Introduction
Invasive free-living species may directly and indirectly influence the biodiversity in newly invaded habitats after their establishment. Their impact is mostly attributed to direct interaction with the local free-living communities (e.g. competition for resources, predation, hybridization with native species etc.) (Mooney and Cleland, Reference Mooney and Cleland2001; Dextrase and Mandrak, Reference Dextrase and Mandrak2006; Shochat et al., Reference Shochat, Lerman, Anderies, Warren, Faeth and Nilon2010; Havel et al., Reference Havel, Kovalenko, Thomaz, Amalfitano and Kats2015). An indirect mode of action could be due to changes in the composition of the parasite communities in the new area (Torchin et al., Reference Torchin, Lafferty, Dobson, McKenzie and Kuris2003; Calhoun et al., Reference Calhoun, McDevitt-Galles and Johnson2018; Hohenadler et al., Reference Hohenadler, Nachev, Freese, Pohlmann, Hanel and Sures2019), as parasites can reduce host density (Anderson and May, Reference Anderson and May1978; May and Anderson, Reference May and Anderson1978; Kuris and Lafferty, Reference Kuris and Lafferty1992; Hudson et al., Reference Hudson, Dobson and Newborn1998) or decrease host body size (Torchin et al., Reference Torchin, Lafferty and Kuris2001). For example, invasive species can co-introduce their own endemic parasites, which can spill over to native host populations (Torchin et al., Reference Torchin, Lafferty, Dobson, McKenzie and Kuris2003; Prenter et al., Reference Prenter, MacNeil, Dick and Dunn2004; Kelly et al., Reference Kelly, Paterson, Townsend, Poulin and Tompkins2009; David et al., Reference David, Staentzel, Schlumberger, Perrot-Minnot, Beisel and Hardion2018; Hohenadler et al., Reference Hohenadler, Nachev, Thielen, Taraschewski, Grabner and Sures2018a). Additionally, invasive species can contribute to the life cycle of native parasites. If an invasive species can serve as a suitable host (e.g. intermediate, paratenic or final host) for local parasites, these parasites may spill back to other local hosts, thereby increasing their infection rates within the native host populations (Kelly et al., Reference Kelly, Paterson, Townsend, Poulin and Tompkins2009; Šlapanský et al., Reference Šlapanský, Jurajda and Janáč2016). In contrast, invasive species might also be responsible for a decrease of the infection risk in the native host populations (e.g. Gagne et al., Reference Gagne, Heins, McIntyre, Gilliam and Blum2016; Šlapanský et al., Reference Šlapanský, Jurajda and Janáč2016).This so-called dilution effect occurs if the invaders serve as inappropriate hosts for local parasites, in which the parasites cannot develop further or if they are not favoured as food item by a predatory definitive host (Ostfeld and Keesing, Reference Ostfeld and Keesing2000; Johnson et al., Reference Johnson, Preston, Hoverman, Henderson, Paull, Richgels and Redmond2012).
Ponto-Caspian gobies (Osteichthyes, Gobiidae) have a high invasion potential, which allowed them to spread into areas distant from their native range. In recent years, they successfully established in the Baltic, Aegean and North Sea basins (Skóra and Stolarski, Reference Skóra and Stolarski1993; Kakareko et al., Reference Kakareko, Pla̧chocki and Kobak2009; Mierzejewska et al., Reference Mierzejewska, Martyniak, Kakareko, Dzika, Stańczak and Hliwa2011; Herlevi et al., Reference Herlevi, Puntila, Kuosa and Fagerholm2017) and even in the North American Great Lakes (Corkum et al., Reference Corkum, Sapota and Skora2004; Kornis et al., Reference Kornis, Sharma and Jake Vander Zanden2013). After the inauguration of the Maine-Danube canal in 1992 several Ponto-Caspian gobiids have invaded the Rhine River system (Stemmer, Reference Stemmer2008; van Kessel et al., Reference van Kessel, Dorenbosch and Spikmans2009; Borcherding et al., Reference Borcherding, Staas, Krüger, Ondračková, Šlapanský and Jurajda2011; Kalchhauser et al., Reference Kalchhauser, Mutzner, Hirsch and Burkhardt-Holm2013), with the round goby Neogobius melanostomus and the bighead goby (Ponticola kessleri) being the most abundant and widespread species (Kottelat and Freyhof, Reference Kottelat and Freyhof2007; Borcherding et al., Reference Borcherding, Staas, Krüger, Ondračková, Šlapanský and Jurajda2011). Although some ecological parameters such as density, fecundity, growth, predation and parasitism were already studied in gobies from several non-native regions of the Danube River and Rhine River (Kvach, Reference Kvach2002; Jurajda et al., Reference Jurajda, Černý, Polačik, Valová, Janáč, Blažek and Ondračková2005; Adámek et al., Reference Adámek, Andreji and Gallardo2007; L'avrinčíková and Kováč, Reference L'avrinčíková and Kováč2007; Kvach and Stepien, Reference Kvach and Stepien2008; Kováč et al., Reference Kováč, Copp and Sousa2009; Ondračková et al., Reference Ondračková, Dávidová, Blažek, Gelnar and Jurajda2009; Mühlegger et al., Reference Mühlegger, Jirsa, Konecny and Frank2010; Kalchhauser et al., Reference Kalchhauser, Mutzner, Hirsch and Burkhardt-Holm2013; David et al., Reference David, Staentzel, Schlumberger, Perrot-Minnot, Beisel and Hardion2018), their impact on species assemblages in the ecosystems throughout Europe and the River Rhine in particular still remains largely unknown. Ponto-Caspian gobies for example were found to negatively affect the population densities of some native fish species (Dubs and Corkum, Reference Dubs and Corkum1996; Mooney and Cleland, Reference Mooney and Cleland2001; Balshine et al., Reference Balshine, Verma, Chant and Theysmeyer2005; Karlson et al., Reference Karlson, Almqvist, Skóra and Appelberg2007; Jakšić et al., Reference Jakšić, Jadan and Piria2016; van Kessel et al., Reference van Kessel, Dorenbosch, Kranenbarg, van der Velde and Leuven2016).
According to previous studies more than 20 different parasite species are known to infest gobiids in the native range of their distribution (e.g. Lower Danube and Black Sea area; see Kvach, Reference Kvach2005; Ondračková et al., Reference Ondračková, Trichkova and Jurajda2006), while a significantly lower number of species is usually reported for non-native areas. Within the well documented and studied Rhine River for example, only 8 different species have been reported for N. melanosomus (Emde et al., Reference Emde, Rueckert, Palm and Klimpel2012, Reference Emde, Rueckert, Kochmann, Knopf, Sures and Klimpel2014; Ondračková et al., Reference Ondračková, Valová, Hudcová, Michálková, Šimková, Borcherding and Jurajda2015) and 7 for P. kessleri (Ondračková et al., Reference Ondračková, Valová, Hudcová, Michálková, Šimková, Borcherding and Jurajda2015). In general, Ponto-Caspian gobies show high infestation rates with acanthocephalans of the genus Pomphorhynchus in both their native and non-native range, with a prevalence often exceeding 90% and correspondingly high intensities (Kvach and Skóra, Reference Kvach and Skóra2006; Francová et al., Reference Francová, Ondračková, Polačik and Jurajda2011; Emde et al., Reference Emde, Rueckert, Kochmann, Knopf, Sures and Klimpel2014; Ondračková et al., Reference Ondračková, Valová, Hudcová, Michálková, Šimková, Borcherding and Jurajda2015). Hohenadler et al. (Reference Hohenadler, Nachev, Thielen, Taraschewski, Grabner and Sures2018a) provided examples of how the acanthocephalan Pomphorhynchus laevis (now most likely to be considered as Pomphorhynchus bosniacus, see Reier et al., Reference Reier, Sattmann, Schwaha, Harl, Konecny and Haring2019), which was introduced by Ponto-Caspian invaders (spill-over), can outcompete a local acanthocephalan species (Pomphorhynchus tereticollis) and thus change the species composition of the parasite communities in the Rhine system. As the evidence for the occurrence of P. bosniacus in Central Europe (Reier et al., Reference Reier, Sattmann, Schwaha, Harl, Konecny and Haring2019) was published after the study by Hohenadler et al. (Reference Hohenadler, Nachev, Thielen, Taraschewski, Grabner and Sures2018a), it cannot be decided with certainty in retrospect whether they found P. laevis or P. bosniacus in the Rhine system although the latter seems to be more likely.
However, individuals of Pomphorhynchus sp. cannot complete their life cycle in gobies and therefore remain encysted in their abdominal cavity as larval or preadult stages. Mainly cyprinids serve as appropriate definitive hosts, with fish species such as barbel (Barbus barbus), chub (Squalius cephalus) or idle (Leuciscus idus) playing the major roles in the River Rhine (David et al., Reference David, Staentzel, Schlumberger, Perrot-Minnot, Beisel and Hardion2018; Hohenadler et al., Reference Hohenadler, Honka, Emde, Klimpel and Sures2018b). Until now, the relevance of Ponto-Caspian gobiids for the transmission of Pomphorhynchus spp. remains unclear. On the one hand, they can reduce the risk of infection for the native fish populations (dilution effect) if the acanthocephalans cannot be transmitted successfully from gobiids to other fish hosts. On the other hand, the gobiids might increase the infection risk, if they serve as appropriate paratenic hosts (spill back), which are preyed upon by local piscivorous definitive hosts (see e.g. Grabowska et al., Reference Grabowska, Błońska, Ondračková and Kakareko2023). A similar case was already demonstrated for larvae of Anguillicola crassus detected in gobies from the Rhine River that remain infectious for the final host, the European eel (Hohenadler et al., Reference Hohenadler, Honka, Emde, Klimpel and Sures2018b).
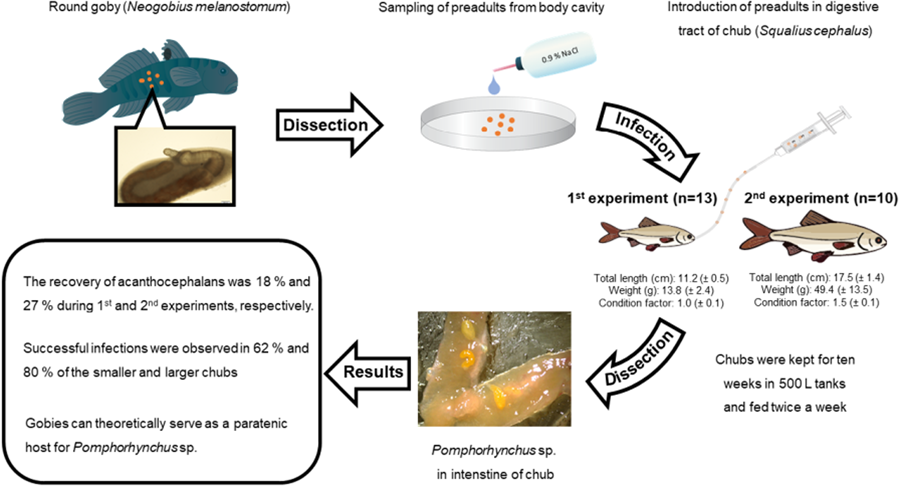
The aim of this study was to investigate whether the preadult Pomphorhynchus sp. detected in invasive gobies remain infectious to the final host, and whether gobies can thus theoretically contribute to the transmission of these acanthocephalans to their final hosts. To evaluate the infection potential of preadult (larval) stages obtained from the abdominal cavity of gobiids, a laboratory infection experiment with chub (S. cephalus) was performed to determine infection success (i.e. recovery rate) and development of the preadult acanthocephalans. The resulting data were compared with results from previous infection experiments (Siddall and Sures, Reference Siddall and Sures1998; Sures and Siddall, Reference Sures and Siddall1999, Reference Sures and Siddall2003; Sures et al., Reference Sures, Dezfuli and Krug2003; Ruchter, Reference Ruchter2012; Le et al., Reference Le, Nachev, Grabner, Hendriks and Sures2016, Reference Le, García, Nachev, Grabner, Balsa-Canto, Hendriks and Sures2018), in which cystacanths of Pomphorhynchus sp. obtained from the amphipod intermediate host were administered to chub.
Materials and methods
Acanthocephalan sampling and fish infection
Infection experiments with acanthocephalans of the genus Pomphorhynchus were performed with chub (S. cephalus), as an appropriate definitive host. The fish were obtained from aquaculture facilities of the Research Institute for Nature and Forest, Belgium, where they were raised in spring water. Accordingly, they could be assumed to be free of any infections with metazoan parasites, which was verified by ten randomly chosen chub, which were killed, dissected and screened with light microscopy for parasites.
Cysts containing preadults of Pomphorhynchus sp. were collected from the abdominal cavity of N. melanostomus. The latter were sampled in March and October 2016 by professional fishermen near Kalkar at the Lower Rhine River (844 river km) and kept alive in aerated water tanks. Prior to the infection experiments, the gobies were sacrificed, dissected and the extracted acanthocephalans were placed in physiological saline (0.9% NaCl) and stored at 5°C overnight. Most of the preadult acanthocephalans were entirely enclosed by a fibrous capsule of variable thickness (see Fig. 1). Worth noticing here is, that some thick-walled cysts were not light transparent at all and the preadults inside appeared almost completely degenerated. Therefore, such cysts with preadults were not considered for the infection experiments.

Figure 1. General occurrence of preadult Pomphorhynchus sp. obtained from the abdominal cavity of gobiids.
Subsequently, the cysts containing the larvae were collected randomly from a Petri-dish and were introduced to the digestive tracts of chubs by using a 2 ml syringe equipped with a 12 cm plastic tubing of 1 mm diameter (for details see Sures and Siddall, Reference Sures and Siddall2003). In total, 23 chubs were experimentally infected, where 1 group of fish (n = 13) was infected with 6 acanthocephalans per fish (1st experiment) and another group of fish (n = 10) was infected with 10 acanthocephalans each (2nd experiment). As both groups were not infected at the same time (approximately 6 months difference) the second group of chubs (n = 13) exhibited on average a larger body size than the other (17.4 cm total vs 11.2 cm length, respectively; see Table 1). However, chubs were from the same brood and were held under the same conditions over time. Before and after the infection, the fish were kept at approximately 20°C water temperature in 500 l tanks with dechlorinated tap water and fed twice per week with commercial pellets. A light cycle with a ratio of 16:8 (light: dark) was simulated in order to provide conditions similar to those in their natural habitats. After 10 weeks the chubs were anaesthetized with 150 mg mL−1 MS-222 (Merck, Darmstadt, Germany) and then sacrificed by cervical dislocation. After dissection, the parasites were removed from the digestive tracts and counted. All infection experiments were carried out in accordance with the relevant guidelines and regulations and were approved by the Ethics Council (Landesamt für Natur, Umwelt und Verbraucherschutz, Nordrhein-Westfalen, permit number: 84-02.05.40.16.017).
Table 1. Morphological data of chub (S. cephalus) and mean recovery rates and ranges obtained for chubs infected with preadults (present study) and cystacanths of Pomphorhynchus sp. (see Siddall and Sures, Reference Siddall and Sures1998; Sures and Siddall, Reference Sures and Siddall1999, Reference Sures and Siddall2003; Sures et al., Reference Sures, Dezfuli and Krug2003; Ruchter, Reference Ruchter2012; Le et al., Reference Le, Nachev, Grabner, Hendriks and Sures2016, Reference Le, García, Nachev, Grabner, Balsa-Canto, Hendriks and Sures2018)

n.a., parameter is not available.
Molecular identification of parasites
As a recent study published after our infection experiments indicates the presence of P. bosniacus in major European rivers (Reier et al., Reference Reier, Sattmann, Schwaha, Harl, Konecny and Haring2019), we sequenced random samples of preadults to check the identity of the Pomphorhynchus species present in the investigated section of the Rhine River.
DNA was extracted from the preadult Pomphorhynchus sp. using a Chelex-protocol. Approximately 0.5 mm pieces of the parasite tissue were cut off and placed in 300 μL of a 10% Chelex 100 resin (Bio-Rad)-solution. Samples were then boiled for 20 min at 95°C and vortexed every 5 min. Subsequently, samples ware cooled on ice and centrifuged at 5,000 ×g for 5 min, whereas the resulting supernatant was used thereafter for PCR. The PCR was conducted with the primers PT/PL COI F and PT/PL COI R according to Tierney et al. (Reference Tierney, Caffrey, Vogel, Matthews, Costantini and Holland2020). The PCR-products were sent for sequencing (Microsynth-Seqlab) and the sequences were compared with the entries for P. laevis and P. bosniacus in the BOLD database (https://www.boldsystems.org/index.php).
Calculations and statistical evaluation
Fulton's condition factor (K) was calculated according to Nash et al. (Reference Nash, Valencia and Geffen2006). The recovery rates were evaluated as a percentage of the acanthocephalans recovered during dissection in relation to the number of administered preadults. In order to compare the infection success of preadult (encapsulated) Pomphorhynchus sp. with those of cystacanths, data from our previous infection experiments with cystacanths (Siddall and Sures, Reference Siddall and Sures1998; Sures and Siddall, Reference Sures and Siddall1999, Reference Sures and Siddall2003; Sures et al., Reference Sures, Dezfuli and Krug2003; Ruchter, Reference Ruchter2012; Le et al., Reference Le, Nachev, Grabner, Hendriks and Sures2016, Reference Le, García, Nachev, Grabner, Balsa-Canto, Hendriks and Sures2018), were considered (for details see Table 1).
The metric parameters of fish from the 1st and the 2nd experiment as well as the recovery rates of acanthocephalans were compared with a Mann–Whitney U-test. Kruskal–Wallis test was applied for comparing the current data with studies of Siddall and Sures, Reference Siddall and Sures1998; Sures and Siddall, Reference Sures and Siddall1999, Reference Sures and Siddall2003; Sures et al., Reference Sures, Dezfuli and Krug2003; Ruchter, Reference Ruchter2012; Le et al., Reference Le, Nachev, Grabner, Hendriks and Sures2016, Reference Le, García, Nachev, Grabner, Balsa-Canto, Hendriks and Sures2018. The recovery rates were correlated with condition factors of fish using Spearman rank correlation analysis.
Results
The subsequent molecular identification of acanthocephalans showed that all sequenced isolates were P. bosniacus according to Reier et al. (Reference Reier, Sattmann, Schwaha, Harl, Konecny and Haring2019), suggesting that the preadults used for the infection experiments can most likely also be classified as P. bosniacus.
The metric parameters of fish used in the infection experiment are presented in Table 1. As the infection experiments were performed in different time periods, the sizes of fish individuals between both experimental groups differed significantly (cf. materials and method) with fish from the second infection experiment being significantly larger with respect to total length and body mass. However, the fish condition factor was similar during both experiments (0.97 and 0.91, respectively).
There were no significant differences between recovery rates of preadults, when comparing both infection experiments (see Fig. 2). However, the smaller chubs in the first experiment showed slightly lower mean recovery rates (17.9%) in comparison to the larger ones (27.0%) from the second experiment (Table 1, Fig. 2). The previous infection experiments with cystacanths of Pomphorhynchus sp. (see Siddall and Sures, Reference Siddall and Sures1998; Sures and Siddall, Reference Sures and Siddall1999, Reference Sures and Siddall2003; Sures et al., Reference Sures, Dezfuli and Krug2003; Ruchter, Reference Ruchter2012; Le et al., Reference Le, Nachev, Grabner, Hendriks and Sures2016, Reference Le, García, Nachev, Grabner, Balsa-Canto, Hendriks and Sures2018) showed a significantly higher overall establishment of Pomphorhynchus sp. in the final host in comparison to the preadults used in the current study (Kruskal–Wallis test, P < 0.05, see Fig. 2). The lowest recovery rates for cystacanths were recovered during the infection experiment of Sures and Siddall (Reference Sures and Siddall1999) and the highest one was obtained by Sures and Siddall (Reference Sures and Siddall2003) being 44.8 and 70.8%, respectively (Fig. 2).

Figure 2. Recovery rates of preadults (current study – 1st and 2nd experiments) and cystacanths of Pomphorhynchus sp. (Siddall and Sures, Reference Siddall and Sures1998; Sures and Siddall, Reference Sures and Siddall1999, Reference Sures and Siddall2003; Sures et al., Reference Sures, Dezfuli and Krug2003; Ruchter, Reference Ruchter2012; Le et al., Reference Le, Nachev, Grabner, Hendriks and Sures2016, Reference Le, García, Nachev, Grabner, Balsa-Canto, Hendriks and Sures2018). Open dots are means, lines within the box are medians, boxes are interquartile ranges, error bars are interdecile ranges and closed dots are outliers.
Discussion
Despite the fact that the acanthocephalans of the genus Pomphorhynchus occur at high prevalence and intensity in Ponto-Caspian gobies in both invasive and native distribution ranges (see Kvach and Skóra, Reference Kvach and Skóra2006), the role of fish in parasite transmission is still unclear. Kennedy (Reference Kennedy2006) stated that acanthocephalans in accidental and paratenic hosts share the same morphological and developmental features. However, unlike those in accidental hosts, the acanthocephalans from a paratenic host can resume development if they are transferred to a suitable definitive host. Even adult acanthocephalans can be transmitted from one vertebrate host to another through a process known as post-cyclic transmission (Nickol, Reference Nickol2003; Kennedy, Reference Kennedy2006), demonstrating the great flexibility of the acanthocephalan life-cycles (Perrot-Minnot et al., Reference Perrot-Minnot, Cozzarolo, Amin, Barčák, Bauer, Filipović Marijić, García-Varela, Servando Hernández-Orts, Yen Le, Nachev, Orosová, Rigaud, Šariri, Wattier, Reyda and Sures2023). The fact that the recovered female acanthocephalans from the infected chubs in the present study harboured already mature eggs (spindle-like form) suggests that preadult individuals of Pomphorhynchus sp. that were collected from the body cavity of N. melanostomus can resume their further development to adults in an appropriate definitive fish host. Considering the high population density of gobies and their high infection rates with Pomphorhynchus spp. in newly invaded regions, it is likely that the gobies can contribute significantly to acanthocephalan's transmission and thus increase the infections levels in native host populations (amphipods and fish). It still remains unclear, which invasion scenario (spill back or spill over) regarding the Pomphorhynchus species in the Rhine River system is valid. Previous parasitological surveys on gobiids from Rhine River and other geographical regions have identified and reported the preadult acanthocephalans as P. laevis (see for example the publications of Ondračková et al., Reference Ondračková, Trichkova and Jurajda2006, Reference Ondračková, Dávidová, Blažek, Gelnar and Jurajda2009, Reference Ondračková, Valová, Hudcová, Michálková, Šimková, Borcherding and Jurajda2015) or P. tereticollis (e.g. Emde et al., Reference Emde, Rueckert, Palm and Klimpel2012). However, according to the recent taxonomic studies on the genus Pomphorhynchus and the published sequences (see Reier et al., Reference Reier, Sattmann, Schwaha, Harl, Konecny and Haring2019), the individuals from gobiids in Rhine River (present study) should be P. bosniacus. Obviously, gobiids can host preadults of different species of the genus Pomphorhynchus, whereas for P. laevis, which is assumed as a native species in Central and Western Europe (see e.g. Kennedy, Reference Kennedy2006; Médoc et al., Reference Médoc, Rigaud, Motreuil, Perrot-Minnot and Bollache2011; Perrot-Minnot et al., Reference Perrot-Minnot, Guyonnet, Bollache and Lagrue2019) a spill-back scenario via invasive gobiids might be possible. According to the original description of P. bosniacus (Kiskároly and Čanković, Reference Kiskároly and Čanković1967) and recent studies (see Nedic et al., Reference Nedic, Smrzlic, Paras and Nikolic2019; Reier et al., Reference Reier, Sattmann, Schwaha, Harl, Konecny and Haring2019), its geographical distribution is assumed to be restricted mainly to the Ponto-Caspian region and the Danube River system in particular. Thus, in the case of P. bosniacus a spill over scenario via gobiids might be possible, however, due to its missing invasion history it remains unknown if it was co-introduced in the Rhine River system with the invasion of gobiids (spill-over) or via other hosts (e.g. Ponto-Caspian amphipods). Additionally, the conflicting taxonomy within the genus Pomphorhynchus based on morphological and genetic identification does not allow a proper estimation of the geographical distribution of different Pomphorhynchus species (see e.g. the studies of Emde et al., Reference Emde, Rueckert, Palm and Klimpel2012; Ondračková et al., Reference Ondračková, Valová, Hudcová, Michálková, Šimková, Borcherding and Jurajda2015; Hohenadler et al., Reference Hohenadler, Nachev, Thielen, Taraschewski, Grabner and Sures2018a, Reference Hohenadler, Honka, Emde, Klimpel and Sures2018b, which were conducted in the lower Rhine River). This is especially true for P. bosniacus, whose identification and records were based solely on molecular data, with no morphological comparison to the type material of its original description was possible (see Reier et al., Reference Reier, Sattmann, Schwaha, Harl, Konecny and Haring2019).
The involvement of paratenic hosts is common in life cycles of acanthocephalans. However, this was mostly reported for species of the classes Archiacanthocephala and Eoacanthocephala (Schmidt, Reference Schmidt, Crompton and Nickol1985; summarized also by Kennedy, Reference Kennedy2006). Among the Palaeacanthocephala, to which species of the genus Pomphorhynchus belong, 4 genera have been reported so far (e.g. Corynosoma, Leptorhynchoides, Andracantha, Bolbosoma) that use paratenic hosts in order to bridge the trophic gap between intermediate and definitive hosts (see also Rocka, Reference Rocka2006). These acanthocephalans require mostly a piscivorous definitive host that do not regularly feed on crustaceans. However, this might be the case also for species of genus Pomphorhynchus, which possibly can also use an complementary transmission route via a paratenic host, as demonstrated by Médoc et al. (Reference Médoc, Rigaud, Motreuil, Perrot-Minnot and Bollache2011). The latter authors also reported very similar infection rates for P. laevis cysts obtained from the body cavity of minnows (Phoxinus phoxinus), which were used to experimentally infect chubs (15–23% vs 17–29% in present study). In contrast to gobies, minnows can serve as definitive host for P. laevis, however, the maturation rate of acanthocephalans in their gut is very low (see Kennedy, Reference Kennedy1999).
Suitable definitive host for acanthocephalans of the genus Pomphorhynchus are usually cyprinids such as chub (S. cephalus) and common barbel (B. barbus) as well as salmonids (see e.g. Kennedy, Reference Kennedy2006; Perrot-Minnot et al., Reference Perrot-Minnot, Guyonnet, Bollache and Lagrue2019), however the latter rarely co-occur in the same habitats with gobies. Small fish might become one of the most important food items in the diet of large chubs or common barbel (Bašić et al., Reference Bašić, Britton, Jackson, Reading and Grey2014; see also Kottelat and Freyhof, Reference Kottelat and Freyhof2007), to which also gobiids are accounted (personal observation). The diet of chub is mostly habitat dependent, with a frequency of occurrence of fish in the diet of the chub of up to 8% in larger rivers (see e.g. Ünver and Erk'akan, Reference Ünver and Erk'akan2011), which in some cases exceeds the weight percentage of all other food items (up to 95% as reported by (Losos et al., Reference Losos, Penaz and Kubièkova1980). The common barbel commonly feeds during night upon benthic associated organisms having also access to prey items under larger stones (Vuković and Ivanović, Reference Vuković and Ivanović1971), where gobiids also hide to avoid predation. Benthic macroinvertebrates, small fish and fish eggs make up the majority of the barbel's diet, with the latter being the most common (see e.g. Losos et al., Reference Losos, Penaz and Kubièkova1980). Considering that gobies might be an essential part of the diet of piscivorous fish after they have become established in newly invaded habitats (reviewed by Grabowska et al., Reference Grabowska, Błońska, Ondračková and Kakareko2023), as reported for pikeperch from the newly colonized Kiel Canal (Thiel et al., Reference Thiel, Horn, Knörr and Tonn2014) or for pikeperch and perch from western Baltic Sea (Oesterwind et al., Reference Oesterwind, Bock, Förster, Gabel, Henseler, Kotterba, Menge, Myts and Winkler2017), they are most likely also used as food source by different large cyprinids and might additionally contribute to the transmission of Pomphorhynchus species.
Adults of Pomphorhynchus that are established in the intestine of a suitable fish definitive host can survive the predation of their fish host and even establish in the intestine of the predator. For example Kennedy (Reference Kennedy1999) investigated such a postcycling transmission of P. laevis by demonstrating the transfer of specimens from one definitive host to another. As the acanthocephalan's proboscis and bulb is commonly surrounded by fibrous tissue in the gut and body cavity of the definitive host, only non-mature adults of Pomphorhynchus sp. can survive the transfer and continue to mature and reproduce in the new fish host. The proboscis and bulbus of preadults obtained from the body cavity of gobiids were not encapsulated and remained intact, thereby being able to be used for establishment in the gut of an appropriate definitive host. However, most individuals were surrounded entirely by a fibrous capsule with variable thickness, whereas the preadults in the thick-walled cysts appeared less vital than the others with thin-walled, or without any cyst (personal observation). Prolonged residence in the body cavity of the gobies is likely to reduce the viability of the acanthocephalans, finally might lead to an inactivation (reduced infectiveness) and even death. In some cases, the parasites inside the thick-walled cysts appeared almost completely degenerated, which presumably was the result from the interaction with the host immune system. Similarly to the observations of Kennedy (Reference Kennedy1999), the preadult acanthocephalans in gobies can remain infectious probably only for a short period before they are surrounded with thick fibrous layer and thus become weakened/inactivated. Furthermore, Dezfuli et al. (Reference Dezfuli, Castaldelli, Bo, Lorenzoni and Giari2011) studied the fate of extraintestinal immature Pomphorhynchus sp. (preadults) encapsulated in the mesenteries and peritoneum of small sheatfish (Silurus glanis) that appeared to be similar to the ones found in gobiids. They found that the cyst wall consisted of 2 distinct layers with an outer one containing collagen fibres infiltrated with mast cells and an inner one, which was in direct contact with the parasite's tegument and comprised a large number of mast cells. Some of the latter were even located directly on the acanthocephalan tegument. As the mast cells were found to be the most common immune cells at attachment sites of Pomphorhynchus sp. and in the cyst wall of the extraintestinal preadults (Dezfuli et al., Reference Dezfuli, Castaldelli, Bo, Lorenzoni and Giari2011) it can be assumed that they are responsible for the inactivation and destruction of parasites as suggested by Murray et al. (Reference Murray, Leggiadro and Douglas2007). Therefore, depending on the duration of the interaction between the parasites and the fish immune system, the acanthocephalans in gobies and other paratenic hosts can exhibit different fitness and capability to infect further fish hosts after predation. This would also explain the differences between the recovery rates of acanthocephalans obtained from the gobies (preadults) and those taken directly from the crustacean intermediate host (cystacanths) observed in the previous infection experiments (Siddall and Sures, Reference Siddall and Sures1998; Sures and Siddall, Reference Sures and Siddall1999, Reference Sures and Siddall2003; Sures et al., Reference Sures, Dezfuli and Krug2003; Ruchter, Reference Ruchter2012; Le et al., Reference Le, Nachev, Grabner, Hendriks and Sures2016, Reference Le, García, Nachev, Grabner, Balsa-Canto, Hendriks and Sures2018). The vitality and infectiveness of the cystacanths in amphipods appears to be less affected over time, due to the lower complexity of the invertebrate immune response (e.g. Dezfuli et al., Reference Dezfuli, Bosi and Rossi1992, Reference Dezfuli, Simoni, Duclos and Rossetti2008) in comparison to those of vertebrates (e.g. for fish Dezfuli et al., Reference Dezfuli, Castaldelli, Bo, Lorenzoni and Giari2011) as well as the immune depression induced by cystacanths in their amphipod host (Cornet et al., Reference Cornet, Franceschi, Bauer, Rigaud and Moret2009).
Conclusions
The outcome of this study revealed that preadults of Pomphorhynchus sp. that occur in the body cavity of gobiids remain infectious and are able to resume their development in an appropriate fish definitive host. Accordingly, gobies and N. melanostomus in particular, might contribute to the transmission success of P. bosniacus, which could lead to a spill back to the local hosts in newly invaded habitats if the gobies are preyed by chub or barbel. However, due to the high abundance of gobies within the local fish community, a dilution-effect scenario might also occur, if they are not favoured by the definitive host as a prey item (effect on population level) or in the case that a longer interaction between the immune system of gobies and preadults lead to a lower infectiveness of the latter (effect on individual level). Therefore, further studies are required in order to extrapolate the findings of the current infection experiment to field conditions in Rhine River system. As no coinfection with other closely related species such as P. tereticollis was detected in the gobies in the present study, the role of gobiids in the transmission of other species of the genus needs to be further investigated. This might shed a light on interspecific competition between the species within the Pomphorhynchus genus occurring in the Rhine River system and thus reveal one of the mechanisms how P. tereticollis was outcompeted over time, as suggested by Hohenadler et al. (Reference Hohenadler, Honka, Emde, Klimpel and Sures2018b).
Data availability statement
Data available on request from the authors.
Authors’ contributions
BS, MN, MH conceived and designed the study. MN, BS, DG, MH was involved in writing process. NB, MH, MN, DG conducted data gathering and processing. All authors were involved in the experimental part of the study as well as in the data analysis and interpretation.
Financial support
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
Competing interests
None.
Ethical standards
All infection experiments were carried out in accordance with the relevant guidelines and regulations and were approved by the Ethics Council (Landesamt für Natur, Umwelt und Verbraucherschutz, Nordrhein-Westfalen, permit number: 84-02.05.40.16.017).