Preamble
Although Richard Granville Bromley passed away in 2018 (Gale, Reference Gale2020; Vallon et al., Reference Vallon, Nielsen, Milàn, Ekdale, Rindsberg and Kjeldahl-Vallon2020), we have chosen to add his name as co-author for a number of reasons. First of all, the present material, which he had been familiar with since 1993, strongly appealed to him. He had photographed the specimens and found this task to be ‘most satisfactory’. In several letters to one of us (JWMJ), he referred to the material as being ‘exquisite’. Richard did not use these terms lightly on a daily basis; thus, they show his fascination for the material. He identified many of the borings, adding handwritten labels to the specimens and mentioned some observations in letters and emails to JWMJ. Unfortunately, owing to the immense workload that he referred to in those letters, he never got round to publishing these borings. The above-mentioned identifications and notes form the basis of the present paper. In 2008, RGB complained, albeit jokingly, about his workload to LHV, noting that he would have material to publish for at least the next 20 years. LHV promised to publish in his name once the notes, material and opportunity had been handed over to him; the present paper fulfils this promise. We also wish to dedicate this paper to the memory of Richard’s wife Ulla Asgaard, a formidable palaeontologist herself, who died on June 18, 2023, while it was still in the early manuscript stages.
Introduction
There are numerous previous studies of trace fossils, both firmground and bioerosional, from the Maastrichtian type area, for instance Jagt (Reference Jagt2003) and Donovan & Jagt (Reference Donovan and Jagt2004, Reference Donovan, Jagt, Mulder, Jagt and Schulp2013a, and references therein). However, these often describe only a few ichnotaxa or a single particular behaviour.
When trace fossils in chalk are not naturally enhanced by silicification (Bromley & Ekdale, Reference Bromley and Ekdale1984), they are usually difficult to examine. The soft chalk needs to be polished first and then treated with a contrast fluid, often motor oil (e.g. Bromley, Reference Bromley1981). This explains why firmground trace fossils from the Maastrichtian have been recorded only in a few instances, for example, from Denmark (Chalk at Stevns Klint) by Ekdale & Bromley (Reference Ekdale and Bromley1983, Reference Ekdale and Bromley1991) and Lauridsen et al. (Reference Lauridsen, Surlyk and Bromley2011).
Bioerosional traces are not restricted to hardgrounds, but quite often occur also in relatively thick carbonatic skeletal material of a variety of organisms. Yet, bioerosional traces share a similar fate to chalk firmground trace fossils. To study them, a similar laborious treatment is required, often in the form of thin section series or casting with rubber and subsequent removal of the surrounding substrate with acid. The bioerosional traces systematically described herein are preserved as natural casts inside bioclasts with the hosting skeletal material being diagenetically dissolved. Most observations can thus be made from a ‘view from within’ of the hosting bioclasts. Owing to their preservation as natural casts, the borings are easily observable without further treatment. However, as it normally is the case with borings in bioclasts, there are two problems (R.G. Bromley, pers. comm., letter of September 1993), which also holds true for the material described herein:
First, there is the phenomenon of xenomorphism. This is imposed on some borings by strong structural trends within the substrate material or by incorporating already existing borings. In our cases, scleractinian corals, in particular, host xenomorphic borings owing to heterogeneous densities within their skeletons. These differences in the substrate fabric may induce the tracemaker to alter its boring strategy which ultimately will change the morphology of that boring. When already existing borings are incorporated, the new bioerosional structure may be overprinted by the morphology of the previous boring (Bromley & D’Alessandro, Reference Bromley and D’Alessandro1984).
Second, stenomorphism is imposed by the sheer lack of space, causing the trace fossil to be ‘bent to size’ within the clast during growth of the tracemaker. Examples are thin shells as substrates or substrates that have been riddled by previous bioeroders. Crowding of borings produces the same morphological problems (Bromley & D’Alessandro, Reference Bromley and D’Alessandro1984).
Microbioerosion occurs often as tiny pustules on the inner surface of mollusc shells but has not been studied in the present paper. The preservation of microborings in the present material is insufficient for determination, especially in the coral substrates. Here the fill is often coarse grained and thus minute diagnostic features have been destroyed diagenetically. Microbioerosion has been systematically treated by Hofmann (Reference Hofmann1996), albeit from other higher stratigraphic levels of the Maastricht Formation. Nevertheless, there is little difficulty in identifying most of the larger specimens, although some features cannot be observed, such as apertures of the borings towards the substrate surface. In view of the fact that the borings are preserved as natural casts and the host substrate is dissolved, the fossils are inverted in comparison to ‘normal’ preservation, where one would be able to observe apertures on the substrate surface but not the boring within that substrate. The present assemblage is dominated by sponge borings (Entobia), followed by some, often very small Gastrochaenolites (bivalve borings) and a few ‘worm borings’ (Maeandropolydora, Talpina).
Material and methods
When not stated otherwise, all specimens are preserved as natural casts in naturally dissolved hard body parts of scleractinian corals or molluscs. The bioerosional traces are encountered mainly on the external surfaces of these biological hardgrounds. Measurements have been taken from photographs using ImageJ software; inaccuracies result from distortions through lenses and human imprecision or errors. All described and illustrated material is housed in the collections of the Natuurhistorisch Museum Maastricht (NHMM, Maastricht, the Netherlands).
Geographical and stratigraphical setting
Provenance of material
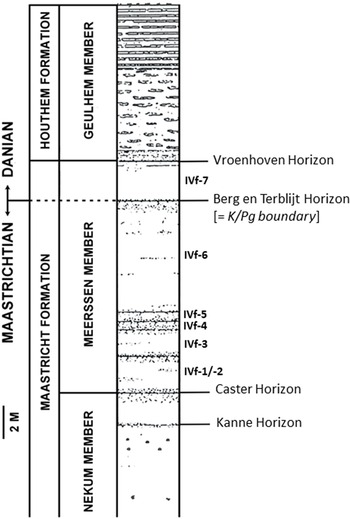
With the exception of Entobia cracoviensis (NHMM JJ 16384, former Ankerpoort-Curfs quarry near Geulhem; Figure 1), Entobia laquea (NHMM JJ 6711) and Entobia megastoma (NHMM JJ 7664), from the basal Meerssen and Nekum members, respectively, at the former ‘t Rooth (Nekami) quarry at Bemelen, all material described and illustrated in the present paper was collected from several levels within the Meerssen Member (Maastricht Formation) at the now disused ENCI-HeidelbergCement Group quarry, south of Maastricht (Felder & Bosch, Reference Felder and Bosch1998, Reference Felder and Bosch2000), over a period of several years in the 1990s. Here, we opt to use the lithostratigraphical log of the former Ankerpoort-Curfs quarry to illustrate the provenance levels (Figure 2) of the ichnofossil taxa described herein because that section also encompassed the Cretaceous–Paleogene (K/Pg) boundary, which is absent from the Maastrichtian stratotype section at the former ENCI quarry. Correlations between the various subunits in the lower and middle portions of the Meerssen Member at both sections, on either side of the River Maas (Meuse) and some 9 km apart, are straightforward (Felder & Jagt, 1998; Keutgen, Reference Keutgen2018; Vellekoop et al., Reference Vellekoop, Van Tilborgh, Van Knippenberg, Jagt, Stassen, Goolaerts and Speijer2020, Reference Vellekoop, Kaskes, Sinnesael, Huygh, Déhais, Jagt, Speijer and Claeys2022).

Figure 1. Map of southern Limburg (the Netherlands) and contiguous Belgian and German territories – the type area of the Maastrichtian Stage. The great majority of ichnofossil suites described herein are from the disused ENCI-HeidelbergCement Group quarry (arrowed).

Figure 2. Lithostratigraphic log of the former Ankerpoort-Curfs quarry at Geulhem, exposing the Meerssen Member (Maastricht Formation), the overlying Geulhem Member (Houthem Formation) and the K/Pg boundary, equating with the Berg en Terblijt Horizon. This section is here used as ‘standard log’ for correlation between the sequences exposed at the Ankerpoort-Curfs and ENCI-HeidelbergCement Group quarries, now both disused (see W.M. Felder & Bosch, Reference Felder and Bosch1998, Reference Felder and Bosch2000).
Lithostratigraphy
The stratotype of the Meerssen Member (Felder, Reference Felder, Zagwijn and van Staalduinen1975; see also Albers & Felder, Reference Albers, Felder and Wiedmann1979) is situated at the now disused Ankerpoort-Curfs quarry at Geulhem, south-east of Meerssen. This unit comprises poorly indurated white yellowish coarse- to very coarse-grained limestones with clearly developed (discontinuous) hardgrounds and fossil hash layers. These lenses and layers comprise to a large extent bryozoan remains and large foraminifera; the total thickness of this unit varies between c. 15 and 20 m. Zijlstra (Reference Zijlstra1994) noted that the upward-coarsening trend in grain size and the increase of average bed thickness indicated a gradual increase of average hydrodynamic energy and deposition rates. The most strongly silicified/lithified layers formed when deposition rate was nil, thus when hydrodynamic energy increased and the consequent increase of erosion equalled the relative sea level rise. During a further increase of hydrodynamic energy, previously lithified sediment was eroded during storms and wavy beds formed. A hardground, a bored and encrusted and mineralised rocky sea bottom, formed when the sediment that was eroded during a storm was not redeposited after the storm, so that the previously lithified layer was continuously exposed.
Villain (Reference Villain1977) described this unit as a gravelly intrabiomicrosparite, deposited ‘sous une tranche d’eau réduite (15 à 2 mètres), une agitation supérieure à celle du Mb permet le déplacement de particules plus grosses (…) déposées en stratification obliques sous les énergies maximales du Md inférieur; elle favorise la prolifération de Lithothamniées dès le Mc, et de Polypiers solitaires au Md.’ The abbreviation ‘Mc’ refers to the Nekum Member, and ‘Md’ to the Meerssen Member in current terminology. Liebau (Reference Liebau1978) typified these deposits as high-energy strata, with an elevated production of carbonate detritus leading to the establishment of a broad, shallow, well-lit, warm carbonate platform with rich phytal associations. Water temperatures were held to have risen to 20–25°C allowing the growth of scleractinian corals, especially in the lower/middle portion of this member (Umbgrove, Reference Umbgrove1925; Leloux, Reference Leloux1999; Baron-Szabo & Leloux, Reference Baron-Szabo, Leloux, Jagt, Fraaije, Jagt-Yazykova and Vellekoop2024; see also Sprechmann, Reference Sprechmann1981). Hofmann (Reference Hofmann1996), on the basis of microborings from the higher portion of the Maastricht Formation, concluded that those traces that could be ascribed to endolithic algae, documented a euphotic to maximally disphotic depositional environment. As borings of phototrophic organisms are rather scarce to not existing in our material, we need to assume that the Meerssen Member was rather disphotic.
Zijlstra (Reference Zijlstra1994) also noted the extreme thickness of the uppermost portion of this member and suggested that this may have been caused by rapid increase of local subsidence rate related to increased tectonic activity connected with the Deccan Trap volcanism. Van Harten (Reference van Harten1972) also pointed out that deposition of the upper Meerssen Member could have occurred in deeper water, in contrast to the continuous shallowing trend up to halfway this member.
Albers & Felder (Reference Albers, Felder and Wiedmann1979) characterised the entire Maastricht tuffaceous facies (e.g. the Maastricht Formation) as follows: biocalcarenites and biocalcirudites, with rare cross-bedding and occasionally with channels. Biocoenoses show a high diversity of tropical-subtropical warm water faunas, mainly consisting of bivalves, and, in comparison with the Kunrade facies (towards the east, close to Heerlen and Kunrade), increased numbers of scleractinians, echinoids and brachiopods. Related to substrate consistency, these biodetritus chalks contain numerous representatives of burrowing endobenthos, and with increased hardground development in the Meerssen Member epibenthos became more dominant. The rich microfaunas comprise a high diversity with a moderate abundance, and rapid evolutionary rates, sharply separated from conditions that prevailed during deposition of the Gulpen Formation. Those authors interpreted the depositional setting to have been fully marine, tropical-subtropical, invariably or generally above wave base in the euphotic zone, very strongly decreased suspension, with rich biocoenoses of high diversity and active biochemical cycle in the formation of exo- and endoskeletons.
In general, faunal diversity increases upwards throughout the Maastricht Formation, culminating in the shallow-water bioherm-like structures (‘patch reefs’) of scleractinian corals, hippuritid and radiolitid bivalves (van de Geijn, Reference van de Geijn1940) and bryozoans of the middle portion (subunit IVf-4) of the Meerssen Member (Figure 2). A combination of ‘Tethyan’ migratory pulses reaching far into northern Europe, attributable to favourable ocean currents, intimately linked with inversion tectonics in some areas and suitable substrates/preferred temperature ranges in (extremely) shallow-water settings, may offer explanations for this trend (Bless et al., Reference Bless, Felder, Meessen, Bless, Dusar and Streel1987; Bless, Reference Bless1989; Jagt, Reference Jagt1999a, Reference Jagt1999b, 2000a, Reference Jagt2000b, Reference Jagt2000c, Reference Jagt2000d).
Biostratigraphy
On palynological and molluscan evidence, the entire Maastricht Formation has been shown to be of late Maastrichtian age, with the exception of the highest subunit (IVf-7) of the Meerssen Member, between the Berg enTerblijt and Vroenhoven horizons (Figure 2), which is of earliest Danian age (Schiøler et al., Reference Schiøler, Brinkhuis, Roncaglia and Wilson1997; Jagt et al., Reference Jagt, Van Bakel, Cremers, Deckers, Dortangs, Van Es, Fraaije, Kisters, Van Knippenberg, Lemmens, Nieuwenhuis, Severijns and Stroucken2013; Vellekoop et al., Reference Vellekoop, Van Tilborgh, Van Knippenberg, Jagt, Stassen, Goolaerts and Speijer2020).
Principal index macrofossils of correlative value include various heteromorph and non-heteromorph ammonites, belemnitellid coleoids and ‘tegulated’ inoceramid bivalves (Keutgen et al., 2017; Jagt & Jagt-Yazykova, Reference Jagt, Jagt-Yazykova, Jagt-Yazykova, Jagt and Mortimore2018, Reference Jagt and Jagt-Yazykova2019). These allow correlations with both Boreal and Tethyan sections in northern/north-eastern and southern Europe, respectively. Some crinoid and cirripede taxa hint at links with the Gulf Coast of North America (Mississippi).
During the past 15 years, collecting has concentrated on the middle and upper Meerssen Member as then exposed at the former ENCI quarry. In the course of these studies, it has become apparent that bed-by-bed correlation in this member is complicated even within this quarry. Storms obviously played an important role during deposition of these sediments and had a considerable impact on (macro-)fossil preservation. Obrution phenomena have been noted on various occasions. Hydrodynamic energy during storm events can be held responsible for the peculiar distribution pattern (convex-down) of the generally extremely fragile exuvia of numerous species of decapod crustacean (Fraaye, Reference Fraaye1996; Jagt et al., Reference Jagt, Fraaije, Van Bakel, Fraaije, Hyžný, Jagt, Krobicki and Van Bakel2014). Faunal studies have also led to the realisation that the Meerssen Member at this quarry was more complete and could be correlated with the Geulhem-Berg en Terblijt area in more detail than previously thought.
Belemnitellid coleoids of the Belemnitella junior group, plus Belemnitella lwowensis, range throughout the entire Maastricht Formation, to be accompanied by the first representatives of the eastern European group of Belemnella (Neobelemnella) kazimiroviensis from the base of subunit IVf-4 of the Meerssen Member onwards. The first appearance datum (FAD) of Bln. kazimiroviensis in the type Maastrichtian apparently coincides with the demise of most, if not all, rudistid bivalves and the majority of hermatypic scleractinian taxa in the area, and thus suggests a (temporary?) incursion of cold-water forms into a very shallow, subtropical setting. Of note also is that this ‘faunal change’ more or less matches the proposed rapid local subsidence to account for the extreme thickness of subunit IVf-6 of the Meerssen Member. This tectonic activity, dated at c. 100,000 years prior to K/Pg boundary, if one accepts Vonhof & Smit’s (Reference Vonhof, Smit, Brinkhuis and Smit1996) estimate of a sedimentation rate of c. 10 cm/kyr, could have resulted in deeper waters and an increased sedimentation rate.
Ammonite ranges in the type Maastrichtian are considerably preservation biased, most material recorded derives from (partially) indurated portions in the sequence. Silicified faunas collected from the lower Maastricht Formation have been shown to comprise ammonites otherwise known exclusively from the upper portion of that unit. The pachydiscid Menuites terminus is amongst the stratigraphically most important species. To date several specimens are known from the middle/upper Meerssen Member. In the Bay of Biscay sections of south-west France and northern Spain, this short-ranging species is the index of the uppermost Maastrichtian terminus Zone.
The stratigraphically important ‘tegulated’ inoceramid bivalve Tenuipteria argentea appears to be restricted to the upper Meerssen Member, with an acme near the top of subunit IVf-6. Below, another taxon, Spyridoceramus tegulatus occurs. This ranges from the Vijlen Member (Gulpen Formation) to the top of the Nekum Member (Maastricht Formation).
Amongst the rich benthic foraminiferal assemblages from the Meerssen Member, large ‘Tethyan’ forms predominate, although some species first occur lower in the section (e.g. Omphalocyclus macroporus in the basal Gronsveld Member). In the literature, such forms have also been recorded from further north in Europe, at the southern limit of the deeper water white chalk facies. A combination of ‘Tethyan’ migratory pulses reaching far into northern Europe on account of favourable ocean currents intimately linked with inversion tectonics in some areas and suitable substrates/preferred temperature ranges in (extremely) shallow-water settings could explain this picture.
The highly diverse selachian faunas of the type Maastrichtian have received ample attention over recent years, but taxonomic work has not been completed yet. Preliminary results are promising as far as intercontinental correlations are concerned. The ‘Tethyan’ pulses apparently had a (?combined) North African/South European, as well as a North Atlantic origin. In this respect it is of importance that Haslett (Reference Haslett1994) noted a palaeoceanographic change for the Bay of Biscay sections from a Tethyan- to a North Atlantic-dominated setting during the latest Maastrichtian. Schönfeld & Burnett (Reference Schönfeld and Burnett1991) noted that the warm-water outflow from the north-west European epicontinental seas through the Channel could well have been a dominant factor in separating the Boreal and Tethyan bioprovinces on the western European shelf. They also considered that the palaeoceanographic setting as well as wind-driven surface currents and bottom currents could also have played an important role. During the late Maastrichtian, tectonic movements resulted in changes in circulation patterns (see Mortimore, Reference Mortimore2018), which were superimposed on the late Maastrichtian sea-level highstand.
Brinkhuis & Schiøler (Reference Brinkhuis, Schiøler, Brinkhuis and Smit1996) noted that the uppermost Meerssen Member in the subterranean Geulhemmerberg section (Geulhem area) yielded, amongst other dinoflagellate species, the markers Thalassiphora pelagica and Palynodinium grallator. This assemblage enables a correlation with the uppermost Maastrichtian in other parts of the North Sea Basin (Schiøler & Wilson, Reference Schiøler and Wilson1993). A detailed dinoflagellate biozonation and sequence-stratigraphical interpretation of the Maastrichtian stratotype section has been proposed by Schiøler et al. (Reference Schiøler, Brinkhuis, Roncaglia and Wilson1997). These authors documented a change from open-marine to marginal marine conditions to have taken place at the boundary between the Lixhe 3 and Lanaye members (Gulpen Formation). In addition, they interpreted changes in lithology and palynological assemblages in the light of a sequence-stratigraphical scheme, noting that parts of four cycles (Haq et al.’s [Reference Haq, Hardenbol, Vail, Wilgus, Hastings, Posamentier, van Wagoner and St C. Kendall1988] UZA 4.5, TA 1.1, TA 1.2 and a fourth of probably higher order) could be recognised at the ENCI quarry.
The strontium isotope profile for the type Maastrichtian (Vonhof & Smit, Reference Vonhof, Smit, Brinkhuis and Smit1996; see also Vonhof et al., Reference Vonhof, Jagt, Immenhauser, Smit, van den Berg, Saher, Keutgen and Reijmer2011) has also been shown to be a useful chronostratigraphic tool in correlating the type section with Bidart and El Kef (Tunisia). Those authors computed for the upper 30 m of the type Maastrichtian (= base Emael Member to top Meerssen Member) a sedimentation rate of ≈ 10 cm/kyr, thus providing a valuable tool for estimating faunal changeover patterns in the late (latest) Maastrichtian of the type area (see also Vellekoop et al., Reference Vellekoop, Kaskes, Sinnesael, Huygh, Déhais, Jagt, Speijer and Claeys2022).
Systematic ichnology
Caulostrepsis cretacea (Voigt, Reference Voigt1971)

Figure 3. A. Entobia cracoviensis, NHMM JJ 16384. B. Entobia laquea, NHMM JJ 14116. C. Entobia magna, NHMM 1996 035. D. Entobia megastoma, NHMM JJ 7664. E. Entobia ovula (larger chambers) and Entobia parva (smaller chambers), NHMM 1996 027. F. Two partly fused chambers of Entobia paradoxa next to E. ovula (left), NHMM JJ 8077. G. Entobia parva, NHMM JJ 8068. H. Entobia volzi and Gastrochaenolites cluniformis, NHMM JJ 8309. J. Several specimens of Caulostrepsis cretacea, NHMM JJ 7308. Scale bars equal 10 mm.
Material
NHMM JJ 7308 (7 specimens; former ENCI quarry, Meerssen Member, basal portion subunit IVf-4), NHMM JJ 7628 (former ENCI quarry, Meerssen Member, basal portion subunit IVf-4), NHMM JJ 8069 (former ENCI quarry, Meerssen Member, basal part subunit IVf-1, directly above Caster Horizon) and NHMM JJ 8310 (former ENCI quarry, Meerssen Member, IVf-3/-4 interval).
Diagnosis
Galleries bent in a long, narrow U-form with the inward-facing walls of the limbs fused by complete removal; the original position of the median wall is sometimes indicated by a very shallow axial depression along the structures. Vane absent. Transverse section always flattened-elliptical but showing gradual decrease in width towards the aperture. Shape of aperture flattened-oval (Bromley & D’Alessandro, Reference Bromley and D’Alessandro1983, p. 291).
Description
The borings consist of long and narrow galleries bent in a U-shape. These typically exhibit a more or less straight to slightly wavy longitudinal axis with an observed length between 4 and 6.5 mm. Their width usually increases towards the distal end of the boring. Here, the maximum width varied between 1 and 1.5 mm. The apertural end is flattened oval and is, with a width of a maximum of 1.1 mm, slightly narrower. Apertures have not been observed. Vanes are absent, but in specimen NHMM JJ 7628, a very shallow axial depression is present. Here each limb of the U-boring measured 0.5 mm in diameter. The axial depression between the two limbs becomes undetectable with increasing distance from the aperture.
Cunctichnus probans Fürsich, Palmer & Goodyear, Reference Fürsich, Palmer and Goodyear1994
Material
NHMM JJ 13752 (former ENCI quarry, Meerssen-Member, subunit IVf-4).
Diagnosis
Cylindrical borings in shells, arcuate to highly sinuous or planspiral, with thin, short, tapering side-branches at points where the tubes abruptly change direction (Fürsich et al., Reference Fürsich, Palmer and Goodyear1994: 162).
Description
Only a single specimen has been recognised in the present lot, although this ichnotaxon has been recorded previously from the study area (Donovan et al., Reference Donovan, Jagt and Nieuwenhuis2015). NHMM JJ 13752 sits inside a large solitary coral associated with indeterminate long, unbranched galleries and a single specimen of Gastrochaenolites orbicularis (see below). Close to this bivalve boring, the characteristic short side branches are visible. Two probing galleries with lengths of 1.0 and 1.2 mm are clearly visible. One of these is the dead-ending main gallery. Both side branches exhibit a tapering ending. The arcuate main gallery has a slightly undulating diameter between 0.5 and 0.7 mm.
Entobia cracoviensis Bromley & Uchman, in Bromley et al., Reference Bromley, Kędzierski, Kołodziej and Uchman2009
Material
NHMM JJ 16384 (3 specimens; former Ankerpoort-Curfs quarry, Meerssen Member, IVf-1–2 interval).
Diagnosis
A single, large, subspherical to ovoid chamber bearing numerous radiating, mostly simple, almost straight, almost invariably unbranched canals of various thickness and generally having a greater length than the diameter of the chamber. The majority of chambers reach more than 20 mm in diameter. The roof of the boring is lacking (Bromley & Uchman, in Bromley et al., Reference Bromley, Kędzierski, Kołodziej and Uchman2009, p. 151).
Description
E. cracoviensis is a single-chambered sponge boring from which numerous unbranched, rather long, almost straight canals radiate in all directions. NHMM JJ 16384 was recovered directly from the quarry face, with the largest of the borings facing up; at least three partial specimens of E. cracoviensis are preserved in this piece of matrix. The chambers are half-open spheroids with their longest diameters being parallel to each other, their counter parts are missing. The specimen was exposed for several years in the quarry face prior to its collection. At the typelocality of E. cracoviensis, all specimens lack their roofs (Bromley et al., Reference Bromley, Kędzierski, Kołodziej and Uchman2009). The chambers have a wide diameter between 11.5 and 35 mm and measure between 10 and 21 mm at their shorter axes. The typical cuspate microsculpture on the chamber’s surface has not been observed. Their inner surface is gently irregular, exhibiting small, irregularly distributed funnel depressions which represent the outlets of the radiating canals. Canals are sparse, long and straight to slightly undulating or winding, mainly unbranched and have diameters between 0.2 and 0.8 mm. Diameters between canals vary in each specimen, but are fairly constant for individual canals. The maximum observed length of these canals measured was 46 mm. One rather thin canal branches under an angle of 34°. Canals have not been observed to intersect.
Remarks
E. cracoviensis and E. resinensis Santos, Mayoral & Bromley, Reference Santos, Mayoral and Bromley2011 exhibit a very similar morphology and may be distinguished mainly on basis of their canals and ecology. From the chamber of E. cracoviensis numerous canals (between 10 and up to 100 per specimen) emerge being mostly simple, almost straight and almost invariably unbranched. The thickness of each canal is fairly constant although different canals of the same specimen may have different diameters. Canal length is usually greater than the diameter of the chamber (Bromley & Uchman, in Bromley et al., Reference Bromley, Kędzierski, Kołodziej and Uchman2009, p. 151). Ecologically, E. cracoviensis has been interpreted as a psammobiont covered by a thin sand layer (Bromley et al., Reference Bromley, Kędzierski, Kołodziej and Uchman2009).
In specimens of E. resinensis, the number of radiating canals is smaller (on average 6 per individual). They never branch but are often bent upwards towards the surface. Canal length is less than the diameter of the chamber (Santos et al., Reference Santos, Mayoral and Bromley2011). That ichnotaxon has been interpreted as fully endolithic (chamber roof is the original surface of the bored rockground) occurring in intertidal to subtidal areas of rocky shores, in contrast to the psammobiont E. cracoviensis which has a roof consisting out of loose sand and occurring in slightly deeper water settings (Bromley et al, Reference Bromley, Kędzierski, Kołodziej and Uchman2009; Santos et al., Reference Santos, Mayoral and Bromley2011).
Although the present specimens exhibit a relatively low number of canals, similar to E. resinensis, they are not bent towards a surface, are longer than the larger diameter of the chambers and at least one canal bifurcates under an acute angle. The environmental interpretation of alternating sedimentation stillstand and elevated depositional rates under high hydrodynamic energy in a subtidal setting given by Zijlstra (Reference Zijlstra1994) is similar to that of the type locality of E. cracoviensis (Bromley et al., Reference Bromley, Kędzierski, Kołodziej and Uchman2009). Hence, we assign our specimens to E. cracoviensis.
Entobia laquea Bromley & D’Alessandro, Reference Bromley and D’Alessandro1984
Material
NHMM JJ 6711 (former ‘t Rooth (Nekami) quarry, Bemelen, basal Meerssen Member), NHMM JJ 14116 (former ENCI quarry, Meerssen Member, subunit IVf-4), NHMM 2008 030 (former ENCI quarry, Meerssen Member, subunit IVf-4), and possibly, NHMM JJ 8077 (former ENCI quarry, basal Meerssen Member, subunit IVf-1, directly above Caster Horizon).
Diagnosis
A camerate entobian composed in mature stages of networks of small chambers arranged in several tiers subparallel to the substrate surface. The chambers, variable in shape, taper abruptly near the constrictions that separate them from neighbours. They are organised in short, more or less arcuate chains that encircle small spaces in a way that resembles lace. The apertures, circular in shape, rarely fused, are small, numerous and distributed irregularly. Phase A well represented by branched exploratory threads that anastomose early to produce a slender network. Furthermore, phase A is usually present at the periphery, even in mature specimens. Phase B is greatly reduced or absent, the enclosed meshes passing almost directly into phase C. The most characteristic growth phase of the ichnospecies is C (Bromley & D’Alessandro, Reference Bromley and D’Alessandro1984, p. 244).
Description
All studied specimens are only partially preserved, even the best specimen, NHMM JJ 14116, is missing initial growth phases. Still, the most characteristic growth phase (phase C) of this camerate entobian is fully developed and clearly recognisable. Usually, the small chambers are arranged subparallel to the substrate surface in a network of several tiers of which only the uppermost is preserved in our specimens. The chambers have a polygonal outline with slightly outwardly bent surfaces, giving the somewhat equidimensional chambers a subglobose or subpyramidal appearance. Diameters were measured with an average of about 2.4 mm, but range between 1.2 and 3.5 mm. The chambers taper abruptly towards their neighbours and are separated from them by sharp constrictions. In specimen NHMM JJ 6711, the lace-like arrangement of these chambers may only be assumed owing to the incomplete preservation. Here, the substrate surface is covered with sediment and apertures, therefore, have not been observed directly. However, some chambers seem to have broken-off and tiny protrusions from the underside of the surface may be interpreted as sediment-filled apertural canals. These are mainly circular, one is oval and another one irregularly shaped (fused). Their diameters range between 0.3 and 0.5 mm. Although the specimen is incomplete, growth stage B is preserved in one branching exploratory canal with slight swellings (width up to 1.2 mm). The smallest diameters between the swellings measure between 0.45 and 0.65 mm, slightly narrower than the constrictions between the chambers of growth phase C which measure 0.65 to 0.9 mm. Apophyses are present but not numerous and usually broken off, so only stubs remain on the outer surfaces of the chamber-casts.
Phase A is preserved in specimen NHMM 2008 030, but has not been observed in others. The exploratory threads are long and slender with a relatively straight course exhibiting swellings at their branching points. Anastomosis is rare, but this might be a preservational artifact. Regular swellings along the exploratory threads mark early stages of phase C in chamber growth.
In specimen NHMM JJ 8077, a boxwork of chambers is preserved. Although the otherwise numerous and diagnostic exploratory threads (Bromley & D’Alessandro, Reference Bromley and D’Alessandro1984) are also missing from this specimen, we favour assignment to the very crowded phase D of E. laquea, rather than to the closely similar and in gerontic stages almost indistinguishable E. ovula. This particular specimen shows stenomorphic growth as it grew in close proximity to a specimen of E. paradoxa. Chambers are so crowded that individual tiers can no longer be recognised. The shape of the chambers is variable and ranges from ovoid to subglobose or subpyramidal (in contrast to rather uniformly shaped chambers of E. ovula), and their diameters are within above given measurements. A pattern in arrangement of the chambers cannot be recognised, but they are not arranged in straight strings as they would be in E. ovula.
Entobia magna Bromley & D’Alessandro, Reference Bromley and D’Alessandro1989
Material
NHMM JJ 8310 (former ENCI quarry, Meerssen Member, IVf-3/-4 interval), NHMM 1996 035 (former ENCI quarry, Meerssen Member, subunit IVf-4), NHMM JJ 16593 (former ENCI quarry, Meerssen Member, subunit IVf-4) and NHMM JJ 13998 (former ENCI quarry, Meerssen Member, subunit IVf-4).
Diagnosis
Strongly camerate entobian fusing in phase D to produce many large, flattened, interlocking, rounded to irregular chambers; these are connected by numerous intercameral canals, mostly slender. Chamber wall relatively smooth, giving rise to abundant thin unbranched apophyses. Apertures large and numerous. The system is dominated by phase D and may extend to cover large areas, but remains generally within a single tier (Bromley & D’Alessandro, Reference Bromley and D’Alessandro1989, p. 286).
Description
Only relatively small parts consisting of merely a few chambers are preserved in the four studied samples. These specimens are interlocked in-between several other entobians and only form a single tier. Nevertheless, the large flattened irregularly rounded and amoeboid chambers with relatively smooth surfaces and abundant remains of apophyses can clearly be assigned to Entobia magna. However, whether these specimens represent growth phases C or D cannot be determined owing to the fragmentary preservation and stenomorphism caused by surrounding other entobians.
According to Bromley & D’Alessandro (Reference Bromley and D’Alessandro1989), Entobia magna produces strong, commonly palmate-branched exploratory threads in growth phase A. During the reduced phase, B round to elongate chambers are being formed at irregular distances. These rapidly swell into rounded, variously shaped chambers during growth stage C and are connected by many intercamerate canals. From the chambers emerge numerous slender, needle-like apophyses. Chambers fuse during phase D to form large amoeboid cavities with smaller chambers situated in-between.
Neither phase A nor B has been recognised in the present specimens. This is not surprising because these phases are largely reduced in Entobia magna according to Bromley & D’Alessandro (Reference Bromley and D’Alessandro1989). In phase C/D, the largest chamber measured approximately 29 × 14.5 mm (specimen NHMM 1996 035) which is about half the size of the largest chamber reported by Bromley & D’Alessandro (Reference Bromley and D’Alessandro1989). Specimen NHMM JJ 16593 consists of three individual chambers. One is irregularly curved and exhibits irregular swellings. The other two also have an irregular outline but are deeply lobed and probably resulted from partial fusion of previously existing cavities. All chamber walls are relatively smooth, apart from numerous fine punctae interpreted as the bases of apophyses. Apertures to the substrate’s surface have not been recognised.
Specimen NHMM JJ 13998 is a fragment of an extremely large chamber of growth phase D. The fragment is 54 mm long and 44 mm wide and has a slightly lobed outline suggesting that several chambers were fused. Although being extremely large, it still is in the range given by Bromley & D’Alessandro (Reference Bromley and D’Alessandro1989, p. 288), who noted, ‘…. rarely exceed 40 mm’. The surface of the cast is relatively smooth, but does exhibit numerous pustules, sometimes emerging from small depressions. These probably are incompletely preserved apophyses. Two large protrusions emerge from one surface. One of these has a circular diameter of about 2.2 mm and rises from the chamber surface for 3.5 mm. The other protrusion has an elliptical to oval cross-section and is broken off at the base. Both probably are apertural canals, although Bromley & D’Alessandro (Reference Bromley and D’Alessandro1989) stated that fusion of apertures was ‘unusual’. At the edges of the relatively flat chamber fragment, a few broken off protrusions with diameters between 0.7 and 1.1 mm are seen. These are probably intercamerate canals connecting to unpreserved neighbouring chambers of the same Entobia-system.
Entobia megastoma (Fischer in d’Archiac, Fischer & de Verneuil, Reference D’Archiac, Fischer, de Verneuil and de Tchihatcheff1866)
Material
NHMM JJ 7664 (former ‘t Rooth quarry, Bermelen, basal Nekum Member, above Laumont Horizon).
Diagnosis
Non-camerate entobian, organised in an irregular boxwork system becoming more or less complexly intermeshed in the mature stages. The galleries are subcylindrical, frequently bifurcated, swollen at nodal points where, usually, several galleries conjoin. Apertures large and numerous, circular or oval in shape, rarely fused, disposed irregularly. Phase A reduced; phases B-D well developed (Bromley & D’Alessandro, Reference Bromley and D’Alessandro1984, p. 250).
Description
One poorly preserved specimen is referred here. This non-camerate entobian usually consists of an irregularly branched gallery-network or -boxwork in growth phases B to E. In our specimen, only a 14 x 9 mm large section of one tier is preserved, probably assignable to the late phase B or early C. The galleries have a circular to strongly flattened elliptical cross-section and variable diameters or widths, respectively (0.7–3.5 mm). However, their diameter or respective width is fairly constant between nodal points. The galleries branch away from each other, but anastomosis does occur. According to Bromley & D’Alessandro (Reference Bromley and D’Alessandro1984), apertures should be large, circular to suboval, numerous and closely spaced, but such have not been observed in our late Maastrichtian specimen. The specimen was constructed in an indeterminate, now dissolved bivalve shell. The strongly flattened elliptical cross-section is therefore probably the result of stenomorphism (compare Bromley & D’Alessandro, Reference Bromley and D’Alessandro1984).
Remarks
In their synonymy for E. megastoma, Bromley & D’Alessandro (Reference Bromley and D’Alessandro1984) noted Cliona megastoma Fischer, 1866 as the first mention of the ichnotaxon, but annotated it with ‘nomen nudum?’, referring to Fischer (Reference Fischer1868) instead for the first valid description. The reasons behind this are unknown to us, as the diagnosis given by Fischer in d’Archiac et al. (Reference D’Archiac, Fischer, de Verneuil and de Tchihatcheff1866) is valid, not only under the rules of the current International Code of Zoological Nomenclature (ICZN) but also under all previous editions. The description only lacks a depiction of the new ichnospecies. However, that was never a requirement of the ICZN (A. K. Rindsberg, pers. comm., 2024). We therefore agree with Wisshak et al. (Reference Wisshak, Knaust and Bertling2019), who considered 1866 to be the date of the first valid reference of E. megastoma Fischer in d’Archiac, Fischer & de Verneuil, Reference D’Archiac, Fischer, de Verneuil and de Tchihatcheff1866.
Entobia ovula Bromley & D’Alessandro, Reference Bromley and D’Alessandro1984
Figure 3E, F
Material
NHMM JJ 7307 (former ENCI quarry, Meerssen Member, subunit IVf-4), NHMM JJ 7309 (former ENCI quarry, Meerssen Member, subunit IVf-4), NHMM 1996 027 (former ENCI quarry, Meerssen Member, subunit IVf-4), NHMM 1996 035 (former ENCI quarry, Meerssen Member, subunit IVf-4), possibly also NHMM JJ 8064 (former ENCI quarry, base Meerssen Member, subunit IVf-1), NHMM JJ 8067 (former ENCI quarry, base Meerssen Member, subunit IVf-1), NHMM JJ 8071 (former ENCI quarry, base Meerssen Member, subunit IVf-1) and NHMM JJ 8077 (former ENCI quarry, base Meerssen Member, subunit IVf-1).
Diagnosis
A camerate entobian composed in the mature stage of small chambers of globose to ovoid shape, greatly crowded, arranged in a boxwork. The chambers are separated from neighbours by a very short intercameral canal, usually reduced to a constriction. In phase C, the chambers are arranged in straight strings, forked at variable angles and anastomosed, giving rise to a network in one or two, poorly distinguishable tiers. A and B phases are reduced. Apertural canals distinct, tapering distally or slightly inflated as a barrel. The openings are relatively small, numerous, rather regularly disposed, rarely fused (Bromley & D’Alessandro, Reference Bromley and D’Alessandro1984, p. 254).
Description
Four specimens are here assigned to Entobia ovula, three further specimens remain slightly doubtful. All specimens are preserved in mature stages (growth phases C and D), forming dense boxworks of globose to ovoid, rarely polygonal chambers arranged in straight to arcuate lines. The diameters of the chambers measure between 1.5 (short axis) and 4.5 mm (long axis). The chambers are separated by sharp constrictions.
According to Bromley & D’Alessandro (Reference Bromley and D’Alessandro1984), each chamber should connect to the surface with at least one, rarely two short, yet distinct canals, usually tapering outwards or with a barrel-like, weakly inflated morphology. Neither apertures nor apertural canals could be observed.
Growth phases A and B are reduced in E. ovula (Bromley & D’Alessandro, Reference Bromley and D’Alessandro1984). It is therefore not surprising that neither has been observed in the larger specimens. However, the tentatively assigned specimen NHMM JJ 8064 shows both phases. Exploratory threads of phase A are thin (about 0.2 mm) and relatively short (about 1 mm). One of these, about 2 mm long, reveals two vague constrictions and therefore needs to be regarded as the beginning of phase B. With a diameter of 0.3–0.4 mm, it is slightly larger than typical phase A threads.
NHMM JJ 8067 is a crowded, stenomorphic specimen wedged into a tubular body fossil. In this specimen, growth is so advanced that there is hardly any space left between individual chambers resulting in their polygonal shape.
Remarks
Most of the doubtful specimens are crowded into tight spaces and therefore stenomorphic. Specimens NHMM JJ 7307 and NHMM JJ 8067, for example, grew inside a cylindrical bioclast. It therefore cannot be decided if the chambers are arranged in a single tier or as a boxwork. E. laquea is often built as distinguishable interconnected tiers parallel to the surface, whereas E. ovula has no such division and is built as a massive boxwork out of interconnected chambers. Surface-parallel growth of tiers in restricted substrates (E. laquea) might therefore be mistaken as a boxwork (usually typical of E. ovula). Entobia ovula can only be separated from E. laquea if surface-parallel sections of the boring can be viewed and a developmental stage is preserved (phase A usually always present in E. laquea; Bromley & D’Alessandro, Reference Bromley and D’Alessandro1984). Neither is the case in the doubtful specimens.
Specimen NHMM JJ 8064 (Figure 4) is a string of chambers, originally constructed within a bivalve shell. From this main branch, three short side arms stretch out, only one to two chambers long. The stem represents growth phase C, whereas at its tip and at the ends of the side branches, phases A and B are determinable.

Figure 4. Growth phases A to C of Entobia ovula, NHMM JJ 8064. Scale bar equals 10 mm.

Figure 5. A. Gastrochaenolites cluniformis, NHMM JJ 8310. B. Gastrochaenolites cor within a plocoid coral, coenchym of which is replaced by Entobia parva, NHMM JJ 5979b. C. Gastrochaenolites dijugus (left) and G. cf. dijugus (right), NHMM JJ 12581. D. Gastrochaenolites lapidicus, NHMM 1996 031. E. Gastrochaenolites torpedo (left) with xenoglyphs (septa of host coral), and two specimens of G. orbicularis (right) with similar xenoglyphs, NHMM JJ 8454. F. Indetermined microboring in gastropod shell, NHMM JJ 1996 035. Scale bar equals 1 mm. G. Cunctichnus probans, NHMM JJ 13752. H. Maeandropolydora elegans, NHMM 1994 149. J. Maeandropolydora sulcans, NHMM 1996 037. K. ?Palaeosabella prisca and incomplete ?Caulostrepsis isp., NHMM JJ 7307. L. Rogerella isp., NHMM JJ 13759. M. Talpina hunanensis, intertwined and branching galleries, two ropes of intertwined galleries in lower left area of picture, on right side of picture several other bioerosional taxa, Entobia isp. (top), Gastrochaenolites cf. lapidicus (centre) and Maeandropolydora sulcans (lower right corner), NHMM 1996 038. N. Talpina hirsuta, NHMM JJ 13714. O. Trypanites solitarius with xenoglyphs imprinted by corallites surrounded by Talpina hunanensis, NHMM JJ 7635. Scale bars (except for 5F) equal 10 mm.
Entobia paradoxa (Fischer, Reference Fischer1868)
Material
NHMM JJ 7628 (former ENCI quarry, Meerssen Member, subunit IVf-4), NHMM JJ 8077 (former ENCI quarry, base Meerssen Member, subunit IVf-1), NHMM 1996 035 (former ENCI quarry, Meerssen Member, subunit IVf-4) and possibly NHMM JJ 13714 (former ENCI quarry, Meerssen Member, IVf-2/-5 interval) and NHMM JJ 5979b (former ENCI quarry, base Meerssen Member, subunit IVf-1, directly above Caster Horizon).
Diagnosis
A camerate entobian composed, in mature stages, of a network of very irregular chambers, somewhat amoeboid in shape, usually arranged in two tiers parallel to the substrate surface. Each chamber is connected to several others; the shape becomes extremely irregular owing to tapering as necks of varying lengths, before the constrictions that separate each chamber from its neighbours. In gerontic forms, a partial fusion among the chambers leads to the development of non-camerate galleries, usually variable in diameter and lacking diagnostic character. Apertures circular in shape, usually relatively small, uncrowded (Bromley & D’Alessandro, Reference Bromley and D’Alessandro1984, p. 259).
Description
Usually, the relatively large amoeboid chambers of E. paradoxa are easily recognised. However, the present specimens are rather small and restricted to only a few chambers. Their preservation is fragmentary. The chambers are interconnected (except in NHMM JJ 7628 which probably consists of several juvenile individuals) and is only preserved in a single tier. They are developed strictly parallel to the substrate surface and show isometry with respect to penetration depth.
Specimen NHMM JJ 7628 is dominated by the juvenile growth stage B. Several isolated, albeit incomplete chambers are seen. They have an elongate basic shape but are often bulging out and thus have an amoeboid outline. The longest measures 11 mm in length and 3 mm in width. Growth phase A is almost not preserved as the long and slender, often branched exploratory threads break off easily. The few threads that are preserved, still connected to the chambers, have a diameter of around 0.4 mm. They run relatively straight, but change directions at branching points where they exhibit swellings.
In growth phase C, best visible in specimen NHMM JJ 8077 (Figure 3F) and in part in NHMM 1996 035, the chambers usually have a polygonal to amoeboid shape and thus fit the definition of growth phase C which is the most characteristic and best-developed phase of E. paradoxa (Bromley & D’Alessandro, Reference Bromley and D’Alessandro1984). The chambers usually measure between 4.8 and 5.6 mm on their longest axes. Apophyses are always present and numerous, although often broken off. Apertures are hidden in the substrate and unobservable. Specimen NHMM JJ 5979b is stenomorphic and hardly recognisable as it completely took over the coenchym of the plocoid coral, competing with E. parva.
Entobia parva Bromley & D’Alessandro, Reference Bromley and D’Alessandro1989
Material
NHMM JJ 5979b (former ENCI quarry, base Meerssen Member, subunit IVf-1, directly above Caster Horizon), NHMM JJ 6711 (former ‘t Rooth [Nekami], Bemelen, basal Meerssen Member), NHMM JJ 8068 (former ENCI quarry, base Meerssen Member, subunit IVf-1), NHMM JJ 8071 (former ENCI quarry, base Meerssen Member, subunit IVf-1), NHMM JJ 8072 (former ENCI quarry, base Meerssen Member, subunit IVf-1), NHMM JJ 8309 (former ENCI quarry, Meerssen Member, IVf-3/-4 interval), NHMM 1996 027 (former ENCI quarry, Meerssen Member, subunit IVf-4), NHMM 1996 032 (former ENCI quarry, Meerssen Member, subunit IVf-4), NHMM 1996 035 (former ENCI quarry, Meerssen Member, subunit IVf-4), NHMM 1996 039 (former ENCI quarry, Meerssen Member, subunit IVf-4), NHMM 2008 030 (former ENCI quarry, Meerssen Member, subunit IVf-4) and possibly, NHMM JJ 7628 (former ENCI quarry, Meerssen Member, subunit IVf-4).
Diagnosis
Diminutive, camerate entobian comprising in phase D a compact boxwork of inflated chambers connected by enlarged intercameral canals. The distal chambers are characterised by a number of tapering, branching projections that give them an irregular or angular appearance. Phase A and B are extremely reduced, the exploratory threads are very short, the system has an abruptly closed growth front. Wide canals opening by large apertures cross the chamber boxwork in all directions (Bromley & D’Alessandro, Reference Bromley and D’Alessandro1989, p. 289).
Description
The diminutive camerate systems of E. parva appear as dense, relatively regular sub-semispherical systems with a diameter or extension of a maximum of 35 mm, but commonly around 10 mm. Individual chambers have diameters around 0.4 mm (range between 0.3 and 0.6 mm) and fusion between chambers occurs rarely. The chambers are covered by numerous branching projections creating a spongy appearance at the closed growth front of phase D. The rather small specimen NHMM JJ 8071 possesses three apertural canals radiating away from the cluster of chambers towards the surface. They have lengths between 1.3 and 1.6 mm and a diameter of 0.2 mm, equal to the chambers of this specimen. Apertures at the surface are not visible. The cluster of chambers itself (without the protrusions of the apertural canals) measures 5.5 x 4 mm. The small measurements and the protrusions might imply a rather juvenile specimen.
Intercamerate canals are rarely observed in other specimens, but when visible (e.g. NHMM JJ 6711) cross-cut the clusters of chambers. These canals have diameters of around 1.4 mm.
Specimen NHMM 1996 027 contains several stenomorph specimens of E. parva. These borings are restricted to the coenosteum of the plocoid coral and therefore exhibit an elongated, rather oval growth between corallites. Individual specimens have lengths of about 6 to 7 mm. In specimen NHMM JJ 5979b, the complete coenosteum of the coral is replaced by stenomorphic E. parva, only avoiding the places of the corallites (Figure 5B).
Entobia volzi Bromley & D’Alessandro, Reference Bromley and D’Alessandro1984
Material
NHMM JJ 8071 (former ENCI quarry, base Meerssen Member, subunit IVf-1), NHMM JJ 8309 (former ENCI quarry, Meerssen Member, IVf-3/-4 interval), NHMM JJ 13714 (former ENCI quarry, Meerssen Member, IVf-2/-5 interval), NHMM 1996 032 (former ENCI quarry, Meerssen Member, subunit IVf-4), NHMM 2008 030 (former ENCI quarry, Meerssen Member, subunit IVf-4), and possibly NHMM JJ 7309 (former ENCI quarry, Meerssen Member, subunit IVf-4), NHMM JJ 7635 (former ENCI quarry, Meerssen Member, subunit IVf-2) and NHMM JJ 8310 (former ENCI quarry, Meerssen Member, IVf-3/-4 interval).
Diagnosis
Diminutively camerate entobian consisting, in phase D, of chambers connected by wide intercameral canals or partially fused, taking a form resembling an irregular, close framework. This system is crossed in all direction by relatively wide, subcylindrical canals that connect with the substrate surface through large apertures. Growth front compact. Phases B and C are considerably reduced, characterised by appearance of irregular chambers or clusters of chambers as small swellings on the walls of the wide canals. Phase A comprises long, slender canals arranged irregularly and branched as a boxwork, having palmate expansions at nodal points. Apertures of two sizes, circular to oval, very irregularly distributed (Bromley & D’Alessandro, Reference Bromley and D’Alessandro1984, p. 262).
Description
At first glance, chambers in E. volzi have diameters commonly around 0.7 mm (ranging between 0.4 and 1.0 mm) and thus are slightly larger in adult growth phase D than the chambers of E. parva. Like E. parva, E. volzi also forms irregular clusters of chambers. The system crossing subcylindrical canals are scarce and have diameters between 0.4 and 1.3 mm. From these, new chambers are created as small swellings in the walls or more commonly (contra Bromley & D’Alessandro, Reference Bromley and D’Alessandro1984) as central protrusions on the outermost chambers (growth phase B). Partial fusion of chambers therefore is common. The long arcuate to sublinear exploratory canals of growth phase A are not preserved in most of our specimens.
Specimen NHMM JJ 8309 (Figure 3H) is our best-preserved individual. The cluster of chambers measures 32 by 14 mm. Thick intercameral canals with a constant diameter of around 1.3 mm connect to the surface as well as slender, arcuate to sublinear canals (exploratory threads) with diameters smaller than the chambers (0.3 mm). These threads often taper towards the surface and may not reach it. However, apertures cannot be observed. With a diameter of only about 5 mm, specimens NHMM JJ 13714 and NHMM 2008 030 are probably rather juvenile borings.
Gastrochaenolites cluniformis Kelly & Bromley, Reference Kelly and Bromley1984
Material
NHMM JJ 8309 (former ENCI quarry, Meerssen Member, IVf-3/-4 interval), NHMM JJ 8310 (2 specimens; former ENCI quarry, Meerssen Member, IVf-3/-4 interval) and NHMM 1996 037 (former ENCI quarry, Meerssen Member, subunit IVf-4).
Diagnosis
Smooth Gastrochaenolites having one principal ridge in the main chamber and a second weakly developed one diametrically opposite. The base is rounded to bilobate. The neck and aperture are rounded to oval (Kelly & Bromley, Reference Kelly and Bromley1984, p. 799).
Description
In the four specimens, the outline of the main chamber is cordiform. The furrow that runs over the bottom of the chamber is deep on one side and clearly visible. In contrast to G. cor (see below), this furrow is placed at the shorter chamber-axis in G. cluniformis. The main chambers measure at their widest extension between 6 and 10 mm on their longer axis and between 5 and 6.5 mm on their shorter axis. Neck and aperture are hidden in all specimens within the casts of surrounding Entobia-borings and the sediment.
Gastrochaenolites cor Bromley & D’Alessandro, Reference Bromley and D’Alessandro1987
Material
NHMM JJ 5979b (former ENCI quarry, base Meerssen Member, subunit IVf-1, directly above Caster Horizon).
Diagnosis
Smooth Gastrochaenolites, the somewhat discoid main chamber having a heart-shaped cross-section that is emphasised by a weak furrow running along both edges. The furrow fades out in the neck region; neck short and aperture round to oval, rarely reniform (Bromley & D’Alessandro, Reference Bromley and D’Alessandro1987, p. 395).
Description
The boring is surrounded by stenomorphic Entobia parva (see above) and measures 3 by 2 mm at the widest extension of the cast; it is relatively small, suggesting a juvenile specimen. The diagnostic heart-shaped cross-section and the furrow running from one to the opposite side of the distal end of this pouch-like boring are weakly developed. The neck is only partially visible, merely from one side. It seems to be rather short. The aperture is hidden in the sediment.
Gastrochaenolites dijugus Kelly & Bromley, Reference Kelly and Bromley1984
Material
NHMM JJ 12581 (former ENCI quarry, Meerssen Member, base of subunit IVf-2).
Diagnosis
Smooth Gastrochaenolites in which neck region is constricted in the form approaching a figure of eight by two opposed ridges (Kelly & Bromley, Reference Kelly and Bromley1984, p. 800).
Description
The single specimen is preserved as a cavity and split open parallel to the penetration axis. The chamber is pouch-like and has a smooth interior wall. The chamber’s width is reconstructed to have been 11 mm, and the penetration depth amounts to 22 mm. Clearly visible is the split aperture, which, in complete specimens, is preserved as a figure of eight constructed by two opposing edges.
Gastrochaenolites lapidicus Kelly & Bromley, Reference Kelly and Bromley1984
Material
NHMM JJ 8454 (former ENCI quarry, Meerssen Member, subunit IVf-4), NHMM JJ 13799 (3 specimens; former ENCI quarry, Meerssen Member, subunit IVf-4), NHMM 1996 031 (2 specimens, 2 possible specimens; former ENCI quarry, Meerssen Member, subunit IVf-4) and, possibly, NHMM JJ 13488 (former ENCI quarry, Meerssen Member, top subunit IVf-1), NHMM 1996 033 (2 specimens; former ENCI quarry, Meerssen Member, subunit IVf-4) and NHMM 1996 038 (former ENCI quarry, Meerssen Member, subunit IVf-4).
Diagnosis
Smooth, clavate boring; elongate ovate; circular cross-section throughout length including the neck region except for the immediate area of the aperture where the section is usually oval, but may be circular; base bluntly paraboloid in longitudinal section; widest diameter located approximately central within the main chamber (Kelly & Bromley, Reference Kelly and Bromley1984, p.798).
Description
Clavate to pouch-like borings with a smooth wall and rounded base. The cross-section throughout the length (parallel to the penetration axis) of the boring is circular. Maximum diameters measured in the boring casts range between 4 and 10 mm. The neck region and aperture are usually hidden in the sediment. In specimen NHMM JJ 8454, a regular decrease in diameter towards the aperture is observed. Xenomorph specimens exist when corallites or interseptal spaces are incorporated into the chambers. However, the overall shape of the borings was not altered by the tracemaker and encountered cavities in the substrate (e.g. corallites) have not been sealed off by calcite precipitation.
Remarks
Gastrochaenolites lapidicus is recorded mainly from horizontal to slightly inclined surfaces, whereas G. torpedo predominantly occurs in vertical to overhanging surfaces (Bromley & D’Alessandro, Reference Bromley and D’Alessandro1987; Bromley & Asgaard, Reference Bromley and Asgaard1993; Gibert et al., Reference de Gibert, Martinell and Domènech1998).
Gastrochaenolites orbicularis Kelly & Bromley, Reference Kelly and Bromley1984
Material
NHMM JJ 8454 (2 specimens; former ENCI quarry, Meerssen Member, subunit IVf-4), NHMM JJ 13752 (former ENCI quarry, Meerssen Member, subunit IVf-4) and NHMM JJ 15165 (2 specimens; former ENCI quarry, Meerssen Member, top of subunit IVf-2).
Diagnosis
Smooth Gastrochaenolites, circular in cross-section throughout; main chamber orbicular; neck region elongate in type specimen but may be short (Kelly & Bromley, Reference Kelly and Bromley1984, p. 801).
Description
NHMM JJ 13752 is preserved as a cast within a solitary coral. The sub-spherical chamber is clearly offset from a long-stretched tapering neck region. The lack of two opposing furrows (equivalent to ridges in the boring) towards the external surface of the coral, suggests that the aperture is circular (in contrast to G. dijugus which exhibits the diagnostic figure of eight aperture). However, the aperture itself cannot be observed because it is hidden inside the sediment.
NHMM JJ 8454 contains next to G. torpedo and G. lapidicus two specimens of G. orbicularis (Figure 5E). All specimens show xenoglyphs from the hosting coral (septa). The diameter of the spherical chamber measures in both specimens around 20 mm. The diameter of the neck decreases in both specimens towards the apertures. It is completely preserved in only one specimen and has a length of 15 mm. The aperture itself is buried in sediment.
The two specimens with inventory number NHMM JJ 15165 are preserved as hollow borings from a hardground. The diameters of the spherical chambers measure in both specimens around 35 mm. The equally long neck is only preserved in one specimen and is tapering towards the aperture. The aperture is circular.
Gastrochaenolites torpedo Kelly & Bromley, Reference Kelly and Bromley1984
Figures 5E, F
Material
NHMM JJ 8454 (former ENCI quarry, Meerssen Member, subunit IVf-4), NHMM JJ 15165 (former ENCI quarry, Meerssen Member, top of subunit IVf-2) and, possibly, NHMM JJ 13362 (former ENCI quarry, Meerssen Member, IVf-4/-5 interval).
Diagnosis
Elongate smooth boring, widest point close to mid-line with the base acutely parabolic. The neck region is markedly compressed, but the aperture itself is oval or approaches a figure-of-eight shape (Kelly & Bromley, Reference Kelly and Bromley1984, p. 802).
Description
These borings exhibit an elongate club-shaped chamber that usually has a smooth wall. However, most of our specimens show xenoglyphs imprinted on the chamber-casts by the interseptal spaces of the host corals. Specimen NHMM JJ 15165 is preserved as an open cavity with a smooth wall. Chambers penetrate up to 50 mm into the substrate. Throughout the chamber, the cross-section remains circular, with a maximum diameter of 17 mm. The base of the chamber is acute parabolic in longitudinal section. The neck in specimen NHMM JJ 13362 is bent, but the long-stretched figure of eight of the aperture (although not preserved) can be envisaged. As noted by Kelly & Bromley (Reference Kelly and Bromley1984), G. torpedo commonly has a lining which thickens towards the aperture and extends above the substrate surface. Such a lining is not present in our specimen and might have been dissolved together with the host shells. However, the cavities in the biogenic substrate (intraseptal spaces) of specimen NHMM JJ 8454 have not been sealed off by the borer because they are recorded as xenoglyphs on the outer surface of the natural casts.
Remarks
Gastrochaenolites torpedo was regarded as a junior, and therefore invalid, synonym of Moniopterus japonicus Hatai, Masuda & Noda, Reference Hatai, Masuda and Noda1974. The latter ichnospecies was transferred to the ichnogenus Gastrochaenolites Leymerie, 1842 by Haga et al. (Reference Haga, Kurihara and Kase2010) whose opinion was reinforced by Wisshak et al. (Reference Wisshak, Knaust and Bertling2019). However, by comparing diagnoses and redescriptions of Gastrochaenolites japonicus (Hatai et al., Reference Hatai, Masuda and Noda1974; Haga et al., Reference Haga, Kurihara and Kase2010) and Gastrochaenolites torpedo (Kelly & Bromley, Reference Kelly and Bromley1984), we cannot agree with such a synonymisation.
The holotype (and the three paratypes which appear to be lost) of G. japonicus lacks important diagnostic features necessary to justify such synonymisation, e.g. the neck area and aperture (incomplete specimens). Already by comparing both pictured holotypes of G. japonicus and G. torpedo, respectively, morphological differences become evident as G. japonicus has an ellipsoid, rather than a torpedo-shaped chamber. Haga et al. (Reference Haga, Kurihara and Kase2010) described the calcareous lining of G. japonicus to thicken towards both ends of its longitudinal axis. Although Haga et al. (Reference Haga, Kurihara and Kase2010, p. 852) conducted a thorough search of the type and other localities, they could not recover new material of G. japonicus, but did succeed in collecting similar material of boring fills detached and isolated from hardgrounds. The authors concluded that the fills of G. japonicus were more resistant to weathering than their host rocks and could have ‘rolled on the sea floor for a considerable period before burial’ (Haga et al., Reference Haga, Kurihara and Kase2010, p. 853). This suggests that the thinned-out lining at the mid-section in the holotype and the additional material collected by Haga et al. (Reference Haga, Kurihara and Kase2010) is a result of erosion rather than a morphological feature. Erosion by rolling around would also explain the lack of both the neck region and aperture in these specimens. According to Kelly & Bromley (Reference Kelly and Bromley1984), the calcareous lining of G. torpedo thickens towards the aperture and may extend above the substrate’s surface as a chimney-like protrusion. The aperture resembles a long ellipse approaching a figure-of-eight shape. The incomplete and heavily eroded material of G. japonicus is insufficient to justify synonymisation. Until more suitable material is recovered, G. torpedo and G. japonicus must be retained as two different ichnospecies.
Palaeoecologically, G. lapidicus is recorded mainly from horizontal to slightly inclined surfaces, whereas G. torpedo predominantly occurs in vertical to overhanging surfaces (Bromley & D’Alessandro, Reference Bromley and D’Alessandro1987; Bromley & Asgaard, Reference Bromley and Asgaard1993; Gibert et al., Reference de Gibert, Martinell and Domènech1998). This suggests that the coral substrates where still in life position when the tracemakers created their borings. As previously observed by Bromley & Asgaard (Reference Bromley and Asgaard1993), this preference has its reason in the low tolerance against sedimentation of the tracemaker of G. torpedo.
Maeandropolydora elegans Bromley & D’Alessandro, Reference Bromley and D’Alessandro1983
Material
NHMM 1994-149 (former ENCI quarry, Meerssen Member, subunit IVf-5).
Diagnosis
System composed of cylindrical galleries of constant diameter, irregularly sinuous, tending to run in paired fashion, the limbs touching but normally not fused. Numerous apertures. (Bromley & D’Alessandro, Reference Bromley and D’Alessandro1983, p. 296).
Description
Owing to its mediocre preservation, it cannot be determined if NHMM 19904 149 contains one or two individuals. The cylindrical galleries have a constant diameter of about 5 mm. Individual galleries or limbs touch each other regularly and are not fused. Galleries are looped or coiled. Vanes have not been observed owing to the grainy surface of the cast (e.g. xenoglyphs imposed onto the boring cast from the ?coral-substrate). If pouches are developed, they cannot be observed with certainty because the pouch-like structures may belong to other borings, e.g. Gastrochaenolites. Apertures are hidden in the sediment, but seem to have a slightly larger diameter than the galleries.
Maeandropolydora sulcans Voigt, Reference Voigt1965
Figure 5J, M
Material
NHMM 1996 037 (former ENCI quarry, Meerssen Member, subunit IVf-4), NHMM 1996 038 (former ENCI quarry, Meerssen Member, subunit IVf-4), NHMM 1996 039 (large; former ENCI quarry, Meerssen Member, subunit IVf-4) and, possibly, NHMM JJ 6431 (former ENCI quarry, Meerssen Member, base of subunit IVf-2).
Diagnosis
Cylindrical gallery having at least two apertures, irregularly contorted, commonly bent in loops, never showing fusion where walls are in mutual contact; vane absent (Bromley & D’Alessandro, Reference Bromley and D’Alessandro1983, p. 295).
Description
The long, cylindrical galleries of each of our specimens have a constant diameter of around 1.4 mm. Galleries are irregularly twisted, often looped or even coiled, to form complicated convolutions. The systems develop freely in three dimensions within coral hosts. Loops and coils are variable in size, having outer diameters between 2.8 and 4.6 mm. When coming into contact with themselves, individual limbs of the gallery remain unfused. No vanes or pouches are developed. Galleries may branch, if they do, this is under right angles. Apertures are usually hidden in the sediment.
?Palaeosabella prisca (McCoy, Reference McCoy1855)
Material
NHMM JJ 7307 (former ENCI quarry, Meerssen Member, subunit IVf-4).
Diagnosis
Long, unbranched, tubular or cylindro-clavate macroboring that expands distally as an acute cone (Villas et al., Reference Villas, Mayoral, Santos, Colmenar and Gutiérrez-Marco2021, p. 4).
Description
One specimen might belong here; it is a natural cast of a long, unbranched but winding boring with a cylindrical morphology over most of its course. At one end, the cylinder gradually widens into a pouch-like chamber, the other end is hidden below sediment. The overall course of the boring is parallel to the substrate surface with a very shallow penetration depth, occurring just below the substrate surface. The boring can be seen over a length of about 15 mm and has a width of about 0.8 mm.
Remarks
The taxonomic status of Palaeosabella Clarke, Reference Clarke1921 was uncertain for decades and has created unnecessary synonyms, e.g. Vermiforichnus clarkei Cameron, Reference Cameron1969 under which the ichnospecies often was described. A short summary and a confirmation of the valid status of Palaeosabella was given by Wisshak et al. (Reference Wisshak, Knaust and Bertling2019), which we follow here.
Rogerella isp.
Material
NHMM JJ 13759 (about 10 specimens; former ENCI quarry, Meerssen Member, subunit IVf-4).
Description
The borings are bilaterally symmetrical along the longitudinal axis and preserved as internal casts only. In lateral aspect, they are similar to a short U, or a shape reminding of an ankle sock tapering on both ends with one end being bluntly rounded while the other abruptly narrows to a lance-like protrusion. The pouch has an extremely short to near-absent neck region leading to the aperture which, in the present specimens, is always hidden in the sediment and thus, cannot be observed. The borings have elongated almond-shaped cross- and longitudinal sections and penetrate into the now dissolved host organism in an arcuate fashion. The lengths of the borings vary between 1.2 and 6.1 mm and their penetration depth between 0.8 and 2.9 mm. The ratio between length to depth ranges from 1.3 to 2.5 with an average of about 1.87 (n = 8).
Remarks
Rogerella isp. has also been recorded from the ambulacral pores of late Maastrichtian holasteroid echinoids from the study area (Donovan & Jagt, Reference Donovan and Jagt2013b; Donovan et al., Reference Donovan, Jagt and Nieuwenhuis2016).
Talpina hirsuta Voigt, Reference Voigt1975
Material
NHMM JJ 13714 (former ENCI quarry, Meerssen Member, IVf-2/-5 interval) and NHMM 1996 039 (former ENCI quarry, Meerssen Member, subunit IVf-4).
Emended diagnosis
Talpina with relatively constant, slightly arcuate to almost straight galleries. Apertures having same diameters as galleries. Branching frequently, but only appearing on convex side of galleries, usually under angles of about 40° to 50°, rarely under obtuse angle. Distal parts of galleries exhibiting short section in which hollow, bristle-shaped protrusions reaching towards substrate surface (emended from Voigt, Reference Voigt1975; Stiller, Reference Stiller2005).
Description
Compared to other ichnospecies of Talpina, T. hirsuta has a rather small diameter and a more regular and more frequent branching. The boring system of T. hirsuta rather spreads two-dimensionally, parallel to the substrate surface than becoming intertwined or entangled with neighbouring galleries. However, as in all ichnospecies of Talpina they avoid cross-cutting or fusion with neighbouring galleries by diving or climbing. The galleries of T. hirsuta have an almost straight to only slightly curved course. Their lengths were measured to be between 1.10 and 1.67 mm, with an average length of about 1.37 mm (n = 10). With values of around 0.23 mm, the gallery diameters are very constant. The average ratio between internodal length and gallery diameter (as proposed by Stiller, Reference Stiller2005) was calculated to be about 6.15. Branching is fairly regularly on the convex side of the galleries and branching angles range from 30° to 70°, with an average of around 53°. In some instances, thin bristle-like processes are rudimentarily preserved on the surfaces of casts of T. hirsuta. Only a few distal endings could be observed, these are bluntly rounded.
Remarks
The original diagnosis by Voigt (Reference Voigt1975) stated absolute size for the gallery diameters. These are ignored in the above emended diagnosis, because absolute size as an ichnotaxobasis should only be used as an auxiliary character under exceptional circumstances, for instance when various ichnospecies differ grossly in measurements. Ratios between morphological parameters are acceptable (Bertling et al. Reference Bertling, Braddy, Bromley, Demathieu, Genise, Mikuláš, Nielsen, Nielsen, Rindsberg, Schlirf and Uchman2006, Reference Bertling, Buatois, Knaust, Laing, Mángano, Meyer, Mikuláš, Minter, Neumann, Rindsberg, Uchman and Wisshak2022). Using photographs of the type material, Stiller (Reference Stiller2005) made additional observations that are included in the emended diagnosis given above. However, in his table summarising morphological characters of most ichnospecies of Talpina, his observation of the distal ends being bluntly rounded are supplied with a question mark. In our material, we have observed bluntly rounded distal ends of galleries. However, because we have not compared our material with the type material, we refrain from adding this information to the emended diagnosis presented above.
Talpina hunanensis Stiller, Reference Stiller2005
Material
NHMM JJ 6431 (former ENCI quarry, Meerssen Member, subunit IVf-2), NHMM JJ 7635 (former ENCI quarry, Meerssen Member, subunit IVf-2), NHMM JJ 7636 (former ENCI quarry, Meerssen Member, subunit IVf-2), NHMM JJ 13714 (former ENCI quarry, Meerssen Member, IVf-2/-5 interval), NHMM 1996 038 (former ENCI quarry, Meerssen Member, subunit IVf-4), NHMM 1996 039 (larger specimens) (former ENCI quarry, Meerssen Member, subunit IVf-4) and, possibly, NHMM JJ 13752 (former ENCI quarry, Meerssen Member, subunit IVf-4).
Diagnosis
Talpina with straight to gently, sometimes also strongly curved tunnels. Cross-section of tunnels generally circular, surface smooth. Branching moderately frequent, occurring in inconstant, mostly longer intervals at variable angles; multiple apertures irregularly spaced. Distal ends of tunnels bluntly rounded. Forms densely intertwined tangles of tunnels (Stiller, Reference Stiller2005, p. 403).
Description
In most of the investigated material, individual specimens cannot be separated from each other owing to the dense and intertwining growth of galleries. Talpina constitutes branching, cylindrical boring systems extending primarily parallel to the skeletal-substrate surface. However, their horizontality is often masked by several tiers of galleries and side branches with a vertical to oblique course, crossing over or under other galleries, creating an entangled mass.
In our specimens, the galleries have a cylindrical to slightly oval cross-section and a straight or slightly wiggly to arcuate course. Diameters are usually fairly constant throughout individual internodal sections, although some specimens (e.g. NHMM JJ 7635) may show variation in the diameter. Internodal sections have lengths between 0.9 and 10 mm (on average 4.18 mm) and diameters between 0.3 and 1.1 mm (on average 0.56 mm). Ratios between internodal lengths and diameters, as suggested by Stiller (Reference Stiller2005) were calculated as being between 1.3 and 12.5 (average 7.48; for all measurements: n = 42).
Branching occurs in irregular distances, often on the convex side of galleries and is often sparse in individual boring systems with angles between 20° and 127° (average angles lying around 60°). When crowded, galleries tend to run parallel for longer distances, they may touch each other but never become fused. Galleries do not cross-cut each other, but rather avoid already existing tunnels by lowering or lifting their course. This may create larger entanglements where individual borings cannot be traced. In specimen NHMM 1996 038, several galleries (up to 8) are plaited into three ropes of intertwined galleries, stretching over a length of approximately 40 mm each (Figure 5M). Fusion and touching are avoided here as well and branching is even more reduced than in other specimens.
An initial apertural canal has also been observed in NHMM 1996 038: It starts by running vertically into the now missing skeletal material. Then it bends rapidly into a horizontal course (parallel to the host-organism’s outer surface) and simultaneously turns into a horizontally lying C-shaped gallery which reconnects to the original surface in a rather oblique manner. Perpendicularly below the initial, but unobservable aperture (at the end of the initial apertural canal), the boring bifurcates and the main branch runs horizontally for 5.6 mm in a slightly arcuate manner to the side, where it then disappears in an entanglement of other Talpina-galleries.
Clearly stenomorphic galleries occur in specimen NHMM JJ 13752. These are bent, closely following the surface of the hosting solitary coral. The occasionally wiggly course of the galleries in this specimen might have been caused by xenomorphism because the galleries seem to follow internal microstructures in the now mainly dissolved septa of the coral.
All observed distal ends in all available specimens are bluntly rounded. Some individuals (e.g. NHMM JJ 6431 and NHMM JJ 7635) show vanes around branching points, especially when the branching angles are around 90°. Whether or not these vanes are an original feature of the borings or are rather linked to the peculiar diagenesis and preservation of these bioerosional trace fossils, cannot be determined with certainty.
Remarks
Measurements and ratios are taken following Stiller (Reference Stiller2005), who compiled characteristics of most of the ichnospecies of Talpina (sensu stricto) described in the literature. Still, most ichnospecies of Talpina are ill-defined, are based on incomplete data (mainly external observations) and often use absolute measurements for their definitions, which was excluded as an ichnotaxobase by Bertling et al. (Reference Bertling, Braddy, Bromley, Demathieu, Genise, Mikuláš, Nielsen, Nielsen, Rindsberg, Schlirf and Uchman2006), but partially reinstated under certain conditions by Bertling et al. (Reference Bertling, Buatois, Knaust, Laing, Mángano, Meyer, Mikuláš, Minter, Neumann, Rindsberg, Uchman and Wisshak2022).
Based on internodal length to diameter ratios provided by Stiller (Reference Stiller2005), our specimens more or less fit into three ichnospecies of Talpina, namely T. bromleyi Fürsich, Palmer & Goodyear, Reference Fürsich, Palmer and Goodyear1994; T. gruberi Mayer, Reference Mayer1952 and T. hunanensis Stiller, Reference Stiller2005. It shows the closest resemblance to T. hunanensis. However, Stiller mentioned that the most common ratio between length and diameter for this ichnospecies lay around 15 or more, while ours has a cluster around 8.6 (average 7.4).
A revision of the ichnogenus is long overdue and highly anticipated. Because Talpina mainly occurs in hard body parts, the borings are expected to be stenomorph, a fact that previous authors of ichnospecies may have ignored. The morphological variety of the different ichnospecies of Talpina is expected to be much larger than described by previous authors and we therefore expect that at least about half of all ichnospecies of Talpina may and will be synonymised in the future.
The galleries in the present specimens seem to have larger diameters than the usually observed dimensions. However, the ratios between internodal lengths and diameter as report by Stiller (Reference Stiller2005) are in the same ranges. Therefore, their overall size is rather of descriptive than ichnotaxonomic value (compare Bertling et al., Reference Bertling, Braddy, Bromley, Demathieu, Genise, Mikuláš, Nielsen, Nielsen, Rindsberg, Schlirf and Uchman2006, Reference Bertling, Buatois, Knaust, Laing, Mángano, Meyer, Mikuláš, Minter, Neumann, Rindsberg, Uchman and Wisshak2022).
Talpina von Hagenow, Reference von Hagenow1840 and Conchotrema Teichert, Reference Teichert1945 were synonymised by Wisshak et al. (Reference Wisshak, Knaust and Bertling2019). The branching U-shaped boring system in specimen NHMM 1996 038 would else have to be regarded as belonging to Conchotrema.
Trypanites solitarius (von Hagenow, Reference von Hagenow1840)
Material
NHMM JJ 7635 (1 specimen and 2 possible specimens; former ENCI quarry, Meerssen Member, subunit IVf-2).
Emended diagnosis
Simple, more or less cylindrical Trypanites with straight or gently curving course generally following close beneath the substrate surface (emended from Bromley, Reference Bromley1972, p. 96).
Description
These unbranched borings have a constant circular diameter of between 1.7 and 2.7 mm. The galleries have a slightly wiggly course parallel to the surface (horizontal) and have been observed over a maximum length of about 16 mm. The wall of the borings shows xenoglyphs of corallites of the host plocoid coral. The tapering, but rounded end could be observed in only a single specimen.
Indeterminable microboring
Material
NHMM 1996 035 (numerous specimens in bivalve shell; former ENCI quarry, Meerssen Member, subunit IVf-4).
Description
Microborings preserved in mollusc shells. Tubular, unbranched boring with a circular cross-section. Penetration starts perpendicularly, but rapidly turns to the side to become ideally parallel to the substrate’s surface. Penetration depth measured at 400–500 µm. Aperture, although not directly observed, is funnel like with a diameter of about 170–180 µm. The neck region has a uniform diameter (about 150 µm). towards the boring’s termination, the cross-section widens again to form a subspherical to club-shaped chamber with a diameter of about 160–200 µm. Maximum observed length of the whole boring is about 500 µm. Single borings appear not to be connected with each other.
Results
The bioerosional trace fossil suite of the Meerssen Member (Maastricht Formation) at the former ENCI-HeidelbergCement Group quarry, which forms the bulk of the material described herein, is dominated by sponge borings, exclusively Entobia. Most abundant seem to be the small-chambered ichnospecies, such as E. parva and E. volzi. Medium chambered entobians, such as E. ovula, E. laquea and E. paradoxa, appear to be the second-common ichnotaxa. Large entobians are rather rare, which might be due to collection bias (e.g. sample vs specimen size). Another reason for the paucity of such larger entobians might be the relatively frequent shifting of suitable substrate owing to storm events (e.g. Liebau, Reference Liebau1978; Albers & Felder, Reference Albers, Felder and Wiedmann1979) and the relatively small size of the patch reefs.
Worm borings are diverse, comprising several ichnogenera, such as Talpina and Maeandropolydora. Caulostrepsis is surprisingly rare which might be another expression of collection or preservation bias. However, because their tracemakers have relatively short lifespans, their vacant burrows may also have been overprinted or destroyed by subsequent settlement of other bioeroders (compare Färber et al., Reference Färber, Titschack, Schönberg, Ehrig, Boos, Baum, Illerhaus, Asgaard, Bromley, Freiwald and Wisshak2016).
The last group of borings was made by bivalves in the form of several ichnospecies of Gastrochaenolites. The predominance of chemical bioeroders (such as the Entobia-tracemakers) compared to mechanical bioeroders (e.g. Gastrochaenolites tracemakers) reflects the carbonate-substrate. However, Entobia is also dominant in substrates that are often moved or turned around, such as boulders (e.g. Gibert et al., Reference de Gibert, Martinell and Domènech1998) or, as in our case, bioclasts. Gastrochaenolites on the other hand predominates in rocky cliffs (e.g. Gibert et al., Reference de Gibert, Martinell and Domènech1998) or, in our case, in upright-standing (probably live) corals. These bivalve borings tend to remain relatively small in a coral substrate compared to rocky cliffs, because the corals may be broken off during storm events probably killing the bioeroders.
Bromley & Asgaard (Reference Bromley and Asgaard1993) simultaneously defined the Entobia and the Gnathichnus ichnofacies. The latter is dominated by shallow tier (‘algoid borings’ by Radtke, Reference Radtke1991) and short-term surface bioerosion by grazers. Our inferred sedimentary environment with sudden burial or flipping around of bioclasts, should rather support short-term bioerosion of the Gnathichnus ichnofacies (compare Bromley et al., Reference Bromley, Hanken and Asgaard1990; Gibert et al., Reference de Gibert, Domènech and Martinell2007). Grazers, feeding on shallow-tier microendoliths, may erode dead corals rapidly (Kiene & Hutchings, Reference Kiene and Hutchings1994) preventing settling and development of larger bioeroders, such as clionaid sponges and boring bivalves. In that way, the constant grinding down of the surface interfered with the deeper substrate levels where larvae of deeper-tier borers might just have settled. Thus, larger boring systems cannot develop in the Gnathichnus ichnofacies (compare Kiene & Hutchings, Reference Kiene and Hutchings1994).
In the Entobia ichnofacies, on the other hand, such grazing is suppressed, either through the presence of predators feeding on grazers (Kiene & Hutchings, Reference Kiene and Hutchings1994), the lack of microendoliths as a food source (Bromley & Asgaard, Reference Bromley and Asgaard1993; Kiene & Hutchings, Reference Kiene and Hutchings1994), or the fact that the food source itself produces a carbonate volume (coralline red algae) equal to the amount being eroded by the grazers (Bromley et al., Reference Bromley, Hanken and Asgaard1990). Only in such environments do larvae of larger and deeper-tier bioeroders have a chance to recruit the substrate and develop larger borings.
The above-described ichnocoenosis is therefore typical of the Entobia ichnofacies (Bromley & Asgaard, Reference Bromley and Asgaard1993), which has been recorded mainly from rocky shores (e.g. Gibert et al., Reference de Gibert, Martinell and Domènech1998). However, it actually is more common in basinal aphotic settings, for instance in the north-western European Cretaceous chalks, especially when hardgrounds, lithoclasts or, as in our case, bioclasts are present (Bromley & Asgaard, Reference Bromley and Asgaard1993). Abdel-Fattah & Assal (Reference Abdel-Fattah and Assal2016) described an Entobia-dominated ichnoassemblage from a Miocene coral reef. They observed that Entobia ichnospecies (and Gastrochaenolites torpedo) dominated the forereef facies in water depths between 10 and 30 m, whereas most Gastrochaenolites ichnospecies (except G. torpedo) are more common at shallower water depths (see also El-Hedeny & El-Sabbagh, Reference El-Hedeny and El-Sabbagh2018). The paucity or lack of endolithic phototrophic borings suggest similar water depths for the Meerssen Member.
The specimens described herein show well-recorded ‘successive colonisation’ sequences (R. G. Bromley, September 1993, written communication) as described by Bromley & Asgaard (Reference Bromley and Asgaard1993) from Ladikó (upper Pliocene to Pleistocene; Island of Rhodes, Greece). From there, they described tiering of bioerosional trace fossils and recognised eight tiers. The first four tiers (A–D) are established in the photic zone where both epilithic and endolithic algae and cyanobacteria thrive. Photic zone in this case means the uppermost section within a skeletal substrate where sunlight can be used for photosynthesis by microbes. Tiers A and B show superficial bioerosion traces reflecting an exploitation of these microbes. The shallowest tier A, with a depth between 20 and 200 µm (Bromley, Reference Bromley, Maples and West1992), is dominated by Radulichnus, a grazing trace made by chitons and limpets. Radulichnus has been previously recorded from the higher Maastricht Formation (Jagt, Reference Jagt2003). Only slightly deeper is tier B where rasping regular echinoids produce Gnathichnus and etched attachment traces such as Centrichnus and Renichnus are made by anomiid bivalves and vermetid gastropods, respectively. All of these ichnogenera are also known from various levels within the Maastricht Formation (Jagt & Dortangs, Reference Jagt and Dortangs2000; Jagt et al., Reference Jagt, Brown, Buchner, Calderon, Hećimović and Kwon2019, Reference Jagt, Deckers, De Leebeeck, Donovan and Nieuwenhuis2020). Tier C preserves the borings of endolithic algae and cyanobacteria that are, with a few millimetres depth, just out of reach of the grazing and etching tracemakers of the most superficial tiers. These borings of photosynthetic-autotrophic endoliths were referred to as ‘algoid borings’ by Radtke (Reference Radtke1991) and are the first to develop, when suitable substrates become available for bioeroders, for instance with the death of a coral (Bromley et al., Reference Bromley, Hanken and Asgaard1990). After the algoids have created a sufficient food source for grazers, chitons, limpets and, in some cases, echinoids exploit these microendolithic algae.
About the same penetration depth as tier C is seen in tier D, represented by borings of acrothoracican cirripedes, such as Rogerella. Apart from one specimen with Rogerella, these four shallowest bioerosional tiers (A–D) have only rarely been observed in the present lot as minute protrusions from the outer surfaces into the hosting molluscs or corals. However, they have previously been recorded from the Maastrichtian type area (see above; also Hofmann, Reference Hofmann1996). The reason for the paucity or even lack of these shallowest tiers within bioclasts in the Meerssen Member either lie in the reduced solarisation at the inferred water depths (deeper than 20 m; compare Kiene & Hutchings, Reference Kiene and Hutchings1994) or are similar to the preservational dominance of deep-tiers in softground trace fossils. Under stable conditions in a marine environment, carbonate hard substrates will be progressively destroyed by bioerosion (and inorganic abrasion), eliminating previously produced (shallower) structures, and favouring preservation of the deeper tiers (Bromley & Asgaard, Reference Bromley and Asgaard1993). The preservational nature of the investigated specimens as casts enhances this effect additionally.
Tiers E–H are bored in the aphotic zones within the substrate and encompass the larger bioerosional trace fossils. Tier E, up to about a centimetre deep, is dominated by sponge borings (e.g. most ichnospecies of Entobia) but also phoronid (Talpina) and some polychaete worm borings (Caulostrepsis contorta in the original observation by Bromley & Asgaard, Reference Bromley and Asgaard1993). Endolithic sponges prefer sedimentation- and grazing-free substrates, under which surfaces they may develop without being exposed by grazers. They establish themselves often within the first year of substrate exposure, but do not grow out to notable sizes until the second to fourth year after colonisation (Bromley et al., Reference Bromley, Hanken and Asgaard1990; Kiene & Hutchings, Reference Kiene and Hutchings1994). Their yearly growth rates have been mentioned as 1 to 4 cm (see Bromley et al., Reference Bromley, Hanken and Asgaard1990).
Tier F penetrates down to about 2 cm. It is characterised by larger entobians, such as E. magna, longer worm borings, such as Trypanites solitarius, Caulostrepsis taeniola (Bromley & Asgaard, Reference Bromley and Asgaard1993) and C. cretacea, and the relatively short bivalve borings (e.g. Gastrochaenolites orbicularis). Larger bivalve borings (most ichnospecies of Gastrochaenolites other than G. orbicularis and G. torpedo), and some polychaete borings (e.g. Maendropolydora decipiens of Bromley & Asgaard, Reference Bromley and Asgaard1993, and M. sulcans) are characteristic of tier G. Only few borers penetrate more deeply (5–10 cm or more). Therefore, tier H is characterised by only few ichnospecies, such as some individual borings belonging to several ichnospecies of Maeandropolydora or large Gastrochaenolites torpedo (Bromley & Asgaard, Reference Bromley and Asgaard1993). These tiers take the longest to develop (e.g. Färber et al., Reference Färber, Titschack, Schönberg, Ehrig, Boos, Baum, Illerhaus, Asgaard, Bromley, Freiwald and Wisshak2016) and were not yet developed during the first six years of the experimental study by Bromley et al. (Reference Bromley, Hanken and Asgaard1990).
Our studies of the present material record all of the aphotic bioerosional tiers, except for the deepest, tier H. The reason for this probably lies in the nature of the substrate containing the above-described bioerosional trace fossils. Such bioclasts are easily flipped around, buried or broken up in shallow-water environments killing off the bioeroders before they can reach gerontic life stages. Most of the observed bivalve borings are rather small compared to the same ichnotaxa in rocky shores and attest to such a scenario. Another reason revolves around the availability of space in bioclasts for the expansion of individual borings, which explains why most of the above-described trace fossils are stenomorphic.
If the above-described bioerosional traces were made while the hosts were still alive or after they had died, cannot be inferred in most cases. In molluscan substrates (e.g. Santos & Mayoral, Reference Santos and Mayoral2008), hosts were often alive when the bioerosion took place only on the outer shell-surfaces, whereas they were dead when also inner surfaces were affected. In our material, the inner surfaces (steinkerns) often were lost through weathering and only the outer surfaces were preserved. In addition, most of our material was recorded in coral substrates. Most bioerosion takes place in the abandoned parts (‘dead zone’) of the skeleton where defence mechanisms against settling larvae of bioeroders do not exist anymore (Beuck et al., Reference Beuck, Freiwald and Taviani2010). Bioerosional structures produced during the hosts’ lifetimes may often be recognised by skeletal intergrowth or interlayering, distortion or other reactions of these biological hardgrounds (Gaaloul et al., Reference Gaaloul, Uchman, Ben Ali, Janiszewska, Stolarski, Kołodziej and Riahi2023). Further, size distribution (e.g. largest at the base) or preferred orientation of the bioeroders along the hosts’ surfaces may provide clues as to whether or not the biological substrate was still alive at the time of colonisation (Santos & Mayoral, Reference Santos and Mayoral2008). In that respect, Gastrochaenolites torpedo is a good indicator of a live coral substrate, as the borings predominantly develop in vertical to overhanging surfaces (Bromley & D’Alessandro, Reference Bromley and D’Alessandro1987; Bromley & Asgaard, Reference Bromley and Asgaard1993; Gibert et al., Reference de Gibert, Martinell and Domènech1998). However, because most of the original substrates have been diagenetically dissolved, most of the above-mentioned information has been lost, and the question whether or not the hosts were still alive when the bioerosion occurred cannot be answered.
The high ichnodiversity, ichnodisparity (Buatois & Mángano, Reference Buatois and Mángano2013) and ichnoabundance of the bioerosional trace fossils reflect an environment with only minor to absent sediment precipitation over longer periods (Bromley, Reference Bromley, Maples and West1992). The diversity and rate of bioerosion decreases with increasing water depth. However, algoid borings or bioerosional grazing trace fossils, which usually are indicators of the photic zone, could only be observed in few cases in the present lot. But, occasional flipping over bioclasts containing bioerosional traces may indicate that at least stronger storms could have affected the sea floor and its inhabitants. Gastrochaenolites ispp. usually occur within the top few metres of the water column (Bromley, Reference Bromley, Maples and West1992), whereas most Entobia ispp. prefer depths of around 20 m (Abdel-Fattah & Assal, Reference Abdel-Fattah and Assal2016).
In an environment characterised by a change between short-term elevated deposition and sedimentation stillstands (Zijlstra, Reference Zijlstra1994), the latter periods probably favoured the sponge-made ichnogenera. Clionaid sponges and boring bivalves need reduced surface grazing and more than two to four years of exposure to establish themselves (Bromley et al., Reference Bromley, Hanken and Asgaard1990; Kiene & Hutchings, Reference Kiene and Hutchings1994).
According to the size of the bivalve borings reported herein, we assume that storm events strong enough to reach the sea floor occurred every 3 to 10 years, in comparison with growing rates of rock-boring bivalves observed by Kleemann (Reference Kleemann1973).
Entobians in general show no environmental preference except for carbonate substrates and sediment-free situations (Bromley, Reference Bromley, Maples and West1992), which are more common in water depths below 15 m (Abdel-Fattah & Assal, Reference Abdel-Fattah and Assal2016). However, Entobia paradoxa seems to be more abundant at bathyal water depths. The few specimens of E. paradoxa reported herein might have occupied cryptic microenvironments and therefore are not significant for an overall bathymetric interpretation. Overall, the ichnocoenosis points to a shallower environment, where sunlight was scarce but still reached the sea floor.
Conclusions
For the first time, a bioerosional trace fossil inventory is carried out for the Nekum and Meerssen members of the Maastricht Formation within the type area of the Maastrichtian Stage. The trace fossils are preserved as natural casts and their host substrates (mainly scleractinian corals and larger bivalves) have been dissolved. The bioerosional trace fossil shows high ichnodiversity and belong to the Entobia ichnofacies. The specimens described herein record a beautifully preserved successive colonisation sequences and bioerosional tiering, ranging between tiers D to G. The shallowest (= photic) tiers and the deepest tier have only been recorded exceptionally. The shallow tiers have either never existed in most studied specimens or they have been erased by continued bioerosion. The deepest tier probably never was initiated because of the small restricted nature of the bioclast substrates.
Acknowledgements
Peter Bromley is thanked for his dedication in protecting and passing on Ulla Asgaard’s and Richard G. Bromley’s collections. Many specimens they had on loan could thus be returned to their respective home institutions. He is also thanked for locating pictures and further notes made by his father. We are grateful to Stephen K. Donovan, Donald H. Goldstein, Dirk Knaust, Andrew K. Rindsberg and Max Wisshak for discussions and helping in accessing hard to find literature, to Sten Lennart Jakobsen, who delivered the specimens back to Maastricht and to John Stroucken for taking the majority of the photographs. The manuscript has been commented upon by Stephen K. Donovan, Hilmar Schnick and an anonymous reviewer, who are all thanked for valuable advice, discussions and additions.