With a total population of c. 1,200 individuals endemic to two islands of the Comoros archipelago, Western Indian Ocean, Livingstone's flying fox Pteropus livingstonii is categorized as Critically Endangered on the IUCN Red List (Sewall et al., Reference Sewall, Young, Trewhella, Rodríguez-Clark and Granek2016). This fruit bat is primarily threatened by habitat loss (Trewhella et al., Reference Trewhella, Rodriguez-Clark, Davies, Reason and Wray2001; Granek, Reference Granek2002; Daniel et al., Reference Daniel, Green, Doulton, Salim, Said and Hudson2017), as timber extraction and unsustainable agricultural practices have led to an 80% decrease in forest cover since 1995 (Boussougou et al., Reference Boussougou, Brou, Mohamed, Baazaoui, Claramunt and Haddad2015; Doulton et al., Reference Doulton, Mohamed, Shepherd, Mohamed, Ali, Maddison, Sarre and Lapstun2015). The local NGO Dahari currently works to protect the species’ 15 known roost sites on Anjouan (Dahari, 2018). We piloted the use of GPS loggers on this bat species to evaluate the applicability of this method for identifying feeding sites and thus improving conservation measures.
Two Livingstone's flying foxes were captured in early 2019 (Fig. 1, Table 1), during 19.00–21.00 using custom-made canopy nets (length × height = 7 × 3 m, mesh size: 10 × 10 cm) mounted 7 m above the ground. The nets were monitored continuously, and captured bats were removed immediately. For each bat we recorded standard measurements (forearm, wing and body length, weight, and reproductive status according to Racey, Reference Racey, Kunz and Parsons2009; Table 1). We fitted GPS loggers (15 g Bird Solar, e-obs, Grünwald, Germany) mounted on collars (handmade, including a weak link allowing the collar to fall off after a maximum of 10 months). Together the GPS logger and collar weighed 20 g, not exceeding the recommended 5% of animal body weight (Aldridge & Brigham, Reference Aldridge and Brigham1988). The loggers were set to record one location every 90 seconds when moving and one location every 6 hours when stationary. Internal accelerometers recorded movement across three axes in bursts of 4 seconds per minute. Data were retrieved at roost sites by remote download via UHF.
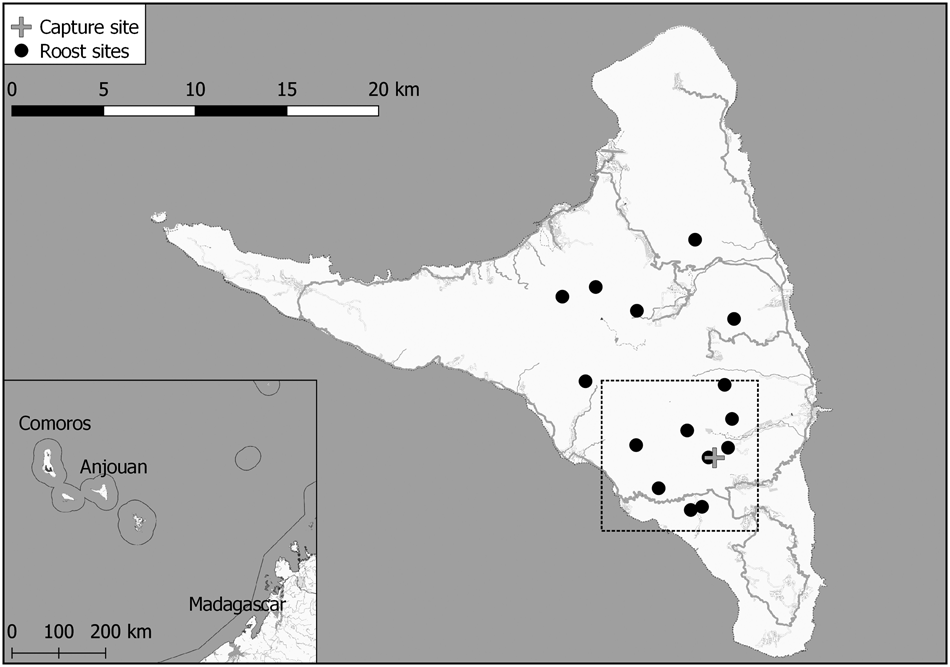
Fig. 1 Anjouan island in the Comoros archipelago, Western Indian Ocean, showing the 15 known long-term roost sites of Pteropus livingstonii and the site where the two individuals were captured. The dashed rectangle indicates the area covered by Fig. 3.
Table 1 Capture information and GPS data for two Pteropus livingstonii tagged with GPS loggers on Anjouan, Comoros.
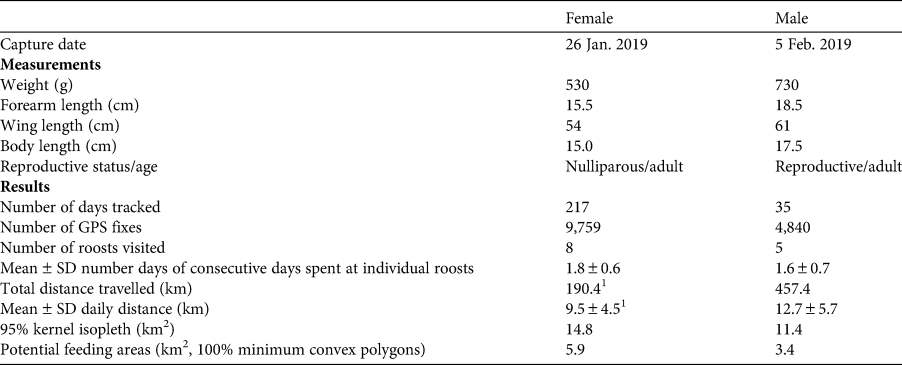
1Based on data for 26 Jan.–14 Feb. 2019; the remaining dates were excluded from path length analysis as the tag lost battery power and subsequently did not record GPS points consistently.
Home ranges were computed as 95% kernel density estimates in R 3.6 (R Core Team, 2019), using the package ctmm (Calabrese et al., Reference Calabrese, Fleming and Gurarie2016). Accelerometer data facilitated an approximate classification of behavioural categories based on the orientations and oscillations of the three axes in the Movebank Acceleration Viewer (Wikelski & Kays, Reference Wikelski and Kays2019): resting (the animal is stationary, head downwards), flying (the animal is moving across the landscape, head parallel to ground), scratching (the animal is stationary, head downwards, using its leg to scratch its fur) and moving/climbing (the animal is changing position within a tree, head position is variable; Fig. 2). These categories were based on Weber et al. (Reference Weber, Duengkae, Fahr, Dechmann, Phensakul and Khumbucha2015) and O'Mara et al. (Reference O'Mara, Scharf, Fahr, Abedi-Lartey, Wikelski, Dechmann and Safi2019), and preliminary behavioural observation of the study individuals: we observed the two collared individuals for 15 minutes after release, noting behaviours continuously until the bats were out of sight. These behaviours were matched with the acceleration data for the time of observation. The remaining acceleration data, and all corresponding location points, were subsequently classed into the behavioural categories. The data were additionally validated by visualization of location using QGIS 2.18 (QGIS Development Team, 2019); for example, flying behaviours only occur along a flight path.
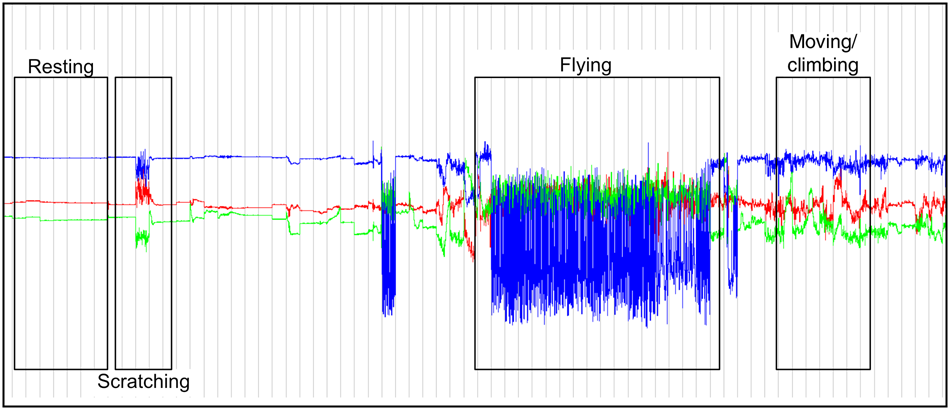
Fig. 2 Screenshot of the Movebank Acceleration Viewer (Wikelski & Kays, Reference Wikelski and Kays2019), showing, from top to bottom, the oscillations along the z, x and y axes recorded by the GPS loggers. The acceleration data were used to distinguish between behavioural categories (resting, scratching, flying and moving/climbing).
The GPS loggers recorded data for 217 and 35 days for the female and the male, respectively. Both individuals covered a mean area of 13.1 ± SD 1.7 km2 and frequented 5–8 known roost sites within the observation period (Fig. 3a). For details of path lengths and home range sizes, see Table 1.

Fig. 3 Movement data of two individual P. livingstonii on Anjouan (Fig. 1) were used to estimate 95% kernel density ranges, presented as low likelihood, high optimal, and maximum likelihood bandwidth home range areas (a), and polygons depicting potential feeding areas (b). Roost sites are named according to the nearest villages. The arrow indicates the area where flying fox feeding behaviour was ground-truthed.
In the complete dataset 4,086 (female) and 1,557 (male) location points were attributed to moving/climbing. As only baseline behaviours could be determined, moving/climbing behaviour probably included translocation within the same tree as well as feeding. All points categorized as moving/climbing were therefore treated as potential feeding sites. We conducted a cluster analysis of these points, grouping them based on distance, with the R package geosphere (Hijmans, Reference Hijmans2019). We computed 100% minimum convex polygons for each of the clusters using the R package adehabitatHR (Calenge, Reference Calenge2006), illustrating potential feeding areas (Fig. 3b, Table 1). Overlap of potential feeding areas between the two individuals was 2.7 km2. We subsequently ground-truthed the area with the greatest overlap (Fig. 3b), and, by conducting opportunistic behavioural observations of Livingstone's flying foxes present in the trees during 17.00–21.00, confirmed that many trees were feeding trees. The feeding trees were located in agricultural fields interspersed with individual native and non-native trees. There was no natural forest in the area.
These findings demonstrate that GPS technology facilitates the detection of feeding sites and the identification of landscape use by Livingstone's flying foxes. Individuals displayed flexibility in roost selection, with both individuals visiting and resting in at least five known roost sites, similar to what has been observed for other flying foxes (Palmer & Woinarski, Reference Palmer and Woinarski2000; Vardon et al., Reference Vardon, Brocklehurst, Woinarski, Cunningham, Donnelly and Tidemann2001; Banack & Grant, Reference Banack and Grant2016; Oleksy et al., Reference Oleksy, Ayady, Tatayah, Jones, Howey and Froidevaux2019).
Natural, undisturbed forest occurs only in fragments across Anjouan, and only three long-term roosts are located in forest habitat (Daniel et al., Reference Daniel, Green, Doulton, Salim, Said and Hudson2017). That feeding sites were in farmed areas was unexpected, based on predictive modelling (Ibouroi et al., Reference Ibouroi, Cheha and Astruc2018). However, Ibouroi et al. (Reference Ibouroi, Cheha and Astruc2018) found that flying fox presence was correlated with tall trees, which may explain their persistence in a landscape dominated by agroforestry that harbours old-growth, native trees (Trewhella et al., Reference Trewhella, Rodriguez-Clark, Davies, Reason and Wray2001).
Our data and analyses of the movement of Livingstone's flying fox have potential to help improve conservation of the species by identifying areas of roosting and feeding, and to support targeted reforestation. We plan to continue research on this Critically Endangered species, using GPS tracking as a way of identifying landscape use and feeding trees, to increase the protection of this threatened bat species and its habitat.
Acknowledgements
We thank Ernest Seamark for providing training and equipment, Marc Büntjen for help with GPS tag programming, and Pascal Fust for scientific input in the planning phase. This study was funded by The Rufford Foundation and the Critical Ecosystems Partnership Fund.
Author contributions
Study design: IM, AH, RO; collar design: KE-P, RO; fieldwork: IM, AH, KE-P, IS, BBAA, A-KF; data analysis: IM; writing: IM, HD, SSAC; project oversight: SSAC.
Conflicts of interest
None.
Ethical standards
This research complied with the Oryx guidelines on ethical standards and with those of Sherwin et al. (Reference Sherwin, Christiansen, Duncan, Erhard, Lay and Mench2003). Animal handling was kept to a minimum and only the required number of individuals were captured. All researchers handling bats wore protective gloves and equipment was disinfected after each use to avoid the spread of disease. The research was conducted by Dahari and the University of Comoros with permission of the Comorian government under permits No. 94-018/AF (22 June 1994) and No. 01/31/MPE/CAB.






