Tea and coffee remain the most widely consumed non-alcoholic beverages in Western society and have gained significant attention in relation to their effects on health. Coffee consumption has been associated with a reduced risk of type 2 diabetes mellitus in several large scale prospective studies(Reference van Dam and Hu1). These associations did not differ by region (USA and Europe), although European cohorts have been drawn mainly from high coffee consumers such as the Netherlands(Reference van Dam and Feskens2, Reference van Dam, Dekker, Nijpels, Stehouwer, Bouter, Heine and Hoorn Study3) and Finland(Reference Tuomilehto, Hu, Bidel, Lindstrom and Jousilahti4–Reference Hu, Jousilahti, Peltonen, Bidel and Tuomilehto7). Tea has also been generally associated with cardio-protective effects with the exception of studies in British cohorts, which are strongly confounded by social status(Reference Peters, Poole and Arab8). Indeed, given that coffee and tea consumption are associated with various unhealthy behaviours (e.g. smoking, alcohol intake) and psychosocial risk factors, the true effects of these beverages might be underestimated.
In one of the only studies that have examined the effects of coffee in a British cohort, increasing coffee consumption was associated with beneficial effects for mortality and coronary morbidity, whereas tea showed opposite trends(Reference Woodward and Tunstall-Pedoe9). Adjustment for confounding from social status accounted for the associations of tea intake, although there appeared to be residual benefits of coffee consumption. Nevertheless, the association between coffee, tea and risk of type 2 diabetes has not been examined in a British cohort. We therefore determined the association between coffee and tea with incident cases of type 2 diabetes among British men and women from the Whitehall II cohort. Given that the primary aim of the Whitehall II study is to examine social inequalities in health, we were able to adjust for possible confounding from social status in our analyses.
Experimental methods
Study population
The Whitehall II cohort consists of London-based office staff that were working in twenty Civil Service departments during recruitment in 1985–8. The initial cohort consisted of 10 308 civil servants (6895 men and 3413 women, aged 35–55 years), with a participation rate of 73 %(Reference Marmot, Smith, Stansfeld, Patel, North, Head, White, Brunner and Feeney10). During a follow-up screening in 1991–3 the FFQ was completed, from which coffee and tea consumption were reported. Thus, for the purpose of these analyses follow-up screening in 1991–3 was regarded as baseline. Further clinical examinations were undertaken in 1997–9 and 2003–4. At baseline, there were 6702 diabetes-free participants with available data for all variables. A further 879 participants were lost to follow-up, leaving 4055 men and 1768 women that were included in the final analyses. Participants that were excluded from these analyses were more likely to be from lower work grades, have a higher BMI, higher systolic blood pressure and a higher 2 h post-load glucose concentration. The study was approved by the University College London Medical School Committee on the Ethics of Human Research and informed consent was obtained at baseline and renewed at each contact.
Measures
In the FFQ coffee and tea intake were reported in nine predefined categories that ranged from never/less than once per month to six or more cups per day. Coffee intake was split into total coffee intake (caffeinated plus decaffeinated) and decaffeinated only categories, although specific coffee preparation methods were not identified. The FFQ has been previously validated in the Whitehall II cohort(Reference Brunner, Stallone, Juneja, Bingham and Marmot11). In this validation study tea and coffee intake were not specifically examined although the mean energy intake from the 7 d diet diary and FFQ were well matched, and there was strong agreement between the estimated intake of various micronutrients using the FFQ and plasma biomarkers. The procedures for the clinical examination have been described elsewhere(Reference Marmot, Smith, Stansfeld, Patel, North, Head, White, Brunner and Feeney10). Briefly, measurements included height, weight, waist and hip circumference, blood pressure, fasting cholesterol and a glucose tolerance test. A questionnaire was completed regarding age, civil service employment grade, smoking habits, health status, medications and physical activity. Vigorous activity was defined as activity strenuous enough to build up a sweat.
Incident cases of diabetes were identified by self-report of doctor's diagnosis, diabetic medication and by 2 h oral 75 g glucose tolerance test (2 h post-load glucose) at the baseline clinical examination and subsequent follow-ups in 1997–9 and 2003–4. All participants underwent oral glucose tolerance testing at both screening phases, unless they reported diabetes. Diabetes was defined as a 2 h post-load glucose concentration of >11·1 mmol/l, based on previous guidelines(12). Incident diabetes was dated at the day of study visit for those first identified through the 2 h post-load glucose. For those identified by self-report, the mid-point between the first instance of self-reported diabetes and the previous phase was used. Person-time of exposure was censored at the mid-point between the last known visit and the first missing visit for those lost to follow-up. Participants with an intermediate missing phase were assumed to have continuous follow-up time. For those who had not developed diabetes, follow-up was censored on 30 September 2004 (final follow-up closing date).
Statistical analysis
Differences in baseline characteristics across coffee- and tea-drinking categories were evaluated using ANOVA and an overall χ2 test for categorical variables. We calculated risk estimates per category of coffee/tea intake using Cox proportional hazard regression models (SPSS version 10.1 program software; SPSS, Chicago, IL, USA). We adjusted the risk estimates for possible confounders. The covariates for the present analyses included 5-year age categories, gender, ethnicity categories (white; South Asian; black; other), employment grade categorised into six levels (as a measure of social status), BMI categories ( ≤ 20; 20·1–25·0; 25·1–30; 30·1–35;>35), waist to hip ratio, smoking categories (yes; never; previously), alcohol intake (units/week) categorised into sex-specific tertiles, physical activity category ( ≤ 1; 1·01–4·0;>4 h/week moderate and vigorous combined), family history of diabetes, hypertension, blood cholesterol, total energy intake, and dietary patterns. Coffee- and tea-drinking categories were defined as non-drinkers (reference category), those drinking no more than one cup per day ( ≤ 1 cup/d), those drinking two to three cups per day (2–3 cups/d) and those drinking more than three cups per day (>3 cups/d). In all models we checked that the proportional hazards assumption was met.
Dietary patterns were identified as in a previous paper(Reference Martikainen, Brunner and Marmot13) using cluster analysis (PROC FASTCLUS; SAS Institute, Cary, NC, USA, 1988) with responses to all food frequency items, except tea and coffee consumption, as input variables. The FASTCLUS procedure minimises the sum of squared Euclidean distances between the observations and the cluster means; 124 (1·5 %) individuals were excluded by not allowing FASTCLUS to assign outlying (distant) observations to a cluster. The R 2 for predicting the frequency response from the clusters was calculated for each FFQ item. The twenty-two FFQ items with the highest R 2 values were used as rationale to label the clusters. The six clusters originally observed were merged to four clusters, described in Table 1.
Table 1 Dietary pattern in relation to coffee and tea intake (%)‡
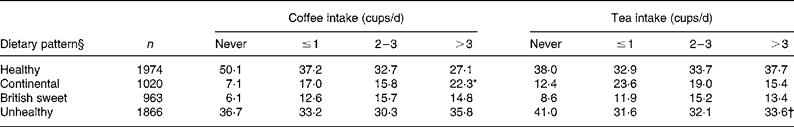
Mean value was significantly different from those of the other coffee intake categories: *P < 0·001.
Mean value was significantly different from those of the other tea intake categories: †P < 0·001.
‡ For details of procedures, see Experimental methods.
§ Dietary cluster definitions: ‘Healthy’ denotes higher than average consumption of wholemeal bread, fruit and vegetables, polyunsaturated margarine and average to low consumption of sweet food, wine and beer; ‘Continental’ refers to higher than average consumption of wholemeal bread, fruit, vegetables, pasta and rice, wine and beer, low intake of full cream milk, but high intake of butter, and average consumption of white bread; ‘British sweet’ denotes higher than average consumption of biscuits, cakes, meat, sausages and savoury pies, white bread, full cream milk, butter, wine and beer, and average intake of fruit and vegetables; ‘Unhealthy’ denotes higher than average consumption of meat and sausages, white bread, chips and full cream milk, average consumption of wine and beer, and very low consumption of fruit and vegetables.
Results
Both men and women most frequently reported consuming 2–3 cups/d coffee and 2–3 cups/d tea, although there were higher proportions of non-coffee and non-tea drinkers among women. Infrequent coffee drinkers were more likely to be of lower employment grade, non-white, non-smokers, have lower levels of blood cholesterol, but a higher prevalence of hypertension (see Table 2). Coffee drinkers (caffeinated and decaffeinated combined) were more likely to consume higher amounts of alcohol, report a greater total energy intake and belong to the ‘continental’ dietary pattern (see Tables 1 and 2). In addition, male coffee drinkers were less physically active, had higher BMI and were younger in comparison to non-coffee drinkers. Infrequent tea drinkers were more likely to be of higher employment grade, non-white, younger, consume less energy but more alcohol and have greater adiposity, higher levels of blood cholesterol and belong to an ‘unhealthy’ dietary pattern (see Tables 1 and 3). In addition, male tea drinkers were more physically active.
Table 2 Baseline characteristics in relation to coffee intake category (caffeinated and decaffeinated combined)‡
(Mean values and standard deviations)

DBP, diastolic blood pressure; SBP, systolic blood pressure.
Mean value was significantly different from those of the other intake categories for men: *P < 0·001.
Mean value was significantly different from those of the other intake categories for women: †P < 0·001.
‡ For details of procedures, see Experimental methods.
Table 3 Baseline characteristics in relation to tea intake category‡
(Mean values and standard deviations)

DBP, diastolic blood pressure; SBP, systolic blood pressure.
Mean value was significantly different from those of the other intake categories for men: *P < 0·001.
Mean value was significantly different from those of the other intake categories for women: †P < 0·001.
‡ For details of procedures, see Experimental methods.
At baseline, 576 cases of impaired glucose tolerance were identified (as a 2 h post-load glucose concentration of 7·8–11·0 mmol/l)(12). Interestingly, there was an inverse association between coffee intake (caffeinated and decaffeinated combined) and 2 h post-load glucose concentration at baseline in men and women (see Table 2). This association persisted (F(1,3) 12·2, P < 0·001) after adjustment for age, gender, ethnicity, employment grade, BMI, waist to hip ratio, smoking, alcohol intake, physical activity, family history diabetes, hypertension, cholesterol, total energy intake and diet pattern. Fasting or 2 h glucose concentrations were not associated with tea intake at baseline. In logistic regression analyses, a lower risk of impaired glucose tolerance was associated with >3 cups/d caffeinated and decaffeinated coffee combined (OR 0·54; 95 % CI 0·38, 0·76; P = 0·001),>3 cups/d decaffeinated coffee (OR 0·79; 95 % CI 0·50, 1·24; P = 0·082) and >3 cups/d tea (OR 0·73; 95 % CI 0·51, 1·04; P = 0·001) with reference to no intake of these beverages, in fully adjusted models.
During follow-up mean (11·7 (sd 3) years) there were a total of 387 incident cases of diabetes among men and women, representing an incidence rate of 6·6 % for the total study period. Approximately half of the cases were identified through self-report of doctor's diagnosis. In unadjusted analyses coffee drinkers (caffeinated and decaffeinated) combined demonstrated a lower incidence of diabetes, which was greatest for participants drinking 2–3 cups/d (hazard ratio (HR) 0·69; 95 % CI 0·51, 0·92; P = 0·013). Similarly, consumption of >3 cups/d decaffeinated coffee only was associated with lower risk (HR 0·52; 95 % CI 0·29, 0·95; P = 0·033). These associations did not persist after adjustment for covariates (see Table 4). Nevertheless, closer examination of the CI, especially in the case of decaffeinated coffee, does not exclude large reductions in risk. Tea intake of >3 cups/d was associated with lower risk of diabetes in analyses adjusted for age, gender, ethnicity and social status (HR 0·66; 95 % CI 0·61, 1·22; P = 0·04) (see Table 4). However, this effect was not robust in the fully adjusted model (HR 0·77; 95 % CI 0·52; 1·14, P = 0·13). In further analyses we adjusted for specific food items (including red and processed meats, whole grains, fruit and vegetables, diary products). The results, however, were largely unchanged, for >3 cups/d caffeinated and decaffeinated coffee combined (HR 0·74; 95 % CI 0·51, 1·08; P = 0·298) or tea (HR 0·75; 95 % CI 0·51, 1·09; P = 0·105).
Table 4 Hazard ratios (HR) of diabetes according to drinking category in men and women*
(Hazard ratios and 95 % CI)

* For details of procedures, see Experimental methods.
† Model 1 adjusted for 5-year age categories, gender and ethnicity (white; South Asian; black; other).
‡ Model 2 adjusted for 5-year age categories, gender, ethnicity, plus employment grade (six levels).
§ Model 3 adjusted for 5-year age categories, gender, ethnicity, employment grade, plus BMI category ( ≤ 20; 20·1–25·0; 25·1–30; 30·1–35;>35), waist to hip ratio, smoking (never; current; previously), gender-specific alcohol intake tertiles, physical activity category ( ≤ 1; 1·01–4·0;>4 h/week moderate and vigorous combined), family history diabetes, hypertension, cholesterol, total energy intake, diet pattern and mutual adjustment for all beverage type.
In further analyses, we examined the combined effects of coffee and tea consumption. In comparison with participants drinking < 1 cup/d tea and coffee (n 493), the HR for intake of ≥ 2 cups/d of both beverages (n 1836) was 0·68 (95 % CI 0·46, 0·99; P = 0·04) in the fully adjusted model. Given the limited number of participants in these analyses, the results should be viewed cautiously.
Discussion
In the present cohort of British civil servants coffee and tea intake were not associated with incidence of type 2 diabetes, despite an inverse association between coffee intake and 2 h post-load glucose concentration at the baseline assessment. There was weak evidence for an association between tea and incident diabetes although this relationship was largely explained by residual confounding from adiposity and dietary pattern. The consumption of tea and coffee in Britain are strongly associated with lower and higher social status, respectively. This trend was also reflected in the present cohort. Previous studies in British cohorts have generally found adverse effects of tea drinking on cardiovascular health, which are however strongly confounded by social status(Reference Peters, Poole and Arab8). In the present study the inclusion of social status as a confounder slightly strengthened the results in relation to the association between tea and diabetes.
Recent attention has focused on the inverse relationship between coffee and risk of type 2 diabetes(Reference van Dam and Hu1). In a meta-analysis of nine cohort studies, overall, participants who drank 4–6 and >6–7 cups/d coffee had a 28 and 35 % lower risk of diabetes compared with those who drank < 2 cups/d(Reference van Dam and Hu1). However, a recent cohort study suggests that the protective effects are largely explained by decaffeinated coffee intake(Reference Pereira, Parker and Folsom14). The present data also appear to suggest greater protective effects for decaffeinated coffee, 0·65 (95 % CI 0·36, 1·16), although our analyses were limited by the relatively small number of decaffeinated coffee drinkers. Given that the British are predominantly tea consumers a possible explanation for our null findings is lower coffee consumption. Indeed, the mean coffee intake of participants in the highest exposure group was approximately 5 cups/d, thus the present findings are not directly comparable with results of previous studies that have examined intakes of ≥ 7 cups/d (Reference van Dam and Hu1). Previous evidence regarding the association between moderate coffee consumption ( ≥ 3 cups/d or 3–4 cups/d) and risk of diabetes is conflicting(Reference van Dam, Dekker, Nijpels, Stehouwer, Bouter, Heine and Hoorn Study3, Reference Reunanen, Heliovaara and Aho6, Reference Saremi, Tulloch-Reid and Knowler15, Reference van Dam, Willett, Manson and Hu16). Furthermore, other recent cohort studies have observed associations in men only(Reference Paynter, Yeh, Voutilainen, Schmidt, Heiss, Folsom, Brancati and Kao17) or among non-elderly adults who had previously lost weight(Reference Greenberg, Axen, Schnoll and Boozer18). The present data may also be more strongly confounded by social status because in other European countries such as Finland, coffee consumption is related to lower social status, as opposed to higher social status in Britain. In addition, a higher proportion of coffee drinkers belonged to the continental dietary pattern and consumed higher amounts of alcohol, which may have provided protective effects and contributed to additional confounding. There is presently limited evidence for the effects of coffee intake on diabetes risk markers from clinical trials. In a randomised controlled trial high coffee consumption for 4 weeks increased fasting insulin concentrations and had no impact on glucose levels compared with coffee abstinence(Reference van Dam, Pasman and Verhoef19). In acute studies the ingestion of decaffeinated coffee had a beneficial effect on glucose metabolism following an oral glucose tolerance test(Reference Battram, Arthur, Weekes and Graham20), although others have reported a deterioration of glucose tolerance after coffee ingestion(Reference Jankelson, Beaser, Howard and Mayer21, Reference Wachmann, Hattner, George and Bernstein22).
Potential health benefits of tea have been attributed to the high flavonoid content, which may act as antioxidants and anti-inflammatory agents(Reference Rietveld and Wiseman23, Reference Steptoe, Gibson, Vuononvirta, Hamer, Wardle, Rycroft, Martin and Erusalimsky24). A feasible hypothesis is that tea may reduce oxidative stress and inflammation that is associated with the progressive impairment of pancreatic β-cell function in the development of diabetes(Reference Ceriello and Motz25). Among a large cohort of US postmenopausal women a 36 % reduction in diabetes risk was observed for participants in the highest tea-drinking category (>4 servings/d)(Reference Pereira, Parker and Folsom14), which is comparable with the present results where tea intake of >3 cups/d was associated with a 34 % risk reduction after adjusting for age, gender, ethnicity and social status. However, previous prospective studies have produced conflicting findings that show no association between black tea intake and diabetes(Reference van Dam, Willett, Manson and Hu16, Reference Iso, Date, Wakai, Fukui, Tamakoshi and JACC Study Group26). Other epidemiological studies have provided mixed evidence for a protective effect of flavonoid intake against type 2 diabetes(Reference Knekt, Kumpulainen, Jarvinen, Rissanen, Heliovaara, Reunanen, Hakulinen and Aromaa27–Reference Nettleton, Harnack, Scrafford, Mink, Barraj and Jacobs29). Dietary antioxidant intake, especially vitamin E at moderate intake levels, has been associated with increased protection against type 2 diabetes in prospective studies(Reference Hamer and Chida30), although this is in contrast to long-term clinical trials that have demonstrated no significant effects(Reference Liu, Lee, Song, Van Denburgh, Cook, Manson and Buring31, Reference Liu, Ajani, Chae, Hennekens, Buring and Manson32). Given that coffee is also thought to be rich in antioxidants(Reference Svilaas, Sakhi, Andersen, Svilaas, Strom, Jacobs, Ose and Blomhoff33), this may explain why combined coffee and tea consumption was associated with lower diabetes risk in the current analyses.
The limitations of the present study should be noted. Coffee and tea consumption was based on self-reported data that did not account for variations in serving size or strength of each beverage. Despite this, the FFQ is a strongly validated questionnaire. Dietary intake was only recorded once at baseline, leaving the possibility that participants changed their habits during follow-up. It should be noted that the present analyses included approximately 56 % of the initial Whitehall II cohort, which is largely due to attrition and incomplete data, although this raises the possibility of selection bias. Indeed, excluded participants were more likely to come from lower work grades and display poorer health profiles thus this may have somewhat diluted the effects. We did not establish the validity of the self-reported diabetes measure thus we cannot exclude the possibility that false-positive cases might have led to an underestimation of the effects. Nevertheless, incident diabetes in the present study is closely matched with the current UK incidence rates. The strengths of the present study include the use of oral glucose tolerance tests at baseline and two further follow-ups to exclude unrecognised prevalent cases of diabetes. In addition, the British Civil Servant employment grade is an accurate indicator of social status which enabled us to adjust effectively for this important confounding variable. In conclusion, coffee and tea intake were inversely associated with impaired glucose tolerance at baseline but not with incidence of type 2 diabetes during 11 years follow-up. The limited range of exposure and beverage consumption according to socio-economic class may explain these conflicting findings.
Acknowledgements
The Whitehall II study has been supported by grants from the Medical Research Council; Economic and Social Research Council; British Heart Foundation; Health and Safety Executive; Department of Health; National Heart Lung and Blood Institute (HL36310), US, NIH: National Institute on Aging (AG13196), US, NIH; Agency for Health Care Policy Research (HS06516); and the John D. and Catherine T. MacArthur Foundation Research Networks on Successful Midlife Development and Socio-economic Status and Health. M. G. M. is supported by an MRC Research Professorship. All authors declare that they have no competing interests. We also thank all participating civil service departments and their welfare, personnel and establishment officers; the Occupational Health and Safety Agency; the Council of Civil Service Unions; all participating civil servants in the Whitehall II study; and all members of the Whitehall II study team. The following are the contributions from each of the authors (all authors contributed to the paper idea): M. H. – preparing data, statistical analyses, writing manuscript; D. R.W. – preparing data (diabetes), contribution to analyses and discussion; A. M. – preparing data (dietary), contribution to analyses and discussion; M. G. M. – principal investigator of the study, contribution to analyses and discussion; E. J. B. – preparing data, contribution to analyses and discussion. M. H. had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.






