There is mounting evidence from both cohort studies and clinical trials that an increased intake of plant foods, specifically those rich in flavonoids, can reduce the risk of CVD(Reference Williamson1). Quercetin (in its glycosylated form) is the most commonly consumed flavonoid compound within the flavonol subclass(Reference Bondonno, Lewis and Blekkenhorst2). The main dietary contributors to quercetin intake include tea, apples, onions and broccoli(Reference Neveu, Perez-Jiménez and Vos3).
Evidence for the health promoting effects of quercetin comes primarily from animal and in vitro studies(Reference Bondonno, Bondonno and Hodgson4). In a randomised-controlled crossover study of men and women, apples with skin significantly reduced systolic blood pressure (BP), improved endothelial function and increased plasma nitric oxide (NO)(Reference Bondonno, Yang and Croft5). These apples with skin provided 184 mg of quercetin and 180 mg of (−)-epicatechin, while the apple flesh only (control) provided less than 5 mg of quercetin and (−)-epicatechin. Previous studies using both pure quercetin aglycone (1095 mg)(Reference Larson, Witman and Guo6) and quercetin glucoside supplementation (50–400 mg)(Reference Bondonno, Bondonno and Rich7,Reference Dower, Geleijnse and Gijsbers8) have shown no effect on endothelial function. This may be due in part to the bioavailability of the quercetin interventions.
Quercetin in foods is usually found in its glycosylated form, with the presence and position of the sugar moiety influencing the site and extent to which it is absorbed(Reference Hollman9). The bioavailability of quercetin glucosides can be enhanced by enzymatic α-oligoglucosylation of this sugar moiety(Reference Murota, Matsuda and Kashino10). Enzymatically modified isoquercitrin (EMIQ®) is a water-soluble glucoside of quercetin produced by the de-glycosylation and subsequent α-oligoglucosylation of rutin(Reference Murota, Matsuda and Kashino10). Plasma concentrations of quercetin metabolites have been shown to be approximately two to three times higher after the consumption of EMIQ® in comparison with isoquercitrin (quercetin-3-O-glucoside), and its bioavailability is 17-fold higher than that of quercetin(Reference Murota, Matsuda and Kashino10,Reference Makino, Shimizu and Kanemaru11) .
EMIQ® has been shown to suppress the increase in systolic BP observed in spontaneously hypertensive rats(Reference Emura, Yokomizo and Toyoshi12) and decrease oxidative stress in a mouse model of atherosclerosis(Reference Motoyama, Koyama and Moriwaki13). EMIQ® is approved in Japan as a food additive and is safe for human consumption. Human trials carried out over a 12-week period indicate a significant reduction in body fat(Reference Yoshimura, Maeda and Takehara14). The effects of EMIQ® on vascular and cognitive function are yet to be investigated.
Therefore, the aim of the present study was to determine whether acute ingestion of EMIQ® improves endothelial function, BP and cognitive function in human volunteers at risk of CVD.
Subjects and methods
Participants
Twenty-five eligible men and women, aged between 50 and 70 years, were recruited by newspaper advertisement from the general population of Perth, Western Australia (Fig. 1). These volunteers attended a screening appointment within the University of Western Australia, School of Biomedical Sciences, located in the Medical Research Foundation building at Royal Perth Hospital. The screening appointment consisted of an electrocardiograph, a standard medical history questionnaire and the measurement of height, weight, BMI and BP. Finally, a blood sample was taken for measurement of fasting serum total cholesterol, HDL-cholesterol, TAG, LDL-cholesterol and glucose. Volunteers were included in the study if they had at least one of the following risk factors for CVD: systolic BP between 120 and 160 mmHg, fasting plasma glucose between 5·6 and 6·5 mm, total cholesterol between 5 and 8 mm or a waist circumference >94 cm for men or >80 cm for women. Exclusion criteria included a BMI <18 or >35 kg/m2, a systolic BP ≤ 100 or ≥160 mmHg, a diastolic BP ≤50 or ≥90 mmHg, diagnosed diabetes, fasting plasma glucose concentrations ≥6·5 mmol/l, use of BP-lowering or cholesterol-lowering medication, alcohol intake >210 g per week for women and >280 g per week for men, current or recent (within previous 6 months) significant weight loss or gain (>6 % of body weight), actively trying to lose weight, current or recent (<12 months) smoking, history of cardiovascular or peripheral vascular disease, psychiatric or any other major illnesses and women who were lactating, pregnant or wishing to become pregnant during the study.
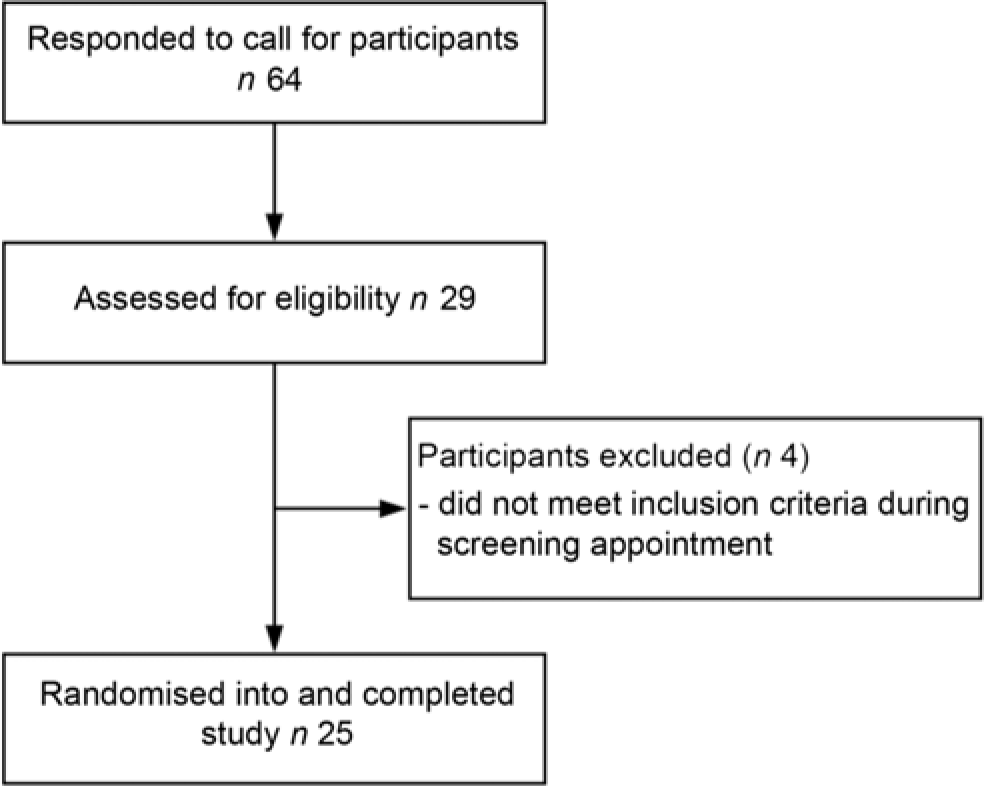
Fig.1. Participant flow diagram.
The study was carried out in accordance with the Declaration of Helsinki and was approved by the University of Western Australia Human Research Ethics Committee (Approval number RA/4/1/9260). All participants provided written informed consent before inclusion in the study. The trial was registered with the Australian New Zealand Clinical Trials Registry (ACTRN12617001202358).
Study design
This acute, randomised, controlled crossover trial was conducted between September 2017 and June 2018. Participants were required to visit the department three times (one practice visit and two study visits). The practice visit involved the completion of a selection of cognitive function tests designed to induce cognitive stress (see description below) three times, in order to control for practice effects and to allow familiarisation with procedure. The practice day data were not included in any analyses. Participants were required to fast for 12 h (water allowed ad libitum) prior to each subsequent study visit. These two visits were separated by at least 7 d to ensure that there were no carryover treatment effects. Participants were instructed to have the same meal the night before, not to consume any alcohol for 24 h prior to the visit, not to take any medication and not to do any exercise on the morning of their visit. Endothelial function was assessed before and 1·5 h post-intervention. A BP measurement was taken prior to and every 10 min for 80 min post-treatment. Measurements of arterial stiffness were taken 2 h post-intervention. Participants completed the selection of cognitive function tests 2·5 h post-intervention, during which BP was measured every 2 min. Finally, a blood sample was taken by venipuncture 3·5 h post-intervention.
Intervention
Participants were randomly assigned to one of two treatment orders generated using a pseudorandom number generator (https://www.randomizer.org/). The EMIQ® was prepared by San-Ei Gen F.F.I., Incorporated. The active treatment consisted of 4·89 mg EMIQ® (2 mg aglycone equivalent)/kg body weight(Reference Murota, Matsuda and Kashino10) plus ½ teaspoon of maltodextrin and 1½ tablespoons of Cottee’s Raspberry flavoured cordial mixed with 250 ml water. The placebo treatment was ½ teaspoon of maltodextrin and 1½ tablespoons of cordial mixed with 250 ml water. The cordial was used to mask the taste and the colour of the EMIQ®. Both treatments were given with a standardised breakfast of two pieces of white bread and cheese. Participants and all researchers performing the tests and analysing the results were blinded to the treatment.
Assessment of endothelial function
In order to assess endothelial function, flow-mediated dilatation (FMD) of the brachial artery was calculated by a trained ultrasonographer dedicated to the research protocol and blinded to the interventions used. Participants were studied in a quiet, temperature-controlled room (21–25°C). Participants rested in a supine position for 20 min prior to the initiation of the FMD measurement. The right arm was extended and supported comfortably on a foam mat. For the ultrasound, a 12-MHz transducer connected to a Philips CX50 Ultrasound Machine was clamped in position over the brachial artery, 5–10 cm proximal to the antecubital crease. After a baseline artery diameter recording of 1 min, a BP cuff placed around the left forearm was inflated to 200 mmHg. After 5 min, the cuff was released, inducing reactive hyperaemia. The brachial artery image was recorded for 4 min (240 s) post-cuff deflation to assess FMD. Images were downloaded for retrospective analysis. FMD analysis was performed using a semi-automated edge detection software (Vascular Research Tools; Medical Imaging Applications LLC), which automatically calculates the brachial artery diameter, corresponding to the internal diameter. Responses were calculated as the percentage change in brachial artery diameter from baseline, at 10-s intervals, for 240 s after cuff deflation.
Measurement of blood pressure and arterial stiffness
BP measurements were taken using a Dinamap 1846SX/P oscillometric recorder (Critikon) attached to the non-dominant arm with participants in a seated position. Following a 10-min rest, one BP measurement was taken immediately prior to treatment consumption. BP was then measured every 10 min post-treatment consumption for 80 min (nine measurements in total).
Office BP, central diastolic BP and arterial stiffness were assessed using the SphygmoCor Xcel (AtCor Medical). Following a 5-min rest in a supine position, BP measurements were taken on three occasions at 1 min intervals, the first measurement was discarded and the second and third measurements were averaged. Arterial stiffness was determined by measuring the augmentation index (AIx) using the Sphygmocor Xcel as described previously(Reference Hwang, Yoo and Kim15) and was standardised to a heart rate of 75 bpm (AIx75).
Cognitive stress test and blood pressure
A selection of cognitive function tests designed to induce cognitive stress using the computerised mental performance assessment System (BPNRC) was used. This series of tests, described in detail previously(Reference Scholey, French and Morris16), comprised two computerised serial subtraction tasks (Serial 3s and Serial 7s) and a Bakan rapid visual information processing (RVIP) task, each repeated three times. Briefly, for the Serial 3 and Serial 7 subtraction tasks, participants were asked to continuously subtract three or seven from a random starting number between 800 and 999. This task lasted for 2 min and both the number of correct responses and the total number of subtractions completed were recorded. For the RVIP task, participants were asked to identify when three odd or three even digits appeared consecutively in series of single digit numbers presented at a rate of 100 per min. This task lasted for 5 min and both the number of correct responses and the average response time were recorded. Participants rested for 20 min prior to and 15 min after the cognitive testing, which took approximately 30 min. For the duration of this task (65 min total), BP was measured every 2 min with a Dinamap 1846SX/P oscillometric recorder (Critikon) attached to the non-dominant arm of participants in a seated position.
Plasma analyses
Plasma samples, obtained 3·5 h post-intervention by venepuncture, were collected into EDTA tubes with added butylated hydroxytoluene and immediately centrifuged at 5000 g, at 4°C for 5 min. One 2 ml aliquot of plasma was kept on liquid N2 for immediate analysis of nitrogen oxides (NOx) and the remaining plasma was stored at −80°C until analysis.
Quercetin metabolites
Plasma quercetin metabolites were measured by liquid chromatography/tandem MS (LC-MS) as described previously(Reference Bondonno, Bondonno and Rich7). Briefly, plasma (250 µl) collected in EDTA was incubated for 2 h with β-glucuronidase enzyme. As β-glucuronidase has both glucuronidase and sulfatase activity but is unable to enzymatically cleave methyl conjugates, isorhamnetin (O-methylated quercetin) was measured. Following solid phase extraction, quercetin aglycone and isorhamnetin were measured on a Thermo Scientific TSQ Quantum Ultra Triple Quadrupole LCMS System (ThermoFisher Scientific). Calibration curves of quercetin and isorhamnetin with the fisetin as the internal standard were used for quantification.
Measurement of plasma total nitrogen oxides
The concentrations of total NOx (NO, nitros(yl)ated species and nitrite (NO2)) in plasma were determined using a previously described gas phase chemiluminescence assay(Reference Bondonno, Yang and Croft5). Blood was collected into N-ethylmaleimide (10 mm) and EDTA (2 mm), mixed and centrifuged at 3000 g (5 min, 4°C). Fresh plasma was kept on ice in the dark and analysed within 1 h. Antifoam (200 µl) was added prior to injection into the radical purger containing potassium iodide (0·125 g) and iodine (0·05 g) in water (2·5 ml)/glacial acetic acid (7·5 ml) at room temperature. Plasma NOx was quantified by the NO signal peak area of samples against a NO2 standard (300 µl, 0·5 µm NaNO2−). Quantification of NO released by the redox reactions occurred by its chemiluminescence reaction with ozone using the Nitric Oxide Analyzer (CLD66; Eco Physics).
Nitrite and nitrate
Nitrate (NO3) and NO2 concentrations were measured using GC-MS as described previously(Reference Yang, Bondonno and Indrawan17), with N15-labelled NO3 and NO2 internal standards.
Glucose
Plasma glucose was measured using a fully automated analyser (Architect ci8200/c16000 Analyser).
F2-isoprostanes
Systemic oxidative stress was determined by measuring plasma F2-isoprostane concentrations as described previously(Reference Mori, Croft and Puddey18). Briefly, F2-isoprostane concentrations were calculated in pmol/l from ratio of the peak areas for the m/z 569 and m/z 573 ions, with a 2H-labelled internal standard, 8-iso-PG F2α-d4.
Statistics
Sample size was calculated with FMD as the primary endpoint. Assuming a within-subject correlation of r 0·25, twenty-four pre-intervention measures, twenty-four post-intervention measures and a within-group sd of 6 %, a sample size of twenty-five subjects would provide more than 80 % power at α = 0·05 to detect a difference of 0·85 % in FMD. Treatment effects for post-intervention FMD were obtained using linear mixed models with adjustment for treatment order, the pre-treatment FMD curve and the time since cuff release with time included as a categorical variable (0–240 s at 10-s intervals). In order to allow the treatment effect to differ over time, we also included a treatment × time interaction term. The subject identifier was included as a random intercept. Assessment of post-treatment BP was similar to that for FMD, with time included as a categorical variable (0–80 min at 10-min intervals). When assessing the effect of EMIQ® on the BP during the cognitive stress test, we additionally adjusted for the rest-period BP. For all other outcomes, treatment effects were obtained using linear mixed models with adjustment for treatment order. Statistical analyses were performed using STATA/IC 14.2 (StataCorp LLC).
Results
Baseline data
Recruitment began on 25 September 2017 and the study concluded on 12 June 2018. Twenty-five participants (twelve males, thirteen females) completed the study (Fig. 1). The baseline characteristics of the study participants are shown in Table 1.
Table 1. Baseline characteristics of study participants*
(Mean values and standard deviations)
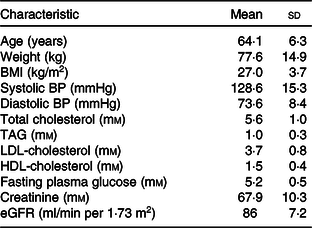
BP, blood pressure; eGFR, estimated glomerular filtration rate.
* n 25: males = 12, females = 13
Endothelial function
Data from one participant were excluded prior to statistical analysis as the clarity of the FMD image was poor. The observed mean percentages of FMD for twenty-four participants, between 0 and 240 s at 1·5 h post-intervention, are presented in Fig. 2. Overall, FMD after the EMIQ® treatment was significantly higher when compared with control (adjusted mean difference = 1·80 (95 % CI 0·23, 3·37) %; P = 0·025).
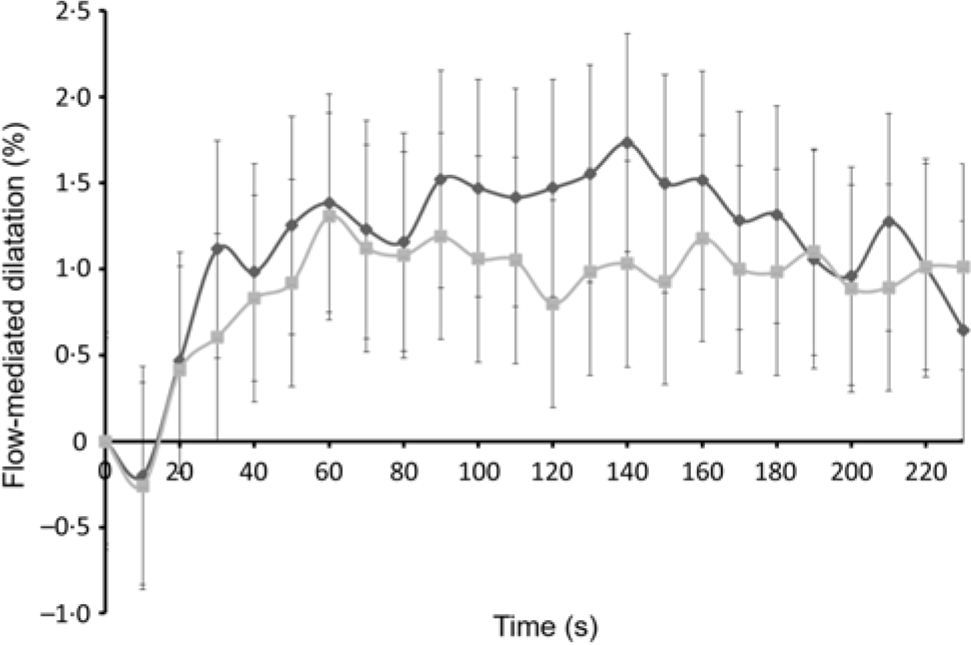
Fig. 2. Acute changes in flow-mediated dilatation (FMD) over 240 s measured 1·5 h post-intervention. Results are presented as means (n 24). P values for treatment effect were obtained using linear mixed models on the differences pre- and post-treatment in FMD (P for treatment effect = 0·025). EMIQ®, enzymatically modified isoquercitrin. ![]() , EMIQ®;
, EMIQ®; ![]() , control.
, control.
Blood pressure and arterial stiffness
Mean systolic BP and diastolic BP, measured immediately before treatment (time = 0) and then every 10 min for 80 min, are shown in Fig. 3. There was no significant difference between EMIQ® and placebo for post-treatment systolic BP (P = 0·916) or diastolic BP (P = 0·073). Similarly, there was no difference in mean arterial pressure (MAP) (P = 0·188) or heart rate (P = 0·290). There was no significant difference between treatments for mean systolic BP, diastolic BP, central diastolic pressure, MAP, heart rate or AIx75 measured 2 h post-treatment (Table 2).
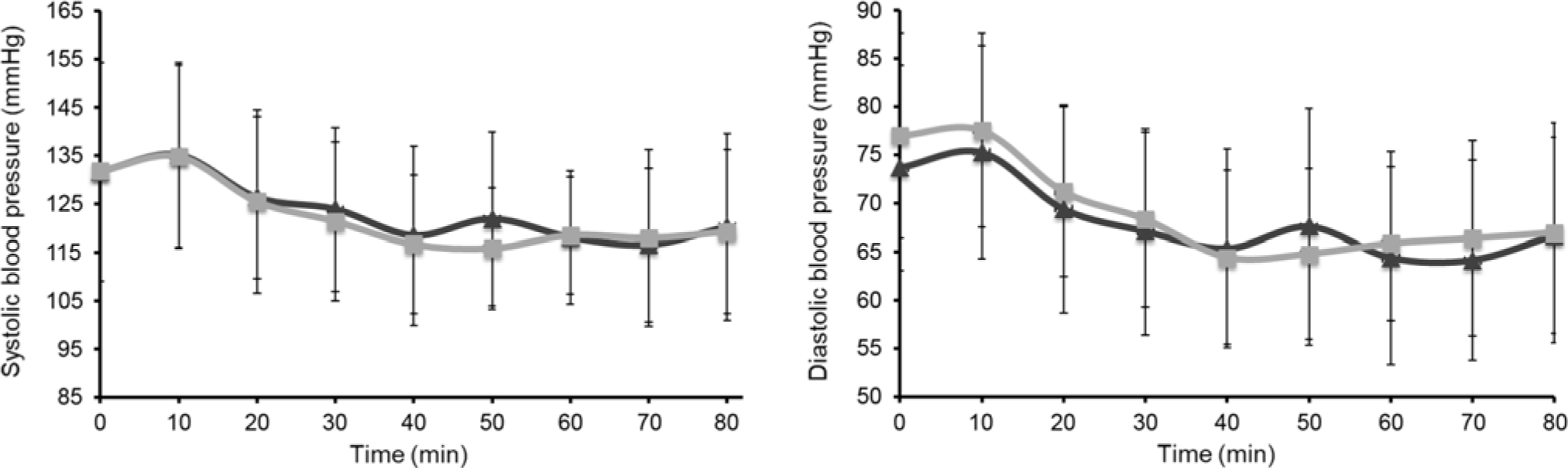
Fig. 3. Acute changes in systolic and diastolic blood pressure over 80 min immediately post-intervention. Treatment was given immediately after the first measurement at time = 0. Results are presented as means and standard deviations (n 25). P values for treatment effect were obtained using linear mixed models with adjustment for treatment order and time. Over the time course, no significant difference was observed between interventions (systolic, P = 0·916; diastolic, P = 0·073). EMIQ®, enzymatically modified isoquercitrin. ![]() , EMIQ®;
, EMIQ®; ![]() , control.
, control.
Table 2. Pulse wave analysis*
(Mean values and standard deviations; n 25)
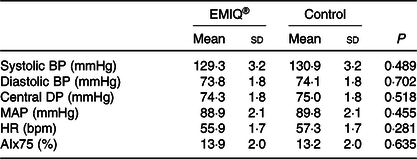
EMIQ, enzymatically modified isoquercitrin; BP, blood pressure; DP, diastolic pressure; HR, heart rate; MAP, mean arterial pressure; AIx, augmentation index.
* P values for treatment effect were obtained using linear mixed models with adjustment for treatment order.
Cognitive stress test and blood pressure
No significant effects were associated with the EMIQ® treatment on any of the cognitive stress test outcomes, nor on BP measurements taken during the cognitive stress tests (Tables 3 and 4).
Table 3. Cognitive function measurements*
(Mean values and standard deviations; n 23)
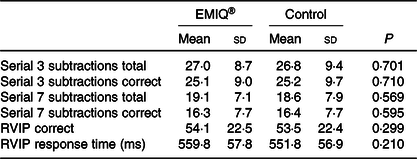
RVIP, rapid visual information processing.
* Cognitive test measurements, 2·5 h post-consumption of enzymatically modified isoquercitrin (EMIQ®) or placebo treatment. P values for treatment effect were obtained using linear mixed models with adjustment for treatment order and time.
Table 4. Blood pressure during cognitive stress testing*
(Mean values and standard deviations; n 25)
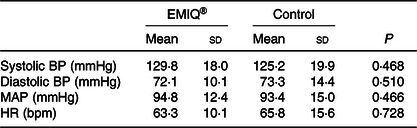
EMIQ®; enzymatically modified isoquercitrin; BP, blood pressure; MAP, mean arterial pressure; HR, heart rate.
* Blood pressure was measured every 2 min for 34 min (seventeen measures total). P values for treatment effect were obtained using linear mixed models with adjustment for treatment order, time and BP during pre-test rest period.
Table 5. Plasma analyses*
(Mean values and standard deviations; n 25)
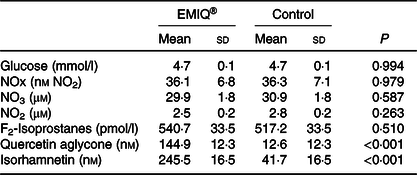
* Measurements of plasma glucose, nitrogen oxide (NOx), nitrate (NO3), nitrite (NO2), F2-isoprostanes and quercetin metabolites were performed 3 h post-consumption of enzymatically modified isoquercitrin (EMIQ®) or placebo treatment. P values for treatment effect were obtained using linear mixed models with adjustment for treatment order.
Plasma analyses
Plasma concentrations of quercetin aglycone and isorhamnetin were significantly higher (P < 0·001) after the ingestion of EMIQ® compared with the placebo (Table 3). No significant difference was observed between EMIQ® and placebo treatments with regard to plasma glucose, NOx, NO2, NO3 or F2-isoprotanes (Table 5).
Discussion
This is the first study to investigate the acute effects of an enzymatically modified isoquercitrin compound on measures of vascular health, BP and cognitive function in humans. In this randomised controlled crossover study, the acute administration of EMIQ® significantly increased plasma quercetin metabolites and improved endothelial function. No changes were observed in BP, arterial stiffness, cognitive function, BP during cognitive stress or biochemical parameters.
In the present study, plasma concentrations of quercetin aglycone and isorhamnetin were significantly higher after the ingestion of EMIQ® compared with the placebo. Previously, the plasma concentration of conjugated quercetin metabolites has been shown to reach a maximal level at approximately 1·5 h after intake of EMIQ®(Reference Murota, Matsuda and Kashino10). For this reason, our primary outcome, endothelial function, was assessed 1·5 h post-intervention.
Endothelial dysfunction, defined as an impairment in endothelium-dependent relaxation, is implicated in prehypertension, hypertension, atherosclerosis and stroke(Reference Cosentino and Volpe19,Reference Yang and Ming20) and is associated with an increased risk of CVD(Reference Halcox, Schenke and Zalos21,Reference Inaba, Chen and Bergmann22) . The results of the present study suggest that EMIQ® improves postprandial endothelial function. In two previous studies, acute quercetin administration (1095 mg quercetin aglycone(Reference Larson, Witman and Guo6) and doses of quercetin-3-O-glucoside ranging from 50 to 400 mg(Reference Bondonno, Bondonno and Rich7)) had no effect on FMD. In the study by Larson et al., FMD was measured 10 h post-quercetin aglycone administration, the time at which quercetin metabolites were shown to peak in the plasma(Reference Larson, Witman and Guo6). The pharmacokinetics of quercetin aglycones and EMIQ are very different; in the present study, FMD was measured 1·5 h post-intervention, at the time quercetin metabolites have been shown to peak in the plasma following EMIQ administration(Reference Murota, Matsuda and Kashino10). Quercetin-3-O-glucoside has been shown to peak in the plasma between 1·5 and 3 h post-intervention; in our previous study(Reference Bondonno, Bondonno and Rich7), FMD was measured 1 h post-intervention. The differences in study design and the form in which the quercetin was administered make the direct comparison with the present study difficult. Another important discrepancy in study design is that EMIQ was administered alongside a meal in the present study, likely influencing the effect of quercetin on endothelial function(Reference de Koning and Rabelink23,Reference Grassi, Draijer and Schalkwijk24) . Overall, FMD in the present study was relatively poor as we recruited participants with at least one risk factor for CVD. Although the improvement observed was subtle (1·80 %), if sustained it could have important clinical implications as a 1 % increase in FMD is associated with a 13 % lower risk of cardiovascular events (relative risk: 0·87, 95 % CI 0·83, 0·91)(Reference Inaba, Chen and Bergmann22).
Evidence suggests that quercetin can improve endothelial health through NO-mediated vasorelaxant activity as well as through the prevention of oxidant-induced endothelial dysfunction(Reference Bondonno, Bondonno and Hodgson4). An acute increase in plasma NO status has been observed after both a high-flavonoid apple intervention(Reference Bondonno, Yang and Croft5) and quercetin (200 mg) intervention(Reference Loke, Hodgson and Proudfoot25). In the present study, we did not observe any increase in plasma NOx or the end products of NO metabolism, NO3 and NO2. This may have been due to the timing of our blood sample, which was 1·5 h after the measurement of endothelial function and 3·5 h post-intervention. Changes in NO are likely to be transient, and because of the difficulty in detecting small changes in NO, the timing of the measurement is likely to be critical.
In our previous randomised clinical trial with healthy men and women, improvements in BP were also observed with the concomitant increase in NO status and FMD, after intake of flavonoid-rich apples(Reference Bondonno, Yang and Croft5). In a meta-analysis of randomised controlled trials, significant reductions in both systolic BP (−3·04 mmHg, 95 % CI −5·75, −0·33, P = 0·028) and diastolic BP (−2·63 mmHg, 95 % CI −3·26, −2·01, P < 0·001) were seen following quercetin supplementation(Reference Serban, Sahebkar and Zanchetti26). Furthermore, in spontaneously hypertensive rats, EMIQ® administered at a dose of 26 mg/kg per d suppressed the increase in systolic BP more effectively than quercetin aglycone(Reference Emura, Yokomizo and Toyoshi12). In the present study, no effect of EMIQ® on post-treatment BP or on BP during the cognitive stress test was observed. This lack of effect may be due to the dose given; in the aforementioned meta-analysis, only a significant effect on BP was observed when quercetin was administered at doses greater than 500 mg/d(Reference Serban, Sahebkar and Zanchetti26). Additionally, the health status of the participants may have played a role as a decrease in systolic BP following quercetin supplementation is generally seen in hypertensive individuals(Reference Larson, Bruno and Guo27,Reference Egert, Bosy-Westphal and Seiberl28,Reference Edwards, Lyon and Litwin29) , not in pre-hypertensive or normotensive individuals(Reference Larson, Witman and Guo6,Reference Dower, Geleijnse and Gijsbers8,Reference Edwards, Lyon and Litwin29,Reference Perez, Gonzalez-Manzano and Jimenez30,Reference Conquer, Maiani and Azzini31,Reference Nakayama, Tsuge and Sawada32) .
Many functions of the endothelium are affected under oxidative stress, including endothelial cell apoptosis(Reference Touyz and Schiffrin33) and adhesion of inflammatory cells(Reference Taniyama and Griendling34), initiating the development of atherosclerosis. Quercetin may decrease oxidative stress through the stimulation of protective defences and repair systems(Reference Forman, Davies and Ursini35). In apoE-deficient atherogenic mice, EMIQ® supplementation for 14 weeks significantly suppressed aortic and aortic sinus atherosclerotic lesion areas and decreased levels of 4-hydroxy-2-nonenal, a marker of oxidative stress(Reference Motoyama, Koyama and Moriwaki13). In the present study, we observed no acute changes in the levels of plasma F2-isoprostanes, an in vivo biomarker of oxidative stress, following the EMIQ® intervention. It may be that long-term interventions are required to observe changes in biomarkers of oxidative stress. Evidence that quercetin may impede the development of CVD by moderating oxidative stress is stronger in animal studies than in human studies(Reference Bondonno, Bondonno and Hodgson4).
There is evidence that flavonols, the flavonoid subclass to which quercetin belongs, can reduce the risk of type 2 diabetes (T2D). This comes from an observational study which found that each 2·5-fold increase in flavonol intake was associated with a 26 % lower incidence of T2D(Reference Jacques, Cassidy and Rogers36), and a meta-analysis demonstrating a significant reduction in fasting plasma glucose (difference in mean = −0·18 mmol/l; 95 % CI −0·29, −0·08) following flavanol intake(Reference Menezes, Rodriguez‐Mateos and Kaltsatou37). However, fasting plasma glucose was not significantly lower in any of the studies with a quercetin intervention. In the present study, we saw no acute effect of EMIQ® on plasma glucose. Given that the study population was relatively normoglycaemic, this may be better investigated in a hyperglycaemic population.
Evidence that quercetin can improve cognitive function comes wholly from animal studies(Reference Sabogal-Guáqueta, Munoz-Manco and Ramírez-Pineda38,Reference Wang, Li and Wu39,Reference Priprem, Watanatorn and Sutthiparinyanont40) . To our knowledge, this is the first study to examine the acute effects of a quercetin derivative on cognitive function in humans. We saw no significant effects of EMIQ® on any measures of cognitive function in the present study. This warrants further investigation; future studies could consider a long-term intervention and a broader range of cognitive function measurements.
Strengths of the present study are that any inter-subject variability was accounted for by our crossover study design and adjustments for baseline measurements of FMD controlled for day-to-day variability. There are, however, several limitations: first, only the primary outcome was measured before and after the intervention. Second, the study was only powered to detect a change in the primary outcome, meaning that we may have been underpowered to detect changes in secondary outcomes. So as not to affect the measurement of any other outcomes, the blood sample was taken 1·5 h after the measurement of FMD (3·5 h post-intervention). Additionally, the present study only investigated one dose of EMIQ®, with the primary outcome measured at only one time point.
In this human intervention study, the acute administration of EMIQ® significantly increased circulating quercetin metabolites and improved postprandial endothelial function. The addition of EMIQ® to commercially available foods and beverages may have positive effects on vascular function. The potential health benefits associated with regular consumption warrants investigation in longer term randomised controlled trials.
Acknowledgements
The authors wish to thank the volunteers who participated in the study.
J. M. H. was supported by a National Health and Medical Research Council Senior Research Fellowship. The present study was funded by San-Ei Gen F.F.I., Incorporated (Osaka, Japan). The funding sponsors had no role in the design of the study; in the collection, analyses or interpretation of data; in the writing of the manuscript and in the decision to publish the results.
N. P. B., C. P. B., J. M. H., N. C. W. and K. D. C. were responsible for the project conception; N. P. B. and C. P. B. conducted the research; N. P. B. and R. J. W. analysed the data; N. P. B. prepared the manuscript; C. P. B., J. M. H., N. C. W., R. J. W. and K. D. C. critically reviewed the manuscript.
The authors declare no conflicts of interest.











