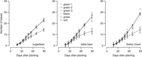Introduction
Most plants absorb light in the blue-violet region (400 to 500 nm) and in the orange-red region (600 to 700 nm) but transmit or reflect light in the far-red (FR) region (700 to 800 nm). Thus, light reflected from plant canopies has a reduced red (R) to FR ratio (R:FR). For example, the R:FR of daylight is about 1.19 compared with 0.13 for an ivy plant (Hedera spp.) (Smith Reference Smith1982). The R:FR is a useful measurement of light quality in agricultural settings, because it relates to crops growing in weedy environments. Plants have evolved the ability to sense and respond to changes in the quality of light using a family of photoreceptors, particularly cryptochromes and phytochromes, which act as molecular switches in response to blue-violet light and R:FR ratio, respectively (Casal Reference Casal2013).
Cryptochromes are blue and ultraviolet light receptors involved in perceiving blue to green light ratios (cryptochrome 1) or photoperiod (cryptochrome 2). Phytochromes exist as either R-absorbing phytochrome (Pr) or FR light–absorbing phytochrome (Pfr). When the stable form of the phytochrome (Pr) absorbs red light (660-nm peak absorbing wavelength), it converts to Pfr. The conformation change is reversible: under FR light (wavelengths neighboring 730 nm), the Pfr form of the phytochrome converts back to the Pr form (Hopkins and Huüner Reference Hopkins and Huüner2008).
Using this phytochrome transformation, plants can detect small changes in R:FR and initiate responses to avoid perceived impending competition. These responses range from growth and morphological changes, such as increased hypocotyl elongation, stem elongation, apical dominance and reduced branching (dicots), reduced tillering (monocots), and longer internode length, to physiological changes, such as assimilate distribution (Ballaré et al. Reference Ballaré, Scopel and Sánchez1990; Kasperbauer Reference Kasperbauer1987; Smith Reference Smith1992). These responses are collectively referred to as the “shade avoidance syndrome” (Roig-Villanova and Martinez-Garcia Reference Roig-Villanova and Martinez-Garcia2016). The ecological importance of shade avoidance responses is clear: by allocating more resources (photosynthates) to elongation growth, plants can overtop their neighbors and avoid being shaded in the future (Ballaré et al. Reference Ballaré, Sanchez, Scopel, Casal and Ghersa1987). However, this advantage comes with costs. Weinig and Delph (Reference Weinig and Delph2001) demonstrated that when briefly exposed to low R:FR light, velvetleaf (Abutilon theophrasti Medik.) seedlings underwent stem elongation that had irreversible consequences on the plant’s ability to respond to environmental cues later in life.
Page et al. (Reference Page, Tollenaar, Lee, Lukens and Swanton2010) observed that corn (Zea mays L.) plants exposed to reflected FR light from early-emerging weeds set fewer kernels and allocated less biomass to the developing ear. In addition, corn plants exposed to reflected FR light had a reduced root-to-shoot ratio, making them more vulnerable to certain stress conditions such as drought or wind. In a related study, corn plants exposed to reduced R:FR, simulating early weed presence, had similar responses (Rajcan et al. Reference Rajcan, Chandler and Swanton2004). Soybean [Glycine max (L.) Merr.] exposed to light reflected by neighboring weeds showed elongated internodes, reduced branching, and reduced root-to-shoot ratio in the early stages of the plant compared with soybeans exposed to light reflected by bare ground (Green-Tracewicz et al. Reference Green-Tracewicz, Page and Swanton2011). Apical dominance and increased stem extension are among the most common shade avoidance responses, as they offer plants a competitive advantage for light. Thus, for rosette-forming plants, shade avoidance responses may differ. For example, Vermeulen and During (Reference Vermeulen and During2010) reported that a rosette-forming plant, creeping cinquefoil (Potentilla reptans L.), increased its petiole length when planted in high densities, although they did not specify whether the petiole elongation was correlated to the environmental R:FR ratio.
Beta vulgaris is physiologically a biennial species grown primarily as an annual crop for its sucrose-rich root, as in the case of sugar beet, but also as a root vegetable (table beet) or leafy vegetable (Swiss chard) (Winner Reference Winner, Cooke and Scott1993). The rosette growth habit of B. vulgaris makes it particularly sensitive to light competition (Dawson Reference Dawson1977; Schaeufele Reference Schaeufele1986; Zimdahl and Fertig Reference Zimdahl and Fertig1967). In addition, the rosette growth habit would limit stem elongation (a typical shade avoidance response) in response to reduced R:FR. Thus, it is still unclear how a shade avoidance response might manifest in B. vulgaris. Shade avoidance responses precede resource competition and thus might influence the critical period of weed control in crops (Page et al. Reference Page, Tollenaar, Lee, Lukens and Swanton2010). Understanding shade avoidance responses in sugar beet could aid in weed management decisions. The objective of this study was to evaluate the response of B. vulgaris to season-long reflected-light quality.
Materials and methods
Field experiments
Large-pail field studies were conducted in 2013 and 2014 to evaluate the response of B. vulgaris to reflected light. Three cultivars of B. vulgaris: sugar beet [Beta vulgaris ssp. vulgaris (L.), ‘BTS 66RR60’], table beet [Beta vulgaris ssp. vulgaris (L.), ‘Detroit Dark Red’], and Swiss chard [Beta vulgaris ssp. vulgaris (L.), ‘Green Fordhook Giant’], were chosen for the study because of different physiology, resource allocation, and economic end products. As was noted earlier, sugar beet is grown for its sucrose-rich root, while table beet and Swiss chard are grown as root and leafy vegetables, respectively (Winner Reference Winner, Cooke and Scott1993). Thus, sugar beet and table beet partition most assimilates in the root, while Swiss chard partitions assimilates mostly into the leaves. In 2013, the study was a 3 by 6 factorial arrangement of three varieties of B. vulgaris (sugar beet, table beet, and Swiss chard) and five plastic mulch (Harris Seed Company, Rochester, NY) treatments (red, blue, green, black, clear colorless) and a nontreated control (bare soil). The experimental design was a randomized complete block (across irrigation line and east–west location gradient) with 15 replicates of each variety by mulch combination. This study used a modified container setup developed by Green-Tracewicz et al. (Reference Green-Tracewicz, Page and Swanton2011). The 19-L black plastic pails (35-cm height, 28-cm top diameter) were filled with peat and perlite potting media (Berger, BM Custom Blend, Saint-Modeste, QC, Canada), and the top was covered with the desired plastic mulch color. A 10-cm-diameter hole was cut into the plastic mulch in the center of the pail, and three B. vulgaris seeds were planted in the center of the hole on June 11, 2013. Shortly after emergence, each pail was thinned to a single B. vulgaris seedling.
In 2014, the study was a 3 by 6 factorial arranged in a randomized complete block design with 16 replications. The treatments included three cultivars of B. vulgaris (sugar beet, table beet, and Swiss chard) and six reflected-light treatments (black plastic mulch, three shades of green plastic mulch, grass to simulate a weedy environment, and bare soil to simulate weed-free conditions). This study used a modified container setup developed by Green-Tracewicz et al. (Reference Green-Tracewicz, Page and Swanton2011) to prevent any root interaction between the grass and B. vulgaris (Figure 1). The 19-L black plastic pails were filled with potting mix, leaving approximately 6 cm of headspace. A hollow cardboard ring (10-cm diameter, 7.5-cm depth) was placed in the center of the pail, and 0.15-mm-thick plastic was taped around the outside. Soil was then added on top of the plastic, filling the rest of the cardboard ring. Kentucky bluegrass (Poa pratensis L.) seed was planted at a high density in the outer ring (covered the entire surface of the outer ring) in the pails receiving the grass treatment 7 d before B. vulgaris was planted to ensure adequate grass growth at the time of B. vulgaris emergence. Squares (60 by 60 cm) of plastic mulch were stretched over the top of the pails receiving a plastic mulch treatment. Three B. vulgaris seeds were then planted in the center of the cardboard ring in each pail on June 6, 2014 and thinned to 1 seedling pail−1 immediately after emergence (within 7 d after planting [DAP]). Because the ring was just 7.5-cm deep and there was no root impediment after the top 7.5-cm layer, the experimental setup allowed B. vulgaris plants to use resources in the entire 19-L pail, except for the area around the 7.5-cm-deep ring that was planted with grass or covered by plastic mulch. The grass was clipped regularly to ensure that the grass did not grow tall enough to shade the crop. Also, the B. vulgaris and grass roots did not interact, and therefore, the effects of the grass treatment were limited to aboveground interactions like reflected light.
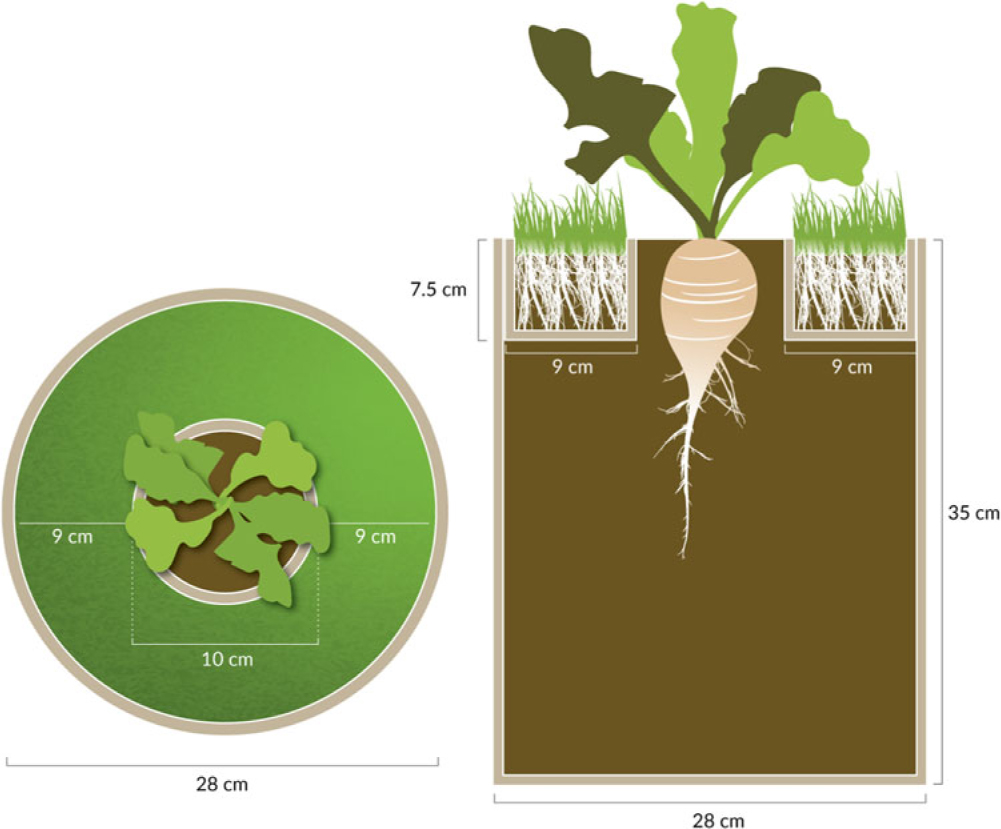
Figure 1. Illustration of the grass treatment used in the field experiment showing the top view (left) and a cross-section (right); modeled after Green-Tracewicz et al. (Reference Green-Tracewicz, Page and Swanton2011). Beta vulgaris was planted into the center ring and was allowed to grow using the full depth (35 cm) of the 21-L pail. Grass roots were constrained to the top 7.5 cm and outer 9 cm of the pail, and were isolated from the B. vulgaris roots using plastic. Grass was clipped as needed to minimize any direct shading of the B. vulgaris plant in the center. For the soil treatment, the design was the same, except no grass was planted into the potting media in the outer ring. Drawing by Jessica Perry.
Pails were arranged in four rows spaced approximately 1 m apart. Within a row, pails were placed 25 cm apart to minimize reflected-light interference among experimental units. In 2013, pails were hand watered daily, and in 2014, B. vulgaris was drip irrigated daily until maturity to ensure no yield limitation due to moisture stress. The grass was also drip irrigated with a separate drip line to ensure green growth and maximal reflection of FR light. The irrigation methods used did not affect growth, because in both years, adequate moisture was applied throughout the season to prevent moisture stress. Beta vulgaris was fertilized with 30 g pail−1 of 14:14:14:5.5% (N:P:K:S) polymer-coated fertilizer (FlorikoteTM NPK, Florikan ESA, Sarasota, FL) at planting to ensure slow and continuous release of nutrients throughout the growing season. Grass was not provided with fertilizer in addition to what was provided by the potting media; the grass remained a healthy green color for the duration of the season, so no supplemental fertilization was needed. Grass treatments were clipped regularly throughout the growing season to prevent direct shading of the B. vulgaris plants.
Reflected-light quality from the plastic mulches, grass, and bare soil was measured midseason (July 29, 2014) using a Jaz spectrometer (Ocean Optics, Dunedin, FL). Reflectance was measured from about 5 cm above the surface from all four cardinal directions and averaged to obtain reflectance per experimental unit. The R:FR of reflected light was calculated as the ratio of R to FR in the reflected-light spectra.
Following emergence, leaf number stage as described by Holen (Reference Holen1998) was recorded for each B. vulgaris plant at 12, 22, 29, 35, 44, 52, 57, and 77 DAP in 2013 and at 18, 26, 34, 41, 48, 55, 69, and 89 DAP in 2014. Leaf stage describes the number of true leaves present and includes the percentage of development of the newest leaves, if they are not fully developed.
All plants were harvested on August 26 to 27 in 2013 and September 3 to 4 in 2014. In 2014, root length, diameter, and fresh weight were measured in table beet and sugar beet at harvest. Diameter was measured at the widest part of the root using a digital caliper, and length was measured from the crown of the root to the root tip. Total leaf area per plant was measured using a Delta-T Leaf Area Meter (Delta-T Devices, Cambridge, UK). Leaf dry weight was measured after leaves had been dried for 48 h at 60 C.
Greenhouse experiment
A greenhouse experiment was conducted twice in 2015 to evaluate shade avoidance responses in sugar beet under controlled environmental conditions. There were two treatments: grass (to simulate a weedy environment) and bare soil (simulating weed-free conditions) arranged in a randomized complete block design with four replications (Figure 2). Each cone tray measuring 28 by 58 cm was considered a block (Figure 2). There were 8 sugar beet (pseudo-replicates) per block. Three sugar beet seeds were planted in February 2015 (first run) and in April 2015 (second run) in individual 158-ml cones (21-cm deep, 4-cm in diameter) and thinned to 1 seedling per Cone-tainer™ immediately after emergence. Each sugar beet was surrounded by either cones containing potting mix (bare-soil treatment) or planted with P. pratensis (grass treatment), as illustrated in Figure 2. In the first run of the experiment, sugar beet and grass were subirrigated by immersing the bottom 5 cm of each Cone-tainer in plastic trays filled with water. In the second run, the sugar beet and grass was watered twice daily by hand using an overhead sprinkler. Sugar beet leaf number as described by Holen (Reference Holen1998) was recorded at 7- to 10-d intervals after emergence. Plants were harvested at 47 DAP (first run) and 55 DAP (second run), and the number of leaves, leaf fresh and dry weights, and root fresh and dry weights were measured.

Figure 2. Greenhouse experiment setup. Each tray was one replicate (with 8 pseudo-replicates) and 28 × 58 cm in size. Each sugar beet was surrounded on all sides by cones either containing potting mix (bare-soil treatment, left) or planted with Kentucky bluegrass (grass treatment, right). Planting into separate cones ensured there was no belowground interaction between sugar beet and grass.
Data analysis
Nonlinear regression analysis was performed using the drc package (v. 3.0-1) in the R statistical language (v. 3.4.4) to quantify the effect of light environment treatment and B. vulgaris variety on leaf number over time (R Core Team 2016; Ritz et al. Reference Ritz, Baty, Streibig and Gerhard2015). A three-parameter Weibull model was used (Equation 1), where Y is the number of B. vulgaris leaves at time x, d is the upper-limit asymptote, x is time in days after planting, e is the value of x at the inflection point on the curve, and b is a slope parameter. The Weibull model provides appropriate statistical parameter estimates of plant growth with meaningful biological interpretations (Shafii et al. Reference Shafii, Price, Swensen and Murray1991). For the 2014 field study, the number of true leaves for each variety and each light environment treatment was estimated from the model, and 95% confidence intervals were calculated.
Harvest data (leaf area, leaf biomass, and root biomass) were analyzed with ANOVA in the R statistical language (v. 3.4.4), and treatment mean differences were separated using Fisher’s protected LSD at alpha = 0.05.
Results and discussion
Reflected light from different-colored plastic mulches did not affect B. vulgaris leaf number in field studies conducted in 2013 (Figure 3; Table 1) or 2014 (Figure 4; Table 1). The R:FR of reflected light from the grass canopy was 0.66 compared with black (1.15) or green (1.16 to 1.55) plastic mulches and the bare soil (1.11). The R:FR values observed for non-grass treatments in this study were similar to the 1.0 and 1.18 for gray-white and brick-red soil surfaces reported by Kasperbauer and Karlen (Reference Kasperbauer and Karlen1994) and similar to the 1.19 of daylight observed by Smith (Reference Smith1982).

Figure 3. Effect of reflected light from colored plastic mulch on leaf number of Beta vulgaris varieties in 2013 field study, Laramie, WY. Regression equation and parameter estimates are provided in Table 1.

Figure 4. Effect of reflected light from bare soil, grass, and colored plastic mulch on leaf number of Beta vulgaris varieties in 2014 field study, Laramie, WY. Regression equation and parameter estimates are provided in Table 1.
Table 1. Parameter estimates describing leaf number of Beta vulgaris varieties following the three-parameter Weibull model for the 2013 and 2014 field experiments, Laramie, WY.

a Y = d* −exp{b* [log(x) − e]}. Parameter estimates are described in text preceding Equation (1).
b Soil plus colored plastic mulch treatments.
In the 2014 field study, the grass treatment substantially reduced leaf number in all B. vulgaris varieties compared with control or plastic mulch treatments (Figure 4), so that by 90 DAP, there were 10 to 14 fewer leaves in B. vulgaris surrounded by grass compared with B. vulgaris in the soil treatment (Figure 5). Craig and Runkle (Reference Craig and Runkle2013) have shown that shade avoidance responses (e.g., stem elongation) can be initiated at R:FR as low as 0.66. This supports the assertion that reduced leaf number in the grass treatment was due to the reduced R:FR, as the effect was not observed for any of the plastic mulch colors, which had a negligible effect on R:FR. In the 2014 field studies, grass treatments increased the time required to reach 6 true leaves by 7, 4, and 3 d compared with soil in sugar beet, table beet, and Swiss chard, respectively (Figure 4). Similar results were obtained in greenhouse studies, where grass increased the time to reach 6 true leaves by 11 to 15 d (Figure 6; Table 2). Previous studies in corn have shown that reflected FR from weeds increased stem elongation and reduced the number of visible leaf tips (Page et al. Reference Page, Tollenaar, Lee, Lukens and Swanton2009, Reference Page, Liu, Cerrudo, Lee and Swanton2011). Thus, the reduction in the number of B. vulgaris leaves might be due to increased allocation to other plant parts.
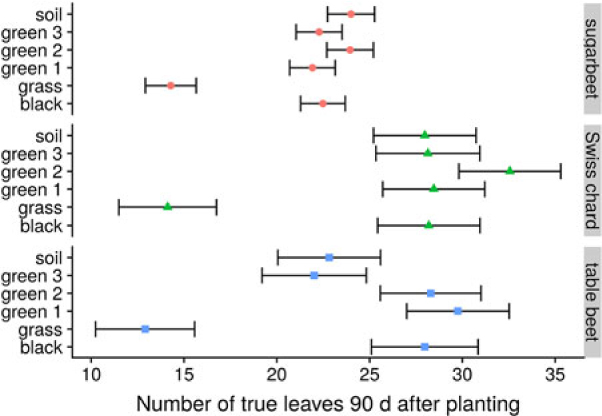
Figure 5. Effect of reflected light from grass and colored plastic mulch on leaf number of Beta vulgaris varieties in at 90 d after planting in 2014 field study, Laramie, WY. Bars represent 95% confidence intervals of the estimates.
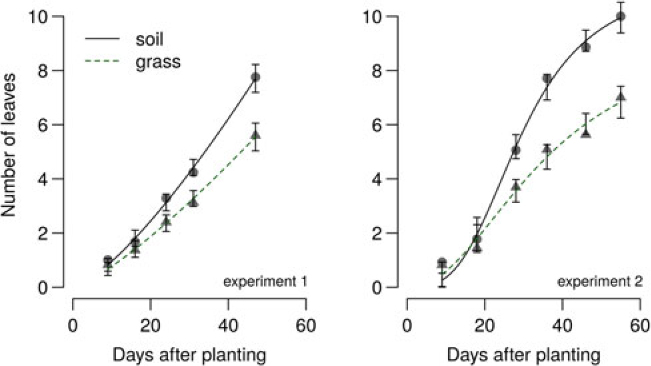
Figure 6. Effect of reflected light from grass on sugar beet leaf number in 2015 greenhouse study, Laramie, WY. Regression equation and parameter estimates are provided in Table 2.
Table 2. Parameter estimates for sugar beet leaf number following the three-parameter Weibull model for 2015 greenhouse experiments, Laramie, WY.

a Y = d* −exp{b* [log(x) − e]}. Parameter estimates are described in text preceding Equation (1).
Differences in sugar beet leaf number were observed after 30 DAP, which corresponded with the appearance of 4 to 8 true leaves (Figures 4 and 6). The difference in leaf number at this stage of development could be due to the transition from leaf canopy–dominated growth to root cambium development (followed by storage root and sugar accumulation–dominated phase). This is because the sixth to seventh cambia, which contribute to the bulk of storage root size and weight, are laid down by the 6-true leaf stage in sugar beet (Milford Reference Milford and Draycott2006). The difference in leaf number at early growth stages of B. vulgaris suggests that shade avoidance responses in B. vulgaris can be initiated early in the growing season, but responses later in the growing season need to be investigated. Thus, early-emerging weeds might reduce growth and yield even in the absence of direct resource (light, water, and nutrients) competition or depletion.
Reflected FR light from grass reduced leaf biomass and leaf area at harvest in all three B. vulgaris varieties (P < 0.001) and root biomass in sugar beet and table beet (P < 0.001) in the 2014 field study (Table 3). The grass treatment reduced B. vulgaris leaf biomass by 21% to 30%, leaf area by 49% to 66%, and root biomass by 70% to 72% compared with the soil treatment. Similar results were observed in the greenhouse study, where sugar beet leaf biomass was reduced by 48% to 57% and root biomass by 35% to 64% in the grass treatment compared with the soil treatment (Table 4). Reduction in leaf area and biomass are common shade avoidance responses. Reduced R:FR resulting from reflected FR light reduced leaf area and biomass in corn (Page et al. Reference Page, Tollenaar, Lee, Lukens and Swanton2009, Reference Page, Tollenaar, Lee, Lukens and Swanton2010, Reference Page, Liu, Cerrudo, Lee and Swanton2011), and leaf area, leaf, root, and total biomass in soybean (Green-Tracewicz et al. Reference Green-Tracewicz, Page and Swanton2011). The reduction in B. vulgaris leaf area was primarily a function of fewer leaves being produced in the grass treatment. Similarly, the fewer leaves and the concomitant reduction in leaf area likely resulted in reduced B. vulgaris root biomass.
Table 3. Effect of reflected light from grass on sugarbeet, table beet, and Swiss chard leaf biomass, leaf area, and root biomass at 90 d after planting in 2014, Laramie, WY.a

a Means followed by an asterisk (*) are statistically different from the same variety grown in a soil environment according to Fisher’s LSD (alpha = 0.05).
b Root fresh weight was reduced by 76% to account for average moisture content before calculating shoot:root biomass. Because this variable was calculated from treatment means, no statistical analysis was performed.
Table 4. Effect of reflected light from grass on sugar beet leaf and root biomass in 2015 greenhouse study, Laramie, WY.

If 76% moisture content of root fresh weight is assumed (McGrath and Trebbi Reference McGrath and Trebbi2007), then shoot-to-root biomass ratio in the 2014 field study was 0.64 to 0.76 for the soil treatment compared with 1.54 to 2.27 for the grass treatment (Table 3). In a related study, Rajcan et al. (Reference Rajcan, Chandler and Swanton2004) similarly observed greater shoot-to-root ratio in corn due to reduced R:FR. The optimality hypothesis suggests that plants allocate more resources to the part that is acquiring the resource that is currently the most limiting (Reich Reference Reich, Waisel, Eshel and Kafkafi2002; Thornley Reference Thornley1972). Thus, perceived impending light competition from plant-reflected light results in more allocation to aboveground plant parts (Poorter et al. Reference Poorter, Niklas, Reich, Oleksyn, Poot and Mommer2012). This is especially problematic in root crops like sugar beet and table beet, because the roots are the primary economic product.
The reduction in root biomass observed here shows that shade avoidance responses have the potential to reduce root yield of sugar beet and table beet even in the absence of resource depletion by weeds. Similarly, leaf yield of Swiss chard might be reduced by shade avoidance responses. However, it is unclear what the total magnitude of these shade avoidance impacts may be in a production setting, because weeds are typically removed multiple times during the growing season. The effects of shade avoidance responses on leaf and root yield under typical growing conditions are almost certainly less than the magnitude observed in this study, in which the shade avoidance signal was allowed to persist from B. vulgaris germination until harvest. But it is also currently unclear how much of the yield loss due to shade avoidance cues is reversible after weeds have been removed. For example, how much yield potential (if any) is sacrificed during the time from B. vulgaris emergence until the 2-true leaf stage when weeds are commonly removed? Future research should explore the relationship between shade avoidance and the critical period of weed removal in B. vulgaris.
Author ORCIDs
Andrew R. Kniss https://orcid.org/0000-0003-2551-4959; Albert T. Adjesiwor https://orcid.org/0000-0002-0047-8771.
Acknowledgments
No conflicts of interest have been declared. This research was funded by a grant from the Wyoming Agricultural Experiment Station through funding received by USDA National Institute of Food and Agriculture (WYO-475-12), a USDA National Institute of Food and Agriculture grant 2016-67013-24912, and funding provided by the Western Sugar Cooperative-Grower Joint Research Committee.