Twin pregnancies are at an increased risk of congenital anomalies compared with singleton pregnancies (Mastroiacovo et al., Reference Mastroiacovo, Castilla, Arpino, Botting, Cocchi, Goujard and Rosano1999; Windham & Sever, Reference Windham and Sever1982). In a majority of cases, the fetal anomaly is discordant and only one twin is affected (Bryan et al., Reference Bryan, Little and Burn1987). This is also true of twin pregnancies complicated by neural tube defects (NTD). The prevalence of NTD in twins is 2.3/1,000. These anomalies are seen in both monozygotic (MZ) and dizygotic (DZ) twin pairs, reflecting the multifactorial pattern of inheritance (Sebire et al., Reference Sebire, Sepulveda, Hughes, Noble and Nicolaides1997).
Twin pregnancies discordant for NTD pose a management dilemma, as the unaffected co-twin is at risk of poor outcomes. Death of an affected twin in utero can have devastating effects on the unaffected co-twin, particularly in monochorionic (MC) pregnancies where the unaffected twin has an increased risk of both neurological damage and death (Fusi & Gordon, Reference Fusi and Gordon1990). In studies involving anencephaly, rates of fetal death in utero (FDIU) of the affected twin have been reported to be approximately 25%, which is accompanied by the death of the unaffected co-twin in the majority of cases (Sebire et al., Reference Sebire, Sepulveda, Hughes, Noble and Nicolaides1997). In addition, the development of polyhydramnios complicates 36–57% of cases (Lipitz et al., Reference Lipitz, Meizner, Yagel, Shapiro, Achiron and Schiff1995; Vandecruys et al., Reference Vandecruys, Avgidou, Surerus, Flack and Nicolaides2006). Expectant management of polyhydramnios has been associated with preterm delivery rates of up to 100% (Leeker & Beinder, Reference Leeker and Beinder2004), exposing the unaffected co-twin to higher rates of perinatal morbidity and mortality (Nassar et al., Reference Nassar, Adra, Gomez-Marin and O'Sullivan2000). Selective termination (ST) of the affected twin is an option offered to parents to minimize risks to the pregnancy and the unaffected co-twin. The technique used is determined by chorionicity. Intracardiac potassium chloride or lignocaine injection is preferred for dichorionic (DC) twins, while cord occlusion methods are preferred for MC twins. ST carries a risk of pregnancy loss that is dependent on the gestation when the procedure is performed, the technique used, and the experience of the operator (Evans et al., Reference Evans, Goldberg, Dommergues, Wapner, Lynch, Dock and Berkowitz1994; Lipitz et al., Reference Lipitz, Meizner, Yagel, Shapiro, Achiron and Schiff1995).
In DC twins, ST is associated with a cumulative loss rate of 5% (Bigelow et al., Reference Bigelow, Factor, Moshier, Bianco, Eddleman and Stone2014). The gestational age at the time of ST is associated with both pregnancy loss rates and preterm delivery rates (Dural et al., Reference Dural, Yasa, Kalelioglu, Can, Yilmaz, Corbacioglu Esmer and Yuksel2017), and it is recommended the procedure is performed prior to 18 weeks’ gestation (Bigelow et al., Reference Bigelow, Factor, Moshier, Bianco, Eddleman and Stone2014).
In MC twins, the risk of pregnancy loss following cord occlusion techniques is higher and the procedures are rarely performed prior to the second trimester. A recent systematic review and meta-analysis by Gaerty et al. (Reference Gaerty, Greer and Kumar2015) has analyzed the perinatal outcomes of MC pregnancies who undergo either radiofrequency ablation or bipolar cord occlusion for ST, and found that neither method was superior to the other. It has also been noted that ST of the presenting MC twin has a higher rate of delivery <34 weeks’ gestation, regardless of the method used (Yinon et al., Reference Yinon, Ashwal, Weisz, Chayen, Schiff and Lipitz2015).
To date, there are no large series assessing twin pregnancies discordant for NTD. The current study aimed, first, to compare two different management options (ST vs. expectant management) in twin pregnancies discordant for NTD and the associated pregnancy and neonatal outcomes; and, second, to contrast outcomes between twin pregnancies discordant for NTD (cases) and twin pregnancies with no NTD (controls) to determine the overall burden of twin pregnancies with discordant NTD relative to twin pregnancies with no NTD.
Patients and Methods
This is a retrospective cohort study of all twin pregnancies affected by a NTD at the Royal Women's Hospital, Melbourne (RWH) over a 10-year period. Cases were obtained from a search of the hospital's ultrasound Picture Archiving and Communication System (PACS) database. All the cases of multiple pregnancy discordant for a NTD were included. Each case was reviewed by an experienced obstetrician with ultrasound expertise to confirm the findings.
Monochorionic diamniotic (MCDA) and dichorionic diamniotic (DCDA) cases only were included. Monochorionic monoamniotic (MCMA) cases were not included as outcomes for these women and their fetuses are complex and largely determined by the risks of monoamnionicity (Shub & Walker, Reference Shub and Walker2015). Each eligible case was matched with three controls who were selected chronologically from the three subsequent multiple pregnancy ultrasounds with the same chorionicity and maternal age (within 5 years). Multiple pregnancies with other major fetal anomalies were excluded from the control group. In addition, the controls were required to have a normal first trimester ultrasound scan result (between 11 weeks and 13.9 weeks). Controls found to have any fetal anomaly or a nuchal translucency (NT) above the 99th percentile (>3.5 mm) were excluded. Further control exclusion criteria included women with significant chronic medical conditions, such as diabetes or hypertension, or those in whom the pregnancy outcome data were unavailable for review. Background and outcome data were collected for the mother and both twins for cases and controls.
The primary outcome was birth before 34 weeks’ gestation. The secondary outcomes were small for gestational age (birthweight <-2 SD relative to Australian twin birthweight percentiles; Li et al., Reference Li, Umstad, Hilder, Xu and Sullivan2015), mode of delivery, admission to a neonatal unit (either the neonatal intensive care unit or the specialcare nursery), or neonatal death.
Data were analyzed using Stata version 14.2. First, we were interested in outcomes confined within the twin pregnancies discordant for NTD between those treated with ST and those treated expectantly where differences between groups were contrasted by chi-square (or Fisher's exact test), or t tests (or Mann-Whitney U Test), as appropriate. Second, twin pregnancies discordant for NTD were compared with those with no NTD, using generalized estimating equations (GEEs) with robust (sandwich) estimates of standard errors to allow for clustering of the three controls for each case. Mean differences with 95% confidence intervals (CIs) or odds ratios (ORs) were calculated from the regression coefficients and their standard errors. For neonatal outcomes, infant characteristics were compared between non-affected cases and their corresponding controls, matched for birth order.
Human Research Ethics Committee (HREC) approval was obtained for a retrospective anonymized audit project that met the criteria for quality assurance activities outlined in the National Health and Medical Research Council guideline ‘Ethical Considerations in Quality Assurance and Evaluation Activities’ (National Health & Medical Research Council, 2014).
Results
Between 2004 and 2014, there were over 1,800 twin pregnancies managed at our hospital. Thirteen were complicated by a discordant NTD: eight with anencephaly, three with an encephalocoele, and two with spina bifida. Nine of the 13 pregnancies were DCDA, three were MCDA, and one was MCMA. The mean gestation at diagnosis was 13.7 weeks (SD 1.0) for anencephaly, 18.1 weeks (SD 0.4) for spina bifida, and 20.4 weeks (SD 3.2) for encephalocoele. Figure 1 shows an ultrasound image of a twin pregnancy discordant for encephalocoele. Among cases with a NTD, six affected fetuses and three unaffected co-twins had diagnostic genetic testing and all were found to have a normal karyotype.
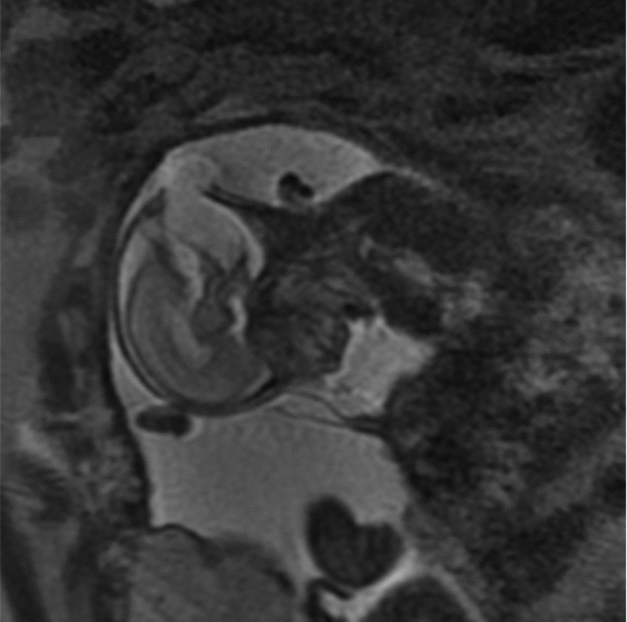
FIGURE 1 MRI of twin with encephalocoele.
The MCMA pregnancy had ST for anencephaly at 15.1 weeks and subsequently underwent a misoprostol induction following demise of the co-twin at 15.9 weeks; this case was excluded from further analysis. In one MCDA twin pregnancy, parents elected to have a termination of the whole pregnancy at 16 weeks’ gestation; this case was also excluded.
Pregnancies Discordant for NTD
Of the remaining 11 affected twin pregnancies, the affected fetus had ST in seven cases (64%), and four were managed expectantly (36%). The seven cases in which ST was performed comprised three for anencephaly, two for encephalocoele, and two for spina bifida. One of the seven cases delivered at 19 weeks following ST of the affected presenting anencephalic twin at 14 weeks’ gestation; although liveborn, the infant died quickly after birth. The remaining six pregnancies where ST was performed resulted in a live term (≥37 weeks’ gestation) delivery.
The four cases managed expectantly included three pregnancies with an anencephalic fetus and one with an encephalocoele. Two women in this expectant management group, both DCDA pregnancies, required tocolysis for preterm labor at 28 weeks’ and 29 weeks’ gestation, respectively. The first woman delivered at 29 weeks’ gestation and the unaffected co-twin was diagnosed with a right grade 1 intraventricular hemorrhage at 7 days of age. The baby was also treated for hyaline membrane disease, a right pneumothorax, and jaundice and was discharged home at 53 days of age. The second woman developed pre-eclampsia and delivered at 30 weeks’ gestation. The unaffected co-twin was diagnosed with a large patent ductus arteriosus, which was treated with indomethacin. The baby also had hyaline membrane disease and jaundice and was discharged home at 46 days of age.
The two remaining MCDA pregnancies managed expectantly delivered at 31 weeks’ and 32 weeks’ gestation, respectively, and both unaffected co-twins had adverse outcomes. One co-twin was diagnosed with sepsis and widespread intraventricular hemorrhage, resulting in a neonatal death at 10 days of age. The other co-twin was diagnosed with periventricular leukomalacia following birth.
Polyhydramnios complicated one (14%) case following ST and three (75%) cases in the expectant management group (OR 0.06; 95% CI [0.003, 1.23]; Fisher's exact test; p = .09).
The mean gestation at delivery in the ST group was 35.5 weeks (SD 7.1) compared with 31.0 weeks (SD 1.5) in the expectant management group, a non-significant difference (mean difference 4.5 weeks; 95% CI [-3.7, 12.9 weeks], p = .25). Rates of delivery <34 weeks’ gestation were 14% (1/7) of those who underwent ST compared with 100% (4/4) of expectantly managed pregnancies (Fisher's exact test; p = .015).
Delivery was by cesarean section in 8 of the 11 cases. Four unaffected twins required admission to the neonatal unit, primarily due to prematurity; all were in the expectant management group (Fisher's exact test; p = .003). Two liveborn infants with anencephaly had birthweight SD scores <-2, whereas the birthweight SD scores of all unaffected co-twins were ≥-2.
Case–Control Analysis
When comparing outcomes of the cases (discordant for NTD) with the controls (not discordant for NTD), there were few differences in demographic or antenatal variables, although more of the cases were smokers and more had polyhydramnios (Table 1). The mode of delivery was similar between the groups with a majority of women undergoing a cesarean section.
TABLE 1 Demographic and Pregnancy Data for Cases and Controls

ART = assisted reproductive technology; TTTS = twin–twin transfusion syndrome; DCDA = dichorionic diamniotic; MCDA = monochorionic diamniotic; NA = not applicable.
*Mann–Whitney U test; †mean difference (95% confidence interval [CI]); ‡odds ratio (OR) (95% confidence interval [CI]).
Primary or secondary outcomes in bold type.
The infant outcomes comparing the unaffected case twins with their matched controls are shown in Table 2.
TABLE 2 Infant Data for Non-affected Cases and Matched Control Twins
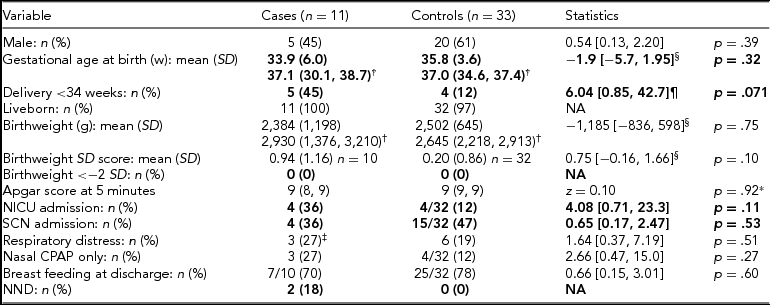
w = weeks; SD = standard deviation; NICU = neonatal intensive care unit; SCN = special care nursery; NND = neonatal death; NA = not applicable.
*Mann–Whitney U test; †median (25th–75th centiles); ‡n = 2 intubated and treated with exogenous surfactant; §mean difference (95% CI); odds ratio (OR) (95% confidence interval [CI]).
Primary or secondary outcomes in bold type.
There were no substantial differences in sex, mean gestational age, birthweight, or birthweight SD score between the groups; no twins in either group had birthweight SD scores <-2. Although more cases delivered <34 weeks than controls, the evidence for a difference was weak after accounting for the lack of independence between controls in the analyses. The groups had similar rates of admission to the neonatal intensive care unit or special care nursery, respiratory distress, and use of respiratory support, and most in both groups were being breastfed on discharge home. The indication for admission to the nurseries in the majority of infants was for prematurity; however, one term infant was admitted with hypoglycaemia at 37 weeks’ gestation. The only two neonatal deaths occurred among the cases, as described above.
Discussion
The main finding in our study comparing management options within twins discordant for NTD was that expectant management resulted in more births <34 weeks, with consequently more infants requiring admission to the nursery than did ST. The main finding from the case–control analysis was that twin pregnancies discordant for NTD were more likely to develop polyhydramnios, with a trend for more to deliver <34 weeks than twin pregnancies with no NTD.
It has long been recognized that twin pregnancies with a single anomalous fetus have poorer outcomes compared with normal twin pregnancies (Sun et al., Reference Sun, Chen, Wen, Fung, Yang and Walker2009). The optimal management of twin pregnancies discordant for NTD is complicated due to the scarcity of evidence in the literature and small sample sizes, and hence a lack of power to detect important differences between groups. A limitation of our study was that it is also small; however, ST resulted in some clinical benefits compared with expectant management. Currently, parents have the option of terminating the entire pregnancy, ST of the anomalous fetus, or expectant management involving monitoring for polyhydramnios and consideration of amniodrainage if required.
ST has previously been shown to prolong gestation at delivery for the unaffected co-twin and this directly correlates with a higher birthweight and better perinatal outcomes (Gul et al., Reference Gul, Cebeci, Aslan, Polat, Sozen and Ceylan2005). It does, however, carry a risk of pregnancy loss of up to 5% in DC twins (Bigelow et al., Reference Bigelow, Factor, Moshier, Bianco, Eddleman and Stone2014) and up to 11% in MC twins (Gaerty et al., Reference Gaerty, Greer and Kumar2015), consistent with the rate of neonatal deaths in the unaffected co-twin in our study. The risk of FDIU of the unaffected co-twin is reported to be greatest in the two weeks post-procedure (Rossi & D'Addario, Reference Rossi and D'Addario2009). These findings must be taken into consideration when counselling parents about management options. ST in MC twins is a viable option and a meta-analysis by Rossi and D'Addario (Reference Rossi and D'Addario2009) showed that outcomes were better for MC twins undergoing ST after 18 weeks, which is contrary to findings for ST in DC twins. Unfortunately, ST does not reduce perinatal mortality overall (Lust et al., Reference Lust, De Catte, Lewi, Deprest, Loquet and Devlieger2008).
Considering the risks associated with ST (including miscarriage, infection, and prelabor preterm rupture of the membranes), as well as the findings of some studies that there is no increased risk of preterm delivery in twin pregnancies discordant for fetal anomalies (Chang et al., Reference Chang, Chao, Cheng, Chung, Chueh, Chang and Soong2004; Linskens et al., Reference Linskens, Elburg, Oepkes, Vugt and Haak2011), it is important to consider expectant management options. Close antenatal monitoring with serial ultrasound examinations for the early diagnosis of polyhydramnios is one option. Although some will develop polyhydramnios, approximately 65% of these cases can be managed expectantly, and amniodrainage or ST can be considered for the remainder of cases (Vandecruys et al., Reference Vandecruys, Avgidou, Surerus, Flack and Nicolaides2006).
When comparing the rates of delivery <34 weeks for the NTD cases (independent of management type) and the control group, 45% of all cases were delivered <34 weeks’ gestation compared with only 12% of controls, but the small sample size reduced the strength of the evidence for a difference between groups. The preterm birth rates in our study overall reflect the known higher rates of preterm delivery seen with normal twin gestations (Goldenberg et al., Reference Goldenberg, Iams, Miodovnik, Van Dorsten, Thurnau, Bottoms and McNellis1996; Petit et al., Reference Petit, Cammu, Martens and Papiernik2011).
With respect to neonatal outcomes, the unaffected co-twin is no more likely to be small for gestational age or require admission to NICU or SCN than matched controls. MC pregnancies have added complexities for management, and it is well described that neurological injury and preterm delivery in MC twins are the most common cause of neonatal morbidity. Death of an affected twin in utero also places the unaffected co-twin at a significant risk (Evans et al., Reference Evans, Goldberg, Dommergues, Wapner, Lynch, Dock and Berkowitz1994). Both of the MC co-twins in our study suffered poor neonatal outcomes.
Cesarean delivery rates were no different for cases and controls, and this supports the findings of Nassar et al. (Reference Nassar, Adra, Gomez-Marin and O'Sullivan2000), who assessed discordant major structural anomalies in twin pregnancies, including NTD. Interestingly, half of the affected twin pregnancies undergoing cesarean section for delivery was for the indication of malpresentation; however, this was likely gestation dependent.
The current study offers insight into the management dilemmas seen with twin pregnancies complicated by NTD. The main strength of the current study is that it is the first case–control analysis of twin pregnancies discordant for an NTD. The major limitation was the small number of cases available for analysis due to the rarity of the condition. The current study may therefore be underpowered to detect clinically important differences. Firm recommendations for management cannot be made without larger randomized controlled trials comparing management options.
Conclusions
Our study has confirmed that polyhydramnios and preterm delivery may complicate twin pregnancies affected with an NTD, but they are not universal. While ST is not without risk, it warrants serious consideration to avoid the potential complications to the unaffected co-twin.
Acknowledgments
This work was supported by the Operational Infrastructure Support Program of the State Government of Victoria.
Disclosure of Interests
None of the authors have any conflicts of interest to report.





