Malnutrition has been associated with increased morbidity and mortality in patients undergoing continuous ambulatory peritoneal dialysis (CAPD)( Reference Han and Han 1 ). Although much progress has been made on improving the nutritional status of CAPD patients, the prevalence of protein–energy malnutrition is still high (ranging from 18 to approximately 56 %, depending on the assessment methods used)( Reference Han and Han 1 ). The anorexia due to abdominal distension and glucose absorption from the dialysate, loss of nutrients in dialysate, inflammation and loss of residual renal function (RRF) could be responsible for malnutrition in CAPD patients.
Individual differences in peritoneal membrane function have been shown to influence the clinical outcomes in patients undergoing CAPD( Reference Rumpsfeld, McDonald and Johnson 2 ). The higher peritoneal transport is a status of a high peritoneal small-solute transport rate, which includes both peritoneal high average and high transport status determined by peritoneal equilibration test (PET)( Reference Chung, Heimburger and Lindholm 3 ). The protein–energy wasting (PEW) reflects malnutrition and wasting caused not only by inadequate nutrient intake but also by depletion resulting from the inflammatory and non-inflammatory conditions that prevail in dialysis population( Reference Riella 4 ). It had been reported that higher peritoneal transport status is associated with high baseline albumin clearance( Reference Balafa, Halbesma and Struijk 5 ), and recently, the Global Fluid Study investigators demonstrated that higher transport status was linked with greater levels of intraperitoneal inflammation( Reference Lambie, Chess and Donovan 6 ). Therefore, it had been suggested that higher peritoneal transport status might result in progressive deterioration of nutritional status in peritoneal dialysis (PD) patients. However, studies on the association between peritoneal transport and nutritional status have presented contradictory results( Reference Harty, Boulton and Venning 7 – Reference Zhang and Ren 10 ). In the present study, we aimed to investigate the relationship between baseline peritoneal transport types and nutritional status at the first year of CAPD in patients of Southern China.
Subjects and methods
Study population
Incident CAPD patients were enrolled in this 1-year prospective study from 15 April 2010 to 31 December 2011 at the PD centre of The First Affiliated Hospital, Sun Yat-sen University, Guangzhou, China. The inclusion criteria were as follows: (1) CAPD therapy for more than 90 d; (2) age ≥ 18 years; (3) use of glucose dialysate (Baxter Healthcare); (4) signed informed consent form. The exclusion criteria were as follows: patients who had episodes of peritonitis within 3 months before PET, or expected to have kidney transplantation within 6 months; or with serious liver disease; or with amputation, or with pacemaker; or with active comorbidities or recent systemic infections; or were not able to accomplish the measurement of body composition in standing position for 3 min. All patients were followed up for 12 months. Icodextrin dialysate was not available in these patients. The study protocols were approved by the Ethics Committee of The First Affiliated Hospital of Sun Yat-sen University.
The following demographic and clinical variables were collected at the start of CAPD: age; sex; body weight and height; primary renal disease; modified Charlson comorbidity index(
Reference Volk, Hernandez and Lok
11
); clinical symptom; blood pressure; medical prescription; CAPD regimen; 24 h urine output; total peritoneal ultrafiltration; night dwell peritoneal ultrafiltration. Data on serum albumin (ALB), pre-albumin (pre-ALB), transferrin, Hb, high-sensitive C-response protein (hsCRP), total cholesterol and Kt/V were obtained at the baseline (0 month), 6 months and 12 months of follow-up, respectively. Prescription of each CAPD patient was recorded by the primary nurses and was used for estimating peritoneal glucose absorption, which was expressed as averaged glucose dose per d
![]() $$(\Sigma glucose dose (g)/vintage (d)), $$
where Σ is the total glucose dose used during the follow-up period. The weekly total Kt/V (urea clearance index used to quantify the dialysis treatment adequacy, where K is the dialyser clearance of urea, t is the dialysis time and V is the volume of distribution of urea approximately equal to total body water of the patient) was measured by the methods described by Lysaght et al.
(
Reference Lysaght, Pollock and Hallet
12
). Residual kidney function was measured by mean values of creatinine clearance and urea clearance and adjusted for body surface area. All patients were followed up for 12 months. The causes and days for hospitalisation, change of medical and PD prescription, and reasons for withdrawal of PD during the follow-up period were also recorded.
$$(\Sigma glucose dose (g)/vintage (d)), $$
where Σ is the total glucose dose used during the follow-up period. The weekly total Kt/V (urea clearance index used to quantify the dialysis treatment adequacy, where K is the dialyser clearance of urea, t is the dialysis time and V is the volume of distribution of urea approximately equal to total body water of the patient) was measured by the methods described by Lysaght et al.
(
Reference Lysaght, Pollock and Hallet
12
). Residual kidney function was measured by mean values of creatinine clearance and urea clearance and adjusted for body surface area. All patients were followed up for 12 months. The causes and days for hospitalisation, change of medical and PD prescription, and reasons for withdrawal of PD during the follow-up period were also recorded.
Peritoneal transport status
A 4 h PET, as described by Twardowski et al. ( Reference Twardowski, Nolph and Khanna 13 ), was performed on each patient within the first 1–3 months after CAPD. Dialysate samples were taken at 0, 2 and 4 h of dwell while blood samples were obtained at 2 h. Ratios of D:P creatinine were calculated using dialysate (D) creatinine concentrations at 4 h of PET divided by the plasma (P) creatinine concentrations (4 h D:P cr). Peritoneal transport status was considered a categorical variable according to the four groupings of 4 h D:P cr values defined by Twardowski et al. ( Reference Twardowski, Nolph and Khanna 13 ): low < 0·50; low average 0·50–0·64; high average 0·65–0·80; high >0·81. Patients were divided into lower peritoneal transport group (including low, low average transporters) and higher peritoneal transport group (including high average, high peritoneal transporters).
Measurement of nutritional status
Subjective global assessment
At the baseline and 12 months of follow-up, subjective global assessment (SGA) score was calculated using the method described by Detsky et al. ( Reference Detsky, McLaughlin and Baker 14 ), which was widely applied in CAPD patients( 15 , Reference Chung, Han and Noh 16 ). According to the score, nutritional status was classified as severe malnutrition (SGA = 1–2, C), mild-to-moderate malnutrition (SGA = 3–5, B) and good nutritional status (SGA = 6–7 A). In the present study, patients were grouped into well-nourished (SGA A) and malnourished (SGA B+C) categories for the purpose of statistical analysis.
Bioimpedance analysis
Multiple-frequency bioelectrical impedance analysis (mBIA; InBody 720) was conducted on the day of PET in fasting condition in the morning, the analysis being conducted three times to get rid of the bias. Phase angle (PA) was applied as the main nutritional parameter, which was calculated directly from reactance and resistance. PA was calculated as arc-tangent reactance/resistance × 180°/π, with a frequency of 50 kHz( Reference Barbosa-Silva, Barros and Wang 17 ). Extracellular water, intracellular water, lean body mass, skeletal muscle mass and fat mass percentage were also measured indirectly by mBIA. Data on mBIA were collected at the baseline, 6 months and 12 months of the follow-up.
Protein–energy wasting score
The diagnostic criteria proposed by the International Society of Renal Nutrition and Metabolism panel( Reference Han and Han 1 , Reference Fouque, Kalantar-Zadeh and Kopple 18 ) were taken into reference; however, some criteria were adjusted for Asian population. The diagnostic criteria of PEW in the present study were as follows: (1) serum chemistry: serum ALB level < 38 g/l, serum pre-ALB level < 300 mg/l, serum cholesterol level < 2·59 mmol/l; (2) body mass: BMI < 18·5 kg/m2( 19 ), unintentional non-oedematous weight loss over time ≥ 5 % over 3 months or ≥ 10 % over 6 months (non-oedematous weight = body weight − excessive water measured by mBIA), total body fat percentage < 10 % (measured by mBIA); (3) muscle wasting: reduced muscle mass ≥ 5 % over 3 months or ≥ 10 % over 6 months (measured by mBIA); (4) protein metabolism: normalised protein catabolism rate < 1 g/kg per d for at least 2 months. A diagnosis of PEW requires that at least three of the four listed categories should be met and at least one test in each of the selected category should be included. PEW was assessed at 12 months of follow-up. During management of the patients, when serum ALB levels of the patients were less than 38 g/l, keto acids (Fresenius Kabi) were prescripted for these patients routinely, and the dosage range was three to four tablets three times per d according to body weight.
Statistical analysis
Data were presented as means and standard deviations or medians and interquartile range for continuous variables and number (percentages) for categorical variables. Differences between the two groups were evaluated by Student's t test or Mann–Whitney test or the χ2 test as appropriate. Serial changes of nutritional parameters were compared with the baseline values according to patient group using ANOVA for repeated measures. Multivariate linear regressions with stepwise, both univariate and multivariate binary logistic regression analysis with forward conditional methods, were performed to determine the variables that significantly affect nutritional status. A P value < 0·05 was considered statistically significant. SPSS version 16.0 for Windows (SPSS, Inc.) was used for the statistical analysis.
Results
Demographic and clinical characteristics of study population
A total of 283 CAPD patients with 171 (60·4 %) male and mean age 47·0 (sd 14·9) years were recruited (Fig. 1). The median PD duration at the time of recruitment was 45 (range 41–72) d. The primary cause of end-stage renal disease was chronic glomerulonephritis (n 162; 57·2 %), followed by diabetic nephropathy (n 58; 20·5 %) and hypertension (n 17; 6·0 %). The average 4 h D:P cr of all patients at baseline was 0·69 (sd 0·16.). Of the total patients, fourteen (4·9 %) were low transporters, 79 (27·9 %) were low average transporters, 140 (49·5 %) were high average transporters and 50 (17·7 %) were high transporters. Compared with lower transporters, higher transporters were predominantly male, had higher proportion of diabetes mellitus and higher Charlson comorbidity index (Table 1). At the initiation of CAPD, patients with higher transport had higher systolic blood pressure, serum hsCRP, serum creatinine, poor appetite, as well as lower total peritoneal ultrafiltration, night dwell peritoneal ultrafiltration and fat mass percentage (Table 2).

Fig. 1 Derivation of the study sample. PD, peritoneal dialysis; PET, peritoneal equilibration test; mBIA, multiple-frequency bioelectrical impedance analysis.
Table 1 Demographic and clinical characteristics of the study population at baseline (Mean values, standard deviations, number of subjects, percentages and ranges)
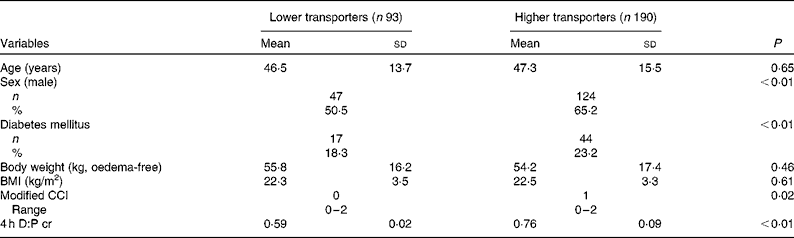
CCI, Charlson comorbidity index; 4 h D:P cr, creatinine ratio of dialysate:plasma at 4 h of the peritoneal equilibrium test.
Table 2 Clinical parameters of higher transporter and lower transporter at 0 and 12 months (Mean values, standard deviations, medians, interquartile ranges, number of subjects and percentages)

SGA, subjective global assessment; PEW, protein–energy wasting; hsCRP, high-sensitive C-response protein; mBIA, multiple-frequency bioelectrical impedance analysis; ECW/TBW, extracellular water/total body water; RRF, residual renal function; SBP, systolic blood pressure.
* SGA B represents a score of 3–5 (mild to moderate malnutrition); SGA C represents a score of 1–2 (severe malnutrition).
At 12 months of follow-up, ten (3·5 %) patients dropped out, among whom two patients with higher transport died, four patients transferred to haemodialysis (one lower transporters and three higher transporters) and four patients with higher transport received kidney transplantation (Fig. 1). Higher transporters had higher rate of poor appetite, lower total peritoneal ultrafiltration, night dwell peritoneal ultrafiltration, fat mass percentage as well as higher peritoneal glucose absorption per d, and higher episodes of peritonitis. There were no significant difference in RRF, weekly total Kt/V urea, Hb and serum transferrin between the two groups both at the baseline or 12 months of follow-up (Table 2).
Nutritional status between the two groups at 0 and 12 months
At the initiation of CAPD, the total malnourished patients defined by SGA B+C were 56·5 %. Compared with lower transporters, higher transporters had lower serum ALB, serum pre-ALB, PA and were more frequent with malnourished conditions defined by SGA (62·6 v. 45·2 %; Table 2; Fig. 2).

Fig. 2 Nutritional parameters of patients at 0 months, 6 months and 12 months (a) serum albumin (ALB), (b) serum pre-albumin (pre-ALB), (c) normalised protein catabolism rate (nPCR), (d) phase angle (PA), (e) skeletal muscle mass (SMM) and (f) lean body mass (LBM). * P< 0·05, compared with lower transporter; † P< 0·05, compared with 0 month. ■, higher transporters; □, lower transporters.
At 12 months of follow-up, the total malnourished patients defined by SGA B+C were 43·2 % and by PEW score were 29·3 %. Patients in both groups had increased level of serum ALB. However, the rate of malnourished conditions defined by SGA and PEW score were significantly higher (52·5 v. 25·0 %, P< 0·001; 37·0 v. 14·1 %, P< 0·001), and the levels of serum ALB (37·1 (sd 4·3) v. 39·6 (sd 4·3) g/l, P< 0·001), serum pre-ALB (356 (sd 99) v. 384 (sd 90) mg/l, P= 0·035), PA (6·15 (sd 0·39) v. 6·27 (sd 0·47)°, P< 0·05), skeletal muscle mass (25·2 (sd 4·3) v. 26·9 (sd 5·1) kg, P< 0·05) were lower than that of lower transporters (Table 2; Fig. 2).
The associations of higher transport status and malnutrition of patients at 12 months of follow-up
The associations of baseline peritoneal transport status and malnutrition of patients at 12 months of follow-up with defined binary logistic regression models are listed in Table 3. After adjustment of ageing (age >65 years), sex, diabetes mellitus, RRF, Kt/V, peritonitis incidence and serum hsCRP, as well as keto acid supplementation, baseline higher transport status was independently associated with malnutrition defined by SGA score (OR 3·43, 95 % CI 1·69, 6·96, P< 0·01). Similarly, baseline higher transport status was independently associated with malnutrition defined by PEW score (OR 2·40, 95 % CI 1·08, 5·31, P< 0·01). Furthermore, after adjusting the same variables as mentioned above, multiple linear regression results showed that baseline higher transporter was independently associated with lower serum ALB (β = − 0·164, P= 0·004) and PA (β = − 0·125, P= 0·009) measured by mBIA.
Table 3 Association between peritoneal transport status and subjective global assessment (SGA) B+C* and protein–energy wasting (PEW) (Odds ratios and 95 % confidence intervals)
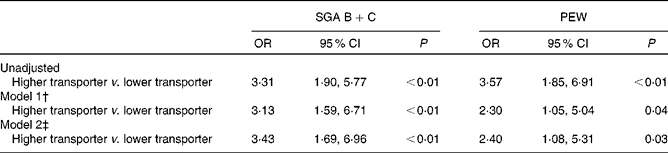
* SGA B represents a score of 3–5 (mild to moderate malnutrition); SGA C represents a score of 1–2 (severe malnutrition).
† Adjusted for age, sex, diabetes mellitus, residual renal function (RRF), Kt/V, peritonitis incidence and serum high-sensitive C-response protein (hsCRP).
‡ Adjusted for age, sex, diabetes mellitus, RRF, Kt/V, peritonitis incidence, serum hsCRP and keto acid supplementation.
Hospitalisation
There were seventy-four hospitalisations among 283 patients during the follow-up period (0·261 time/patient year). Primary causes for hospitalisation included peritonitis (twenty-seven hospitalisations, 0·095 time/patient year), inadequate dialysis (nineteen hospitalisations, 0·067 time/patient year) and non-peritonitis infection (eight hospitalisations, 0·028 time/patient year). Patients with higher peritoneal transporter status had significantly higher total rate of hospitalisation (0·32 (0–3) v. 0·13 (0–2) time/patient year, P< 0·01) and the hospitalisation rate due to peritonitis (0·14 (0–3) v. 0·01 (0–1) time/patient year, P< 0·01). Because only two patients died, the multivariate Cox regression was not analysed.
Discussion
The present prospective study showed that both at the baseline or 12 months of follow-up, higher transporters had higher rate of malnutrition than that of lower transporters, although nutritional status of the two groups was improved during the follow-up. After adjustment of age, sex, diabetes mellitus, RRF, Kt/V, peritonitis incidence and serum hsCRP, as well as keto acid supplementation, baseline higher peritoneal transport status was independently associated with poor nutritional status of patients at 12 months of follow-up.
Previous clinical studies on whether higher peritoneal transport status had been associated with overall worse nutritional status in CAPD patients have shown discrepant results. A 2-year prospective study that recruited both prevalent (177 cases) and incident (fifty-eight cases) patients by Szeto et al. ( Reference Szeto, Law and Wong 9 ) has found that peritoneal transport status had not been associated with longitudinal change of nutritional parameters assessed by serum ALB level and percentage of lean body mass. An earlier cross-sectional study (sample size: 147 CAPD patients) by Harty et al. ( Reference Harty, Boulton and Venning 7 ) has found no significant relationship between 4 h D:P cr and nutritional parameters. In two cross-sectional studies with 147 and 104 prevalent CAPD patients, respectively, Kang et al. ( Reference Kang, Yoon and Choi 8 ) and Zhang & Ren et al. ( Reference Zhang and Ren 10 ) found that the 4 h D:P cr is an independent factor affecting the overall nutritional status measured by composite nutritional index and SGA, respectively. In our prospective study, 283 incident patients on CAPD were observed and followed up for 1 year. It was found that higher peritoneal transporter was independently associated with worse nutritional status defined not only by comprehensive nutritional assessment tools such as SGA and PEW score but also by single nutritional parameters, including serum ALB level, serum pre-ALB and PA measured by bioelectrical impedance analysis, which was consistent with the results reported by Kang et al. ( Reference Kang, Yoon and Choi 8 ) and Zhang & Ren et al. ( Reference Zhang and Ren 10 ).
The mechanisms of patients with higher peritoneal transport had worse nutritional status were complicated. It was noticed that patients with higher peritoneal transport status had higher serum hsCRP levels and higher modified Charlson comorbidity index score, which indicated more systemic inflammation and comorbidity status. Pro-inflammatory cytokines could cause muscle wasting( Reference Mitch, Du and Bailey 20 ), and inflammation might suppress appetite and induce anorexia( Reference Han and Han 1 , Reference Mak, Cheung and Cone 21 ). In the present study, baseline higher transporters were more frequent with a decreased appetite than lower transporters both at the initiation of CAPD and follow-up period, which might indicate that a decreased appetite caused by inflammation was an important reason for the poor nutritional status at baseline. During the follow-up, patients with peritoneal higher transport had significantly higher estimated peritoneal glucose absorption from dialysate, and higher glucose absorption from dialysate might cause loss of appetite( Reference Chung, Carrero and Lindholm 22 , Reference Zheng, Sederholm and Anderstam 23 ). These patients also had higher incidence of peritonitis during the follow-up period. It was reported that patients with PD-related peritonitis were often with worse nutritional status, which might result from the combined effects of increased protein catabolism, increased peritoneal ALB losses and elevated acute-phase responses associated with peritoneal inflammation( Reference Han and Han 1 ). In another cross-sectional study, researchers also find out that both peritonitis and 4 h D:P cr were two independent risk factors for overall nutritional status( Reference Kang, Yoon and Choi 8 ).
Keto acid has been used as therapy recommendations in PD patients with low nutrient intakes to improve the nutritional status. In the present study, patients with serum ALB less than 38 g/l in both groups used keto acid for the treatment of malnutrition. Although nutritional statuses of the two groups were improved, the patients with higher peritoneal transport still had poorer nutritional status compared with that with lower peritoneal transport at the end of the study. After adjustment of keto acid supplementation and covariables such as age, sex, diabetes mellitus, RRF, Kt/V, peritonitis incidence and serum hsCRP, baseline higher peritoneal transport status was independently associated with poor nutritional status of patients at the 12 months of follow-up, which indicates that higher peritoneal transport status was an independent risk factor of malnutrition in CAPD patients.
Limitations
The present study has several noteworthy limitations. First of all, it was a single-centre study; second, we did not measure mid-arm muscle circumference in PEW assessment, but just used mBIA on measurement of muscle wasting, which probably resulted in a lower incidence of PEW than using the International Society of Renal Nutrition and Metabolism criteria. Third, the supplementation of keto acids may affect the study parameters. And yet keto acid supplementation had been adjusted both in defined binary logistic regression models and in multiple linear regressions. Finally, the study had a short follow-up duration, and longer study period is needed to observe further changes of nutritional status affected by baseline peritoneal transport status.
Conclusion
The present study demonstrated that higher transporters had higher rate of malnutrition than that of lower transporters, although nutritional status of the two groups were improved during the 12 months of CAPD. Baseline higher peritoneal transport status was an independent factor associated with worse nutritional status of CAPD patients. However, longer follow-up is needed to evaluate the long-term effect of baseline higher peritoneal transport status on malnutrition of CAPD patients.
Acknowledgements
We are indebted to all nephrologists and nurses in our PD centre for their excellent management of PD patients. We also thank the patients, PhD candidates and staffs involved in the prospective cohort study.
This study was supported by the Key Clinical Program of the Ministry of Health, China (2010-439), U.S. Baxter's Renal Discoveries Extramural Grant Program (EGP GRANT #09AP012-OG), the National Key Technology R&D Program (no. 2011BAI10B08), the National Basic Research Program of China (grant no. 2011CB504000) and the Guangzhou Committee of Science and Technology, China (grant no. 2010U1-E00831).
The authors' contributions are as follows: Y. L. contributed to the study design, data collection and drafting of the manuscript; R. H. conducted the mBIA test and SGA on patients; Q. G. and Q. Y. involved in the analysis and interpretation of the data; C. Y. and J. L. followed up the patients; X. Y. and X. Y. involved in the study design, revision of the manuscript critically for important intellectual content and final approval of the manuscript submitted.
None of the authors has any conflict of interest to declare.







