INTRODUCTION
Deforestation and forest degradation, particularly in tropical regions, are significant contributors to two of the most pressing global environmental challenges, namely biodiversity loss and climate change (Strassburg et al. Reference Strassburg, Kelly, Balmford, Davies, Gibbs, Lovett, Miles, David, Orme, Turner and Rodrigues2009; Talbot Reference Talbot2010). Tropical forests are the most species diverse terrestrial ecosystems (Parmentier et al. Reference Parmentier, Malhi, Senterre, Whittaker, Alonso, Balinga, Bakayoko, Bongers, Chatelain, Comiskey, Cortay, Djuikouo Kamdem, Doucet, Gautier, Hawthorne, Issembe, Kouamé, Kouka, Leal, Lejoly, Lewis, Nusbaumer, Parren, Peh, Philips, Sheil, Sonké, Sosef, Sunderland, Stropp, Steege, Swaine, Tchouto, Van Gemerden, Van Valkenburn and Wöll2007); the majority of the 34 global biodiversity hotspots identified worldwide occur within tropical forests (Mittermeier et al. Reference Mittermeier, Robles-Gil, Hoffman, Pilgrim, Brooks, Mittermeier and Da Fonseca2004). Tropical forests are also some of the most important natural sinks of carbon, accounting for as much as 40% of the carbon stored as terrestrial biomass worldwide, and thus playing a fundamental role in the global carbon cycle (Philips et al. Reference Phillips, Malhi, Higuchi, Laurance, Nunez, Vasquez, Laurance, Ferreira, Stern, Brown and Grace1998; Pan et al. Reference Pan, Birdsey, Fang, Houghton, Kauppi, Kurz, Philips, Schivdenko, Lewis, Canadell, Ciasis, Jackson, Pacala, McGuire, Piao, Rautiainen, Sitch and Hayes2011).
The spatial distribution of biomass and biodiversity is influenced by a range of environmental factors such as climate, soil and disturbance (Talbot Reference Talbot2010; Thompson et al. Reference Thompson, Garnder, Guariguata, Koh, Okabe, Pan, Schmitt, Tylianakis, Barlow, Kapos, Kurz, Parrotta, Spalding, van Vliet, Parrotta, Wildburger and Mansourian2012). Experimental data and direct observation of ecosystems have revealed positive relationships between species diversity, biomass and productivity (Tilman et al. Reference Tilman, Lehman and Thomson1997; Erskine et al. Reference Erskine, Lamb and Bristow2006; Cardinale et al. Reference Cardinale, Wright, Cadotte, Carroll, Hector, Srivastava, Loreau and Weis2007; Midgley et al. Reference Midgley, Bond, Kapos, Ravilious, Scharlemann and Woodward2010). However, it is difficult to examine these relationships in complex ecosystems such as tropical forests, and there is still considerable debate regarding the extent of the relationship between biomass and diversity within forest ecosystems (Szwagrzyk & Gazda Reference Szwagrzyk and Gazda2007; Talbot Reference Talbot2010; Thompson et al. Reference Thompson, Garnder, Guariguata, Koh, Okabe, Pan, Schmitt, Tylianakis, Barlow, Kapos, Kurz, Parrotta, Spalding, van Vliet, Parrotta, Wildburger and Mansourian2012).
Tropical forests are predominantly located in developing countries and are often subject to activities such as logging and conversion to agriculture (Lewis Reference Lewis2006). Africa has some of the highest rates of net forest loss worldwide (FAO [Food and Agriculture Organization of the United Nations] 2010) with Western and Central Africa estimated to having the highest global rates of primary forest loss over the last 20 years (FAO 2010). The fragmentation of tropical forests may be the single greatest threat to global biodiversity (Hill & Curran Reference Hill and Curran2003). Deforestation and degradation of tropical forests are also significant contributors to climate change and within many tropical countries are the largest source of carbon emissions (Gibbs et al. Reference Gibbs, Brown, Niles and Foley2007; Pan et al. Reference Pan, Birdsey, Fang, Houghton, Kauppi, Kurz, Philips, Schivdenko, Lewis, Canadell, Ciasis, Jackson, Pacala, McGuire, Piao, Rautiainen, Sitch and Hayes2011). Saatchi et al. Reference Saatchi, Harris, Brown, Lefsky, Mitchard, Salas, Zutta, Buermann, Lewis, Hagen, Petrova, White, Silman and Morel(2011) estimated that deforestation and forest degradation, located mainly in the tropics, contributed 12–20% of global greenhouse gas emissions over the last 20 years.
Biodiversity and its relationship with the carbon cycle has become an important consideration in international efforts to mitigate the loss of climate change, through reducing the conversion of natural ecosystems (Midgley et al. Reference Midgley, Bond, Kapos, Ravilious, Scharlemann and Woodward2010). The United Nations programme for Reducing Emissions from Deforestation and Forest Degradation (UN–REDD) is focused on maintaining carbon storage within tropical forests in developing countries (Gibbs et al. Reference Gibbs, Brown, Niles and Foley2007). In order for REDD to be effectively implemented, accurate estimates of forest carbon stocks are required (Miles & Dickson Reference Miles and Dickson2010; Maniatis et al. Reference Maniatis, Malhi, Saint Andre, Mollicone, Barbier, Saatchi, Henry, Tellier, Schwartzenberg and White2011). Above-ground biomass (AGB) estimates provide information on the location of sources and sinks of carbon, and allow the quantification of the amount of carbon lost from sinks through deforestation and degradation (Houghton Reference Houghton2005). Recent studies, using estimates of AGB, have indicated that tropical forests are likely to act as a growing carbon sink and therefore may help buffer the increase in levels of atmospheric CO2 (Philips et al. Reference Phillips, Malhi, Higuchi, Laurance, Nunez, Vasquez, Laurance, Ferreira, Stern, Brown and Grace1998; Baker et al. 2004a; Lewis et al. Reference Lewis, Lopez-Gonzalez, Sonké, Affum-Baffoe, Baker, Ojo, Philips, Reitsma, White, Comiskey, Djuikouo, Ewango, Feldpausch, Hamilton, Gloor, Hart, Hladik, Lloyd, Lovett, Makana, Malhi, Mbago, Ndangalasi, Peacock, Peh, Sheil, Sunderland, Swaine, Taplin, Taylor, Thomas, Votere and Wöll2009; Pan et al. Reference Pan, Birdsey, Fang, Houghton, Kauppi, Kurz, Philips, Schivdenko, Lewis, Canadell, Ciasis, Jackson, Pacala, McGuire, Piao, Rautiainen, Sitch and Hayes2011).
The REDD scheme also has the potential to provide significant benefits to biodiversity conservation, through the protection of species diverse forests (Harvey et al. Reference Harvey, Dickson and Kormos2009; Gardner et al. Reference Gardner, Burgess, Aquilar-Amuchastegui, Barlow, Berenguer, Clements, Danielsen, Ferreira, Foden, Kapos, Khan, Lees, Parry, Roman-Cuesta, Schmitt, Strange, Theilade and Vieira2012). REDD funding for carbon sequestration is likely to be significantly greater than that currently available for biodiversity conservation within tropical regions. The areas of forest that could be protected through REDD schemes may also be significantly larger than the area currently receiving protection for the purposes of conservation (Harvey et al. Reference Harvey, Dickson and Kormos2009). The need for REDD to address biodiversity conservation was recognized during negotiations in the ad hoc Working Group on Long Term Cooperative Action (AWG-LCA), when the scope of REDD schemes was broadened to include incentives for a wide array of forest management practices including conservation (Blom et al. Reference Blom, Sunderland and Murdiyarso2010). However, an understanding of the relationship between species diversity and carbon stocks is required if REDD schemes are going to fulfil their potential for biodiversity conservation (Midgley et al. Reference Midgley, Bond, Kapos, Ravilious, Scharlemann and Woodward2010; Strassburg et al. 2010). Direct relationships between biodiversity and the carbon cycle in mature tropical forests have not yet been extensively studied (Talbot Reference Talbot2010). A greater understanding of the congruence between biodiversity and carbon pools is therefore necessary on a local and regional scale in order to inform policy considerations (Midgley et al. Reference Midgley, Bond, Kapos, Ravilious, Scharlemann and Woodward2010; Miles & Dickson Reference Miles and Dickson2010; Gardner et al. Reference Gardner, Burgess, Aquilar-Amuchastegui, Barlow, Berenguer, Clements, Danielsen, Ferreira, Foden, Kapos, Khan, Lees, Parry, Roman-Cuesta, Schmitt, Strange, Theilade and Vieira2012).
The rainforests of West and Central Africa are the second largest block of rainforest in the world (Baccini et al. Reference Baccini, Laporte, Goetz, Sun and Huang2008) and contain the highest levels of biomass per hectare (c. 250 t ha−1) worldwide (Lewis et al. Reference Lewis, Lopez-Gonzalez, Sonké, Affum-Baffoe, Baker, Ojo, Philips, Reitsma, White, Comiskey, Djuikouo, Ewango, Feldpausch, Hamilton, Gloor, Hart, Hladik, Lloyd, Lovett, Makana, Malhi, Mbago, Ndangalasi, Peacock, Peh, Sheil, Sunderland, Swaine, Taplin, Taylor, Thomas, Votere and Wöll2009; FAO 2010). Tree diameter and height data from forest plots can be used to estimate carbon stocks through the calculation of AGB (Kettering et al. Reference Ketterings, Coe, van Noordwijk, Ambagau and Palm2001; Chave et al. Reference Chave, Andalo, Brown, Cairns, Chamber, Eamus, Fölster, Fromard, Higuchi, Kira, Lescure, Nelson, Ogawa, Puig, Ríera and Yamakura2005). Forests plots can also provide information regarding tree species diversity and forest structure. This study uses data collected from an established network of 1-ha permanent plots located within mature tropical forests in five central African countries. The aim was to explore the relationship between tree species diversity and biomass using this network of 1-ha survey plots.
METHODS
Study sites
Seven study sites in four countries within Central Africa were used for the purposes of this analysis (Table 1; Appendix 1, Table S1, see supplementary material at Journals.cambridge.org/ENC). The sites mainly comprised moist semi-deciduous mixed forest, although a range of forest types, including montane forest and monodominant Gilbetiodendron dewevrei forest (one plot, CON-01), were studied. Average rainfall across the sites was 1400–3500 mm yr−1. Mean annual temperature was 20–27 °C.
Table 1 Locations, area, number of plots, survey years and main forest types present for the sites used in the study. Forest types based on Letouzey Reference Letouzey(1985) and Kindt et al. Reference Kindt, Osino, Orwa, Nzisa, van Breugel, Graudal, Lillesø, Kehlenbeck, Dietz, Nyabenge, Jamnadass and Neufeld(2011).

Six sites (Campo Ma’an, Takamanda, Monts De Cristal, Nouabalé Ndoki, Waka and Monte Mitra) were located within existing or recently established national parks (NP). The remaining site in Cameroon is located in a former forest reserve (Ejagham). The Nouabalé Ndoki and Waka sites contained plots that had experienced varying levels of selective logging in the past.
Data collection
A total of 33 1-ha survey plots were established across the study sites (Table 1). Plots were established following the standardized methodology of Dallmeier Reference Dallmeier(1992). Within each plot all individual trees and tree stems with a diameter at breast height (DBH) ≥10 cm were identified and measured. The total height of each individual tree/stem was also estimated using a clinometer. Voucher specimens were collected during plot measurement for identification at national/regional centres of botanical expertise, notably the Herbier National du Gabon (Libreville) and the Herbier National (Yaoundé, Cameroon).
Data analysis
Diversity
Species diversity for each plot was calculated using the Shannon Wiener (SW) index:
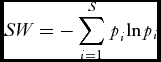 \begin{equation}
SW = - \sum\limits_{i = 1}^S {p_i^{} {\rm ln}p_i }
\end{equation}
\begin{equation}
SW = - \sum\limits_{i = 1}^S {p_i^{} {\rm ln}p_i }
\end{equation}
The diversity indices were then converted into the ‘effective number of species’ for each plot by calculating exp(SW) (Jost Reference Jost2006). The effective number of species is the number of equally-common species required to give a particular value of the index (Jost Reference Jost2006). In order to prevent bias due to sample size, rarefaction was applied to all samples prior to the calculation of SW, 250 species were randomly selected from each plot and the Shannon Wiener values and the species richness were calculated from these random samples. The Shannon equitability index (ESW ) was also calculated for each plot:
Above-ground biomass
AGB of stems with a DBH >10 cm was estimated using the moist forest equation of Chave et al. Reference Chave, Andalo, Brown, Cairns, Chamber, Eamus, Fölster, Fromard, Higuchi, Kira, Lescure, Nelson, Ogawa, Puig, Ríera and Yamakura(2005), which uses the largest available dataset of 2410 trees harvested in 27 sites across the tropics. The Chave equation has been found to be accurate by several site-specific studies that have developed allometric equations for calculating biomass within Africa, and its use has been recommended for regional scale studies in Africa (Djomo et al. Reference Djomo, Ibrahima, Saborowski and Gravenhorst2010; Henry et al. Reference Henry, Besnard, Asante, Eshun, Adu-Bredu, Valentini, Bernoux and Saint-Andre2010; Vieilledent et al. Reference Vieilledent, Vaudry, Andriamanohisoa, Rakotonarivo, Randrianasolo, Razafindrabe, Rakotoarivony, Ebeling and Rasamoelina2012).
Biomass of stems < 10 cm, litter and climbers were calculated at 3%, 5% and 3%, respectively, of the total biomass of stems ≥10 cm (Brown Reference Brown1997; IPCC [Intergovernmental Panel for Climate Change] Reference Eggleston, Buendia, Miwa, Ngara and Tanabe2006). We did not estimate biomass within dead wood in the study plots. We used a carbon conversion rate of 0.47 tonne of carbon per tonne of dry biomass (derived from IPCC2006).
We compiled data on wood mass density of species identified within the plots predominantly from the Global Wood Density Database (Zanne et al. Reference Zanne, Lopez-Gonzalez, Coomes, Ilic, Jansen, Lewis, Miller, Swenson, Wiemann and Chave2009). Additional species densities were obtained from the wood density database (see http://worldagroforestry.org/sea/Products/AFDbases/WD/Index.htm). We calculated densities from wood with 12–15% moisture content using (Chave et al. Reference Chave, Coomes, Jansem, Lewis, Swenson and Zanne2009):
Species used to calculate the diversity indexes and biomass estimates were checked for orthography and synonymy using the African Flowering Plants and Tropicos databases (Lewis et al. Reference Lewis, Lopez-Gonzalez, Sonké, Affum-Baffoe, Baker, Ojo, Philips, Reitsma, White, Comiskey, Djuikouo, Ewango, Feldpausch, Hamilton, Gloor, Hart, Hladik, Lloyd, Lovett, Makana, Malhi, Mbago, Ndangalasi, Peacock, Peh, Sheil, Sunderland, Swaine, Taplin, Taylor, Thomas, Votere and Wöll2009). Species-specific wood density was used where possible (33% of stems). If a species-specific wood density was not available, we used the mean value for the genus (46% of stems) or the family (16% of stems). For unidentified stems, or where wood density information was not available for the species, genus or family, we used the overall mean wood density obtained from the database of species compiled for this study (5% of stems) (Baker et al. 2004b).
Species diversity, richness and above-ground biomass
Partial mantel tests considering the geographic distances between plots were used to explore the relationship between species diversity and AGB, between AGB and species similarity and between species richness and AGB using the Vegan package in R. The regression residuals were checked for spatial autocorrelation using PASSaGE 2 (Rosenberg & Anderson Reference Rosenberg and Anderson2011). The Bray-Curtis index was used to determine the level of species dissimilarity between the plots.
We used an independent sample t-test (t) to compare Shannon Wiener index and species richness data in the 16 plots within the lower and upper quartiles of the AGB estimates. We condicted preliminary analyses to ensure a normal distribution and equal variances.
We used principal components analysis (PCA), using Fitopac 2.1 software, to determine any associations between AGB, species diversity, species richness, evenness (Shannon's equitability EH ) and tree density.
RESULTS
Species diversity
A total of 671 species were recorded across all of the survey plots; we identified 46% to generic level only, while 19% were identified to family level only or could not be authoritatively identified.
The Shannon Wiener index ranged from 0.44 to 4.03, or 1.6 to 56.5 for the effective number of species. The mean effective number of species across all plots was 38.56 (SD = 10.62). The lowest diversity was found within the plot located in monodominant Gilbertiodendron dewevrei forest in Nouabale-Ndoki (Congo). Plot EQ-03 within the Monte Mitra Forest in Equatorial Guinea had the highest species diversity (Table 2).
Table 2 Species diversity and biomass estimates per plot, including equivalent carbon content. Mean DBH and mean height for stems within each plot is also shown.

Above-ground biomass
Total biomass varied considerably between plots, ranging from 102.25 to 619.54 Mg ha−1 (Table 2). Mean biomass for all sites was 350.25 Mg ha−1 (SD = 125.29).
The mean wood density of stems was 0.62 g cm−3 (SD = 0.13). Stem density ranged from 0.211 to 0.981 g cm−3; approximately 85% of stems had a wood density between 0.5 and 0.8 g cm−3.
Mean DBH and height of stems in the study was 24.38 cm (SD = 2.47) and 15.01 m (SD = 2.75), respectively (Table 2). Most biomass was found within tall trees, trees > 15 m in height accounting for approximately 90% of the biomass across all of the plots (Fig. 1). The greatest biomass was found in trees with a larger DBH, although this trend was less pronounced than height. Trees with a DBH > 50 cm contained approximately 65% of the biomass across all plots (Fig. 1).

Figure 1 Total above-ground biomass levels for different (a) height and (b) DBH classes across all 33 survey plots (midpoint of each height and DBH class is shown).
Species diversity, richness and above-ground biomass
We removed the monodominant Gilbertiodendron dewevrei forest from all analyses, as it was a clear outlier in terms of species diversity (Table 2). We found no significant spatial autocorrelation between AGB and species diversity (Moran's I = –0.0313, p = 0.082). The Bray Curtis distances ranged from 0.3 to 0.98, and indicated that the study sites had well defined species compositions. The partial Mantel tests indicated weak positive correlations between AGB and the Shannon Wiener index (r = 0.21, p = 0.03) and between AGB and the species similarity index (r = 0.18, p = 0.013; Fig. 2). There was no significant relationship between AGB and species richness (r = 0.12, p = 0.17).

Figure 2 Plot of effective number of species calculated from the Shannon Wiener index against above-ground biomass estimates across 32 survey plots. Linear regression line is shown (y = 0.0323x + 28.363). Plot CON-01, located within a monodominant forest type, is excluded.
The eight plots within the upper quartile of AGB estimates had a significantly higher mean effective number of species (M = 44.38, SD = 5.55), compared to the eight plots within the lower quartile of AGB estimates (M = 35.22, SD = 10.01; t = 2.26; df = 14; p = 0.04), but did not exhibit significantly higher mean species richness (M = 74, SD = 6.37) than the plots within the lower quartile of AGB estimates (M = 64.63, SD = 11.13; t = 2.07; df = 14; p = 0.057).
The first two axes of the PCA accounted for almost 90% of data variance (Tables 3 and 4). All the descriptors (aside from tree density) were positively correlated with principal component 1 (PC1), which explained approximately 60% of the variance of the dataset. The diversity measures effective number of species, equitability and species richness displayed the highest correlations with PC1. AGB was also correlated with axis 1 and therefore those diversity measures. Principal component 2 (PC2) accounted for almost 30% of the variance between the plots, with all variables apart from the equitability index showing positive correlations with this axis. Tree density and biomass showed higher correlations with this axis, which had no real relation to the diversity measures.
Table 3 Eigenvalues and percentage of variance explained by different principal components.

Table 4 Correlation of descriptors along the first two principal component axes

DISCUSSION
Our results show a complex and highly variable relationship between biomass and species diversity within Central African rainforests. Some plots with high diversity had relatively low biomass, and some plots with low diversity had high biomass. Despite this variability, in our survey plots there was evidence of positive correlations between biomass and species diversity. The top 12 plots in terms of AGB estimates included seven plots with the highest species diversity and eight plots with the highest species richness. Clearly, a number of variables simultaneously influence the distribution of biodiverse and carbon-rich forests, and it should be emphasized that our survey plots encompassed different forest types, management designations, altitudinal and climate conditions. Historical forest disturbance is likely to have a significant impact on standing biomass and tree species diversity, and some of our survey plots have been previously impacted by differing degrees of selective logging.
The biomass estimates presented here are likely to be conservative, as we used the lower range limits for estimating the biomass content of stems with DBH <10 cm, litter and climbers. We also did not include estimates of biomass within dead wood or below-ground biomass. The biomass of roots in particular can vary considerably and be a significant component of total biomass (Brown Reference Brown1997). However, underestimation of these parameters is expected to have impacted all study plots equally. The biomass estimates we produced are within the range found for other studies within Central Africa (Lewis et al. Reference Lewis, Lopez-Gonzalez, Sonké, Affum-Baffoe, Baker, Ojo, Philips, Reitsma, White, Comiskey, Djuikouo, Ewango, Feldpausch, Hamilton, Gloor, Hart, Hladik, Lloyd, Lovett, Makana, Malhi, Mbago, Ndangalasi, Peacock, Peh, Sheil, Sunderland, Swaine, Taplin, Taylor, Thomas, Votere and Wöll2009; Djuikouo et al. Reference Djuikouo, Doucet, Charlemagne, Lewis and Sonké2010; Maniatis et al. Reference Maniatis, Malhi, Saint Andre, Mollicone, Barbier, Saatchi, Henry, Tellier, Schwartzenberg and White2011). The mean species wood density from this study (0.62 g cm−3) was similar to the 0.60 g cm−3 reported by Djuikouo et al. Reference Djuikouo, Doucet, Charlemagne, Lewis and Sonké(2010) from the Dja Biosphere Reserve (Cameroon) and Henry et al. Reference Henry, Besnard, Asante, Eshun, Adu-Bredu, Valentini, Bernoux and Saint-Andre(2010) from Boi Tano (Ghana).
A positive relationship between biomass and species diversity has important policy implications, as it would support the assertion that REDD schemes can provide significant co-benefits for biodiversity conservation. The present biomass estimates demonstrate the high variability of carbon storage within different forest areas. The forest plot with the highest AGB estimate contained more than six times the biomass of the plot with the lowest estimate. There are therefore instances where forests high in species diversity are not carbon rich, and vice versa. In these cases, there may be trade-offs between carbon and biodiversity conservation. In addition, our study only examined tree species diversity, which may not show great congruence with the diversity of other taxa (Lawton et al. Reference Lawton, Bignell, Bolton, Bloemers, Eggleton, Hammond, Hodda, Holt, Larsen, Mawdsley, Stork, Srivastava and Watt1998; Heino et al. Reference Heino, Kimmo, Tolonen, Kotanen and Paasivirta2009). Tchouto et al. Reference Tchouto, De Boer, De Wilde and Van Der Maesen(2006) found that diversity of tree species did not always reflect the diversity of shrub and herbaceous species in the Campo-Ma’an Forests in Cameroon, although tree diversity could be used to predict the diversity of other floral layers to some extent. High diversity of tree species may not mean high diversity of other species groups, or of particularly rare or threatened species. Different taxa also respond differently to forest disturbance and degradation (Lawton et al. Reference Lawton, Bignell, Bolton, Bloemers, Eggleton, Hammond, Hodda, Holt, Larsen, Mawdsley, Stork, Srivastava and Watt1998; Schulze et al. Reference Schulze, Waltert, Kessler, Pitopang, Shahabuddin, Veddeler, Mühlenberg, Gradstein, Leuschner, Steffan-Dewenter and Tscharntke2004).
Despite complexities in biomass and biodiversity relationships, REDD schemes should still be able to provide significant co-benefits between carbon and biodiversity conservation. In order to maximize the benefits to biodiversity conservation, where possible, REDD funding should prioritize mature forests, as they are key to maintaining biodiversity in the tropics (Gibson et al. Reference Gibson, Lee, Koh, Brook, Gardner, Barlow, Peres, Bradshaw, Laurance, Lovejoy and Sodhi2011). The conservation of large intact primary forests is also key to reducing greenhouse gas emissions, as natural ecosystems are generally more carbon dense as well as biologically diverse (Thornley & Cannell, Reference Thornley and Cannell2000; SCBD [Secretariat Convention on Biological Diversity] 2009). Mature forests are likely to have a greater incidence of large trees. Trees with large diameters (> 10 cm DBH) often make the largest contribution to AGB (Kirby & Potvin Reference Kirby and Potvin2007; Baishya et al. Reference Baishya, Barik and Upadhaya2009; Djuikouo et al. Reference Djuikouo, Doucet, Charlemagne, Lewis and Sonké2010). The majority of AGB in our study was found within taller trees and trees with a larger diameter. As well as holding the largest stocks of carbon within biomass, mature trees are often most impacted by forest degradation and deforestation (Gibbs et al. Reference Gibbs, Brown, Niles and Foley2007). Mature and more biodiverse forests are also likely to provide greater ecosystem resilience, providing a better guarantee of the long-term persistence of forests and therefore the carbon pool, which is to be protected (Thompson et al. Reference Thompson, Mackey, McNulty and Mosseler2009).
Several authors have stressed that REDD schemes may also carry inherent risks to biodiversity conservation (Strassburg et al. Reference Strassburg, Kelly, Balmford, Davies, Gibbs, Lovett, Miles, David, Orme, Turner and Rodrigues2009; Gardner et al. Reference Gardner, Burgess, Aquilar-Amuchastegui, Barlow, Berenguer, Clements, Danielsen, Ferreira, Foden, Kapos, Khan, Lees, Parry, Roman-Cuesta, Schmitt, Strange, Theilade and Vieira2012). Biodiversity threats from REDD could include leakage or displacement of deforestation and forest degradation activities to other species diverse forest areas or ecosystems (SCBD 2011). Another potential risk is carbon stock enhancement leading to plantation forestry and a subsequent loss of biodiversity at plantation sites (Miles & Dickson Reference Miles and Dickson2010). However, if properly managed, carbon stock enhancement can also provide additional benefits to biodiversity through forest restoration and afforestation (SCBD 2011). Gardner et al. Reference Gardner, Burgess, Aquilar-Amuchastegui, Barlow, Berenguer, Clements, Danielsen, Ferreira, Foden, Kapos, Khan, Lees, Parry, Roman-Cuesta, Schmitt, Strange, Theilade and Vieira(2012) suggested a framework for integrating biodiversity conservation into REDD schemes and argued that this was essential if biodiversity considerations were to be workable within REDD schemes. Including biodiversity considerations at planning stages is also necessary to ensure risks to biodiversity are avoided and any trade-offs between biodiversity conservation and REDD activities are properly managed and negotiated.
It is important to recognize that less species-diverse forests, such as the monodominant forest type observed in this study, may also provide significant stores of carbon (Djuikouo et al. Reference Djuikouo, Doucet, Charlemagne, Lewis and Sonké2010). Safeguards should be put in place to ensure that biodiverse, but carbon-poor, habitats do not suffer from a lack of funding or land conversion due to an emphasis on conservation of high carbon ecosystems (Midgley et al. Reference Midgley, Bond, Kapos, Ravilious, Scharlemann and Woodward2010). If REDD schemes are successful in conserving forests, the best use of limited conservation funds may be to protect low-carbon and non-forest ecosystems from land-use change, including change that may be caused by the displacement of activities from forested areas (Miles & Kapos Reference Miles and Kapos2008). There are a number of knowledge gaps in terms of carbon and biodiversity linkages in forests ecosystems; for example the impact of species composition on ecosystem function is not well known. Further work on the relationship between plant species richness, functional diversity and biomass accumulation in diverse forest ecosystems is required (Thompson et al. Reference Thompson, Garnder, Guariguata, Koh, Okabe, Pan, Schmitt, Tylianakis, Barlow, Kapos, Kurz, Parrotta, Spalding, van Vliet, Parrotta, Wildburger and Mansourian2012). The significant variability in the relationship between carbon and biodiversity in our study plots supports this conclusion.
CONCLUSION
Our results demonstrate that high variability exists between standing biomass and tree species diversity in different forest areas in Central Africa. Despite this variability, on average, forests with a greater species diversity also tend to be more likely to have a higher biomass content, and therefore greater carbon storage. This suggests that REDD policies should be prioritized towards species-diverse forests. Biodiversity also plays an important role in the long-term stability and resilience of tropical forests. As such, biodiverse forests provide a better long-term guarantee of persistence (if protected from conversion), than less diverse forests. However, we found that the relationship between biomass and diversity was highly variable in our survey plots; more research is required into the causes of this variation and evidence of the relationship between diversity and carbon in additional locations, at different spatial scales and on a range of taxa. Only then will it be possible to ascertain whether biodiversity is important for carbon storage.
ACKNOWLEDGEMENTS
The fieldwork for this paper was funded by two grants to the Smithsonian Institutions’ Monitoring and Assessment of Biodiversity Programme (MAB) from the International Conservation of Biodiversity Group (ICBG) and United States Agency for International Development (USAID's) Central Africa Regional Programme for the Environment (CARPE). Both grants were administered by TS as an Honorary Research Associate of the SI/MAB. Special thanks are extended to Francisco Dallmeier, Alfonso Alonso and James Comiskey of SI/MAB, and also to the many field assistants and scientists who helped with data collection and analysis, notably Michael Balinga, Yves Issembe, Gretchen Walters, Miguel Leal, Thomas Nzabe, Philip Nkeng, Betafor Maurice, Zach Nitua, Ndumbe Paul, Anacletus Koufani, Augstin Njiamnshi, Dinga Njingum, Mvogo Guillaume Akogo, Moise Makaki, Salomon Minty Ang, Andre Minsta Ella, Bienvenue Minkoa, Francois Jounde, John Njie, Raphael Ebot, Clement Fultang, Michael Otang, Anne Henderson, Adele Rowden, Lee White, Brian Curren, Emma Stokes, Mark Gately, Phillippe Auzel and Christopher Kernan. We also thank USAID for funding CIFOR biodiversity-related research, through which the analysis for this paper was undertaken.








