small reversal of protein expression in G3 and G4 by genistein intervention may have an interesting implication for the cell cycle modulating properties of genistein. Loss of phosphatase and tensin homologue function and/or constitutive activation of Akt/protein kinase B by the phosphoinositide kinase-3 pathway, commonly found in PCa, will reduce the expression of p27Kip1 ( Reference Li, Yen and Liaw 23 , Reference Medema, Kops and Bos 24 ). In vitro studies on genistein indicate that it inhibits protein kinase B constitutive activation by re-establishing phosphatase and tensin homologue expression( Reference Li and Sarkar 25 , Reference Kikuno, Shiina and Urakami 26 ). Although the increase of p27kip1 in G3 and G4 by genistein was not significant, this result may indicate the mechanism for its G1 cell cycle arrest and proliferation inhibition of tumour cells( Reference Shen, Klein and Wei 27 ). Further research is needed in this connection.
The proliferation biomarker Ki67 was clearly up-regulated in tumour tissue, although it was not modulated by genistein. Our detection of 1 % Ki67 positive luminal cells in normal and 4–6 % in tumour tissue corroborates with previous publications( Reference Barbisan, Mazzucchelli and Santinelli 28 ). The increasing levels of Ki67, p21Waf1/Cip1 and p53 may indicate that loss of p53 function already is present in some G3 cancers.
The apoptotic biomarkers BAX and BCL-2 were both significantly or near-significantly up-regulated in PCa, corroborating a previous report on Gleason score 5–10( Reference Krajewska, Krajewski and Epstein 29 ). We observed no significant regulation of BAX or BCL-2 by genistein intervention.
The role of neuroendocrine markers as prognostic factors is controversial, although the serum level of CgA and NSE seems to have a prognostic value in castration-resistant PCa( Reference Taplin, George and Halabi 30 – Reference Hvamstad, Jordal and Hekmat 32 ). Genistein treatment of LNCaP human prostatic adenocarcinoma cells has been shown to induce several positive biomarkers for neuroendocrine differentiation including CgA( Reference Pinski, Wang and Quek 19 ). We detected very few CgA- and NSE-positive cells in tumour tissue. The reduction of CgA-positive cells in tumour tissue corroborates earlier results from serum, showing a reduction in localised PCa and an increase in castration-resistant PCa( Reference Kamiya, Akakura and Suzuki 31 ).
The ability of genistein to modulate the progression of existing PCa is not clear. In the present study, we have investigated the effects of pure genistein. As opposed to the majority of chemopreventive reports of genistein treatment, some recent studies on mouse xenograft models indicate genistein to promote increased metastasis of PCa, whereas isoflavones containing genistein and daidzein do not( Reference Hillman, Wang and Kucuk 33 – Reference El Touny and Banerjee 37 ). Other studies show that dietary genistein inhibits metastases of human cancer, including PCa, in mice and that the discrepancy may be related to methodology( Reference Lakshman, Xu and Ananthanarayanan 38 , Reference Gu, Zhu and Dai 39 ). In addition, Setchell et al. ( Reference Setchell, Brown and Zhao 40 ) raised doubts about the use of rodent models for gaining insight into the effect of isoflavones in humans due to differences in the metabolism of genistein. We did not detect any signs of cancer-promoting effects in our study with pure genistein intervention in our human study subjects having early localised PCa.
Overall, genistein intervention at nutritionally relevant levels in patients with early PCa modulated several biomarkers which may be related to cancer prediction and progression. Genistein may have an inhibitory effect on androgen-related biomarkers. We also suggest a possible mechanism as to how genistein may induce cell cycle arrest and inhibition of proliferation in PCa. A limitation in our study is the small number of cases included and also the relative short time of intervention. Further studies on the effects of genistein in PCa are warranted, including clinical studies examining biomarkers and in vitro studies investigating its mechanisms of action.
Acknowledgements
The authors are grateful for the technical assistance by Olov Ogren and the assistance by DSM Nutritional Products Limited in the process of obtaining approvals and for supplying the study capsules. O. K. is a Georgia Cancer Coalition Distinguished Cancer Scholar. This was an investigator-initiated and -driven study. The work was performed at Oslo University Hospital, Aker and the study was solely financed with official grants from the hospital. B. L. and S. J. K. designed the study and had overall responsibility for the project. O. K., F. S., K. A. T. and A. S. assisted in the design of various parts of the study. B. L. was responsible for the subject selection, data collection and final data analysis. B. L. and H. R. conducted the PCR analysis. C. H. and A. S. performed the laser capture microdissection and conducted the immunohistochemical analysis. J. Y. conducted the androgen-related gene immunohistochemistry. L. M. D. conducted the statistical analysis. B. L., S. J. K., K. A. T. and A. S. drafted the paper, and all authors contributed to the final completion of the manuscript. The authors have no conflicts of interest.












