INTRODUCTION
Staphylococcus aureus and its resistant form, methicillin-resistant S. aureus (MRSA), rank top among the clinically most relevant nosocomial pathogens, leading to increased morbidity, mortality, length of hospital stay and costs [Reference Diekema1–Reference Pittet and Wenzel4]. Colonized healthcare workers (HCWs) are capable of developing clinical S. aureus infections [Reference von Eiff5, Reference Wertheim6], transmitting S. aureus to patients [Reference Wertheim6–Reference Tammelin8], and introducing S. aureus into their families [Reference Lu9–Reference Wagenvoort11]. Understanding S. aureus colonization patterns in HCWs may aid in the development and reinforcement of infection control strategies and add to the understanding of S. aureus epidemiology.
Interestingly, recent reports have revealed higher S. aureus nasal carriage rates in surgeons than in high-risk patient groups [Reference Schwarzkopf12] and more frequent MRSA nasal carriage in nurses than other HCWs [Reference Elie-Turenne13, Reference Suffoletto14], supporting the view that healthcare service may be a risk factor for carriage. However, studies in the healthcare setting generally lack a broader perceptive on the ecological context of HCWs in the community and how background prevalence in the general population and households may bias the results. Importantly, S. aureus nasal carriers may ‘impose’ their carrier status upon other household members [Reference Mollema10, Reference Wagenvoort11, Reference Peacock15], and the bacterium can be reintroduced into the hospital by intrafamilial spread from and to HCWs [Reference Lu9, Reference Wagenvoort11]. Moreover, with novel technologies for molecular typing of S. aureus there are better opportunities for detailed epidemiological studies [Reference Lu9].
In The Tromsø Staph and Skin Study (TSSS), including 4026 adults aged 30–87 years, participating in a population-based health screening survey, we recently reported overall nasal carriage rates of 35% in men and 20% in women [Reference Olsen16], and a heterogeneous distribution of S. aureus spa types by gender and age [Reference Sangvik17]. Importantly, we found overlapping population structures for S. aureus nasal isolates in this population-based screening and clinical S. aureus isolates in septic patients from the same geographical area and time period [Reference Sangvik17]. TSSS was designed to study the host–microbe–environment relationships in S. aureus carriage within an unselected general population, focusing on healthcare-associated environmental risk factors (i.e hospital stay, work in healthcare services) reported at the screening. Thus, to explore S. aureus epidemiology in HCWs, we compared screening data on S. aureus nasal carriage, spa types and residing with children, for HCWs and non-HCWs from the working-age population, 30–69 years, participating in TSSS.
METHODS
Population and study design
The study subjects in TSSS were men and women who participated in the sixth survey of the Tromsø Study (Tromsø 6) conducted in 2007–2008. The Tromsø Study is an ongoing population-based cohort study with five previous surveys undertaken between 1974 and 2001 [Reference Jacobsen18]. Based on the official population registry, residents of the municipality of Tromsø were invited to take part in the survey. In Tromsø 6 all subjects aged 40–42 and 60–87 years, a 40% random sample aged 43–59 years, and a 10% random sample aged 30–39 years were invited. In addition to the random samples, 295 participants were invited because they attended the second visit in the fourth survey in 1994. Invitation letters were sent randomly, avoiding selection bias during the sampling period. A total of 12 984 men and women aged 30–87 years attended Tromsø 6 (attendance rate 65·7%). Due to lack of capacity and funding, young adult residents (aged 18–29 years) could not be invited [Reference Jacobsen18].
For the study of S. aureus nasal carriage in a general population, we considered a more evenly distributed sampling across age groups to be most suitable and the inclusion of 4000 observations to be sufficient for analysis of host–microbe–environment relationships. Thus, in Tromsø 6, during October 2007–June 2008, all attendees aged 30–49 years (n = 1730) and random samples of older attendees aged 50–87 years (n = 2629, relative distribution of birth cohorts as in the municipality) were asked to participate in TSSS, including a nasal swab culture at the screening (first visit) and a repeated nasal swab culture at a second visit within a few weeks [Reference Olsen16]. A total of 4026 men and women aged 30–87 years (30–49 years, n = 1597, agemean = 41·1, agemedian = 41; 50–87 years, n = 2429, agemean = 62·6, agemedian = 61) had at least one nasal swab culture taken and were included in TSSS, and of these, 2997 subjects (30–49 years, n = 1118, agemean = 41·1, agemedian = 41; 50–87 years, n = 1879, agemean = 62·4, agemedian = 61) had a repeated culture taken (Fig. 1). Mean interval between cultures was 33 days and for 90% of the observations the interval was >12 days.
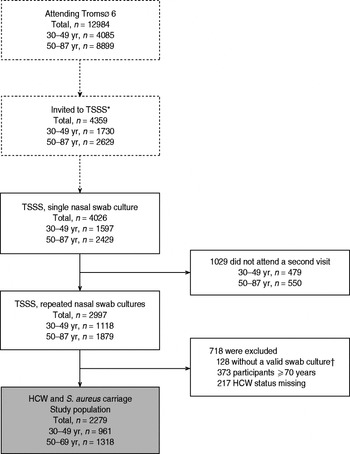
Fig. 1. The study population. TSSS, The Tromsø Staph and Skin Study; HCW, healthcare worker. * Invited to the TSSS. Age group <50 years: all subjects. Age group 50–87 years: random samples of subjects. † Not valid swab culture: 91 had no growth in swab culture, 37 had taken antibiotics last 24 h before visit (systemic or eye drops/ointments).
For the present study of S. aureus epidemiology in HCWs, 37 subjects who had taken antibiotics within 24 h of the first or second visit, were excluded. According to the working age in Norway, 373 subjects aged ⩾70 years were excluded. The age of retirement in Norway is normally from 67 years, but may be postponed until age 70 years. In 2008, 22% of men and 14% of women aged 67–69 years were registered in the Norwegian labour force [19]. Of the remaining subjects aged <70 years, a total of 217 (30–49 years, n = 116, agemean = 40·9, agemedian = 41; 50–69 years, n = 101, agemean = 59·7, agemedian = 59) with missing data on HCW status were excluded. Thus, for the present analysis 2279 participants aged 30–69 years (30–49 years, n = 961, agemean = 41·1, agemedian = 41; 50–69 years, n = 1318, agemean = 58·7, agemedian = 59) were included (Fig. 1).
The study was approved by the Regional Committee for Medical and Health Research Ethics, North Norway, and all attendees signed an informed consent form. Interviews and clinical examinations were performed at the screening centre by specially trained technicians according to standardized procedures. Body height and weight were measured, and body mass index (BMI) calculated (kg/m2) [Reference Jacobsen18]. Two self-administered structured questionnaires covered a broad range of issues related to health and lifestyle, including the question ‘Are you residing with children aged <18 years?’ (yes/no), referred to as ‘residing with children’ in the present analysis.
Assessment of HCW status
Self-reported information on current healthcare-associated environmental exposure was obtained by the interview question ‘Do you work in healthcare services?’(yes/no), asked at the second visit. Healthcare services were defined as hospital, nursing home, senior care service, general practitioner (GP)'s office, and public health centre. The large-scale setting with multiple screening items in Tromsø 6 allowed for only one screening question on workplace. As an open interview question on workplace (all kinds of professions) turned out to be too time-consuming during the initial 4 weeks of the survey, it was decided to narrow it down to the specific question on work in healthcare services. This change in data collection led to missing information on HCW status until the fifth week of the survey. Thus, for the present study only the data collected after the inclusion of the specific question on work in healthcare services were analysed. The majority of HCWs in Tromsø work at the University Hospital of North Norway (UNN). UNN has about 4400 employees and is the only hospital within a radius of >200 km.
Detection of S. aureus nasal carriage
Both vestibuli nasi were sampled by the same NaCl-moistened sterile rayon-tipped swab and placed in Amies charcoal transport medium (Copan, Italy). Within 3 days, all specimens were cultured at UNN, using blood agar (Oxoid, UK), chromIdTMS. aureus, and chromIdTM MRSA agar plates (bioMérieux, France). In order to ensure high sensitivity, all culture plates were incubated for 42–48 h at 37 °C. To retain high specificity, colony morphology was thoroughly examined and the most dominating suspected green colony on the chromID plates was selected for further confirmation as S. aureus by the Staphaurex Plus (Remel, USA) agglutination test. All confirmed S. aureus isolates were frozen at −70 °C in glycerol-containing liquid media for molecular analysis. No MRSA was isolated [Reference Olsen16].
S. aureus carrier status was defined by the results of both nasal swab cultures at the first and second visits; persistent carrier = two positive cultures; non-carrier = one or no positive culture [Reference van Belkum20]. Our validation in 120 participants in TSSS, showed excellent inter-method reliability for S. aureus nasal carrier status using 1-week interval vs. 2–6 weeks between the nasal swab cultures [weighted kappa = 0·85, 95% confidence interval (CI) 0·77–0·92], also implying a low rate of transient colonization [Reference Haldorsen21].
spa typing
S. aureus nasal isolates from the first (99·6%) and second (99·9%) visits were spa typed as described elsewhere [Reference Sangvik17]. Primers recommended by Ridom GmbH (Germany) were used, i.e. spa-1113f and spa-1514r as described previously by Strommenger et al. [Reference Strommenger22]. Polymerase chain reaction (PCR) products were sequenced on both strands by Macrogen Korea or Macrogen Europe and spa types were assigned using Ridom StaphType software (Ridom GmbH, Germany) [Reference Harmsen23].
According to the Ridom StaphType software 264 and 268 different spa types were assigned in the first and second visits, respectively. In total, six and seven isolates were not typed due to repeated negative spa PCR amplification or deviating repeat length, respectively.
Among S. aureus nasal carriers 92·5% (98/106) of HCWs and 93·0% (453/487) of non-HCWs had the same spa type in both samples (data not shown), also in accordance with the literature [Reference Sakwinska24].
Statistical analysis
Logistic regression models stratified by gender were used to estimate odds ratios (ORs) and 95% CIs for S. aureus nasal carriage overall and by spa types associated with HCW status. The analysis of spa types was restricted to the nasal swab cultures of the first visit.
Selected characteristics of HCWs and non-HCWs were compared by two-sided Student's t test for continuous variables and two-sided Pearson's χ2/Fisher's exact test for categorical variables.
We evaluated model fit and plausibility of several covariates and the final multivariable models included age, BMI, current daily smoking (yes/no), alcohol intake (<2 or ⩾2 times a week), recreational physical activity (three levels), household income (<or ⩾€37000/year), education level (<or ⩾college/university), and residing with children (yes/no). We tested whether residing with children modified the association between HCW status and S. aureus nasal carriage in stratified logistic regression analysis. Testing for interaction was done by inclusion of the multiplicative terms of the two predictor variables in the models. Tests of reliability of the final analyses were done by the Hosmer–Lemeshow goodness-of-fit test.
All tests were two-sided. Stata version 11.0 (StataCorp, USA) was used for statistical analyses.
RESULTS
HCWs comprised 334 (25·7%) of 1302 women and 71 (7·3%) of 977 men. HCWs were younger than non-HCWs in both women and men (both P values <0·05) (Table 1). Among women, HCWs were more often residing with children and reported higher household income, education level, and recreational physical activity level, and less frequent alcohol intake than non-HCWs. Among men, HCWs reported more frequent alcohol intake and hospitalization over the last 12 months than non-HCWs (all P values <0·05) (Table 1).
Table 1. Characteristics of women and men by healthcare worker status. The Tromsø Staph and Skin Study (n = 2279*)
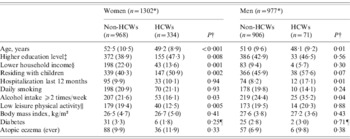
HCW, Healthcare worker.
Values are given as means (standard deviation), and numbers (%).
* Numbers may vary due to missing information.
† Two-sided Student's t test for continuous variables. Pearson's χ2 test for categorical variables.
‡ ⩾College/university degree.
§ <€37000/year.
|| Mostly sedentary recreational physical activity, e.g. watching TV.
¶ Fisher's exact test.
Risk of S. aureus nasal carriage
The overall prevalence of S. aureus nasal carriage was 26·2% in HCWs and 26·0% in non-HCWs. The corresponding sex-specific rates were 22·5% and 18·4% in women (P = 0·11), and 43·7% and 34·1% in men (P = 0·10), respectively (Table 2).
Table 2. Estimated risk of S. aureus nasal carriage by healthcare worker status. Logistic regression analysis. The Tromsø Staph and Skin Study (n = 2279*)
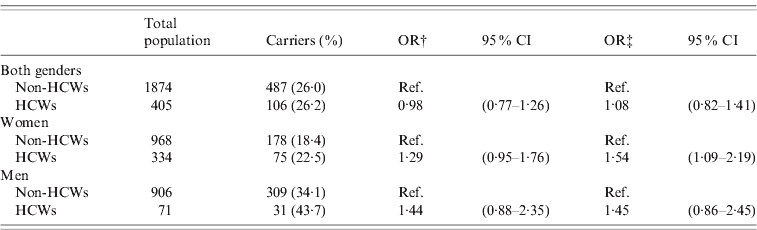
HCW, Healthcare worker; OR, odds ratio; CI, confidence interval.
* Numbers may vary due to missing information.
† Age-adjusted.
‡ Multivariable logistic regression model including: age, current daily smoking (yes/no), alcohol intake (<2 or ⩾2 times a week), recreational physical activity (three levels), household income (<or ⩾€37000/year), residing with children (yes/no), body mass index, education level (<or ⩾college/university degree).
In multivariable analysis, HCW status was not associated with S. aureus nasal carriage in the total population (Table 2). However, female HCWs had a 54% increased risk of S. aureus nasal carriage vs. non-HCWs (OR 1·54, 95% CI 1·09–2·19), while male HCWs did not differ from non-HCWs with respect to risk of S. aureus carriage.
For women residing with children, HCWs had an 86% increased risk of S. aureus nasal carriage compared to non-HCWs (multivariable analysis: OR 1·86, 95% CI 1·14–3·04; P for interaction = 0·42) (data not shown), while in women not residing with children there was no difference in risk by HCW status. For men, there was no pattern of effect modification by family status.
Risk of S. aureus nasal carriage by spa type
At first visit, a total of 61, 41, 32, 26, 22 and 15 participants were colonized with spa types t012, t084, t065, t021, t015 and t002, respectively, which were the predominant spa types including almost one third of the S. aureus nasal isolates. The distribution of the 31 most common (⩾4 observations) spa types in the first sample according to HCW status is shown in Figure 2. The majority of these spa types were observed in both HCWs and non-HCWs, while seven spa types were restricted to non-HCWs, among these spa type t002. None of the most common spa types were found in HCWs only.
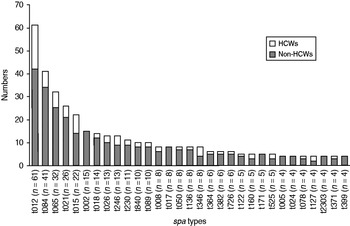
Fig. 2. Distribution of spa types in non-healthcare workers (non-HCWs) and healthcare workers (HCWs) at first visit, nasal swab cultures. The Tromsø Staph and Skin Study. spa types with ⩽3 observations are not shown (233 different spa types in 298 participants).
Among S. aureus nasal carriers, HCWs had 2·17 and 3·16 times higher risk of spa types t012 and t015 in the first sample, respectively, compared to non-HCWs (multivariable analysis: OR 2·17, 95% CI 1·16–4·08 and OR 3·16, 95% CI 1·13–8·87) (Table 3).
Table 3. Estimated risk of the six most common spa types at first sample by healthcare worker status. S. aureus nasal carriers (n = 593), The Tromsø Staph and Skin Study
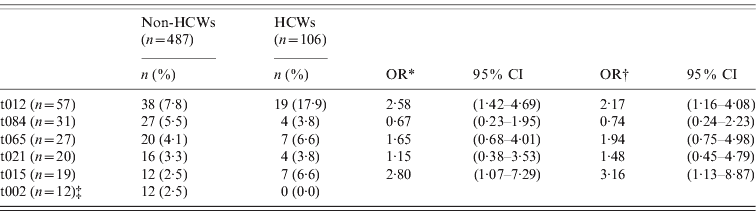
HCW, Healthcare worker; OR, odds ratio; CI, confidence interval.
* Logistic regression model (unadjusted).
† Multivariable logistic regression model including: age and gender.
‡ No OR estimates due to no spa type t002 in HCWs.
When restricting the analysis to S. aureus nasal carriers residing with children, HCWs had a 2·42 times higher risk of spa type t012 in the first sample compared to non-HCWs (age- and gender-adjusted analysis: OR 2·42, 95% CI 1·03–5·70). The association for spa type t012 was particularly strong in male nasal carriers (age-adjusted analysis: OR 4·61, 95% CI 1·36–15·61) (Table 4). Among S. aureus nasal carriers not residing with children, HCWs had a fourfold increased risk of spa type t015 in the first sample compared to non-HCWs (age- and gender-adjusted analysis: OR 4·28, 95% CI 0·99–18·43) (Table 4).
Table 4. Estimated risk of spa types t012 and t015 nasal carriage by healthcare worker status and residing with children. Logistic regression analysis. The Tromsø Staph and Skin Study
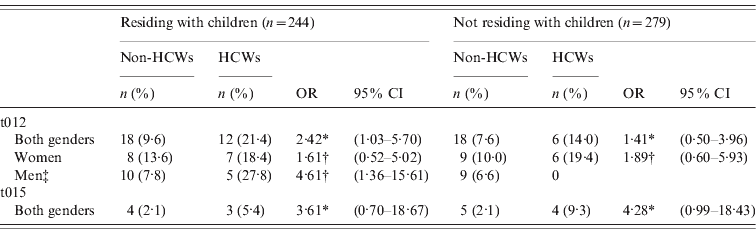
HCW, Healthcare worker; OR, odds ratio; CI, confidence interval.
* Multivariable logistic regression model adjusted for age and gender.
† Multivariable logistic regression model adjusted for age.
‡ No OR estimate due to no spa type t012 in male HCWs not residing with children.
DISCUSSION
In this large population-based study with participants aged 30–69 years with repeated nasal swab cultures, working in healthcare services was associated with a 54% increased risk of S. aureus nasal carriage in women. The risk was even higher in women residing with children. For men, work in healthcare services and residing with children were associated with increased prevalence of common spa types. Our study suggests that a synergism between environmental risk factors (work and household) is of importance for S. aureus carrier status in HCWs.
To our knowledge, this is the first study which reports that female HCWs have a higher risk of S. aureus nasal carriage than female non-HCWs in a general working-age population. Interestingly, other studies confined to HCWs, observed higher nasal carriage rates of MRSA in nurses than in other healthcare professionals, and cited increased patient contact as a cause [Reference Elie-Turenne13, Reference Suffoletto14]. Our screening study did not include information about the HCWs' profession. However, the majority of the Norwegian healthcare workforce are nurses and auxiliary nurses, and about 90% of these are women [Reference Køber and Vigran25, 26]. Thus, our findings suggest that work in healthcare services is an environmental risk factor for S. aureus nasal carriage in women, due to a substantial amount of contact with patients and the elderly in residential care when working as nurses and auxiliary nurses. No MRSA was isolated in our study. However, epidemiological evidence supports a similar mechanism for transmission of methicillin-sensitive S. aureus (MSSA) and MRSA usually via direct contact with patients and other close contacts (household members, etc.) [Reference Calfee27, Reference Skov28].
We observed that working in healthcare services in combination with residing with children was a high risk cluster for S. aureus carriage in women. Our findings are in agreement with current literature supporting the view that contact transmission within HCWs' families may affect the burden of MSSA and MRSA infections in hospitals [Reference Lu9, Reference Wagenvoort11]. Importantly, residing with children per se was not associated with S. aureus nasal carriage in our study, and number of children aged <18 years and age did not differ between HCWs and non-HCWs (results not shown). Information on childcare was not included in our study, and attending kindergarten has been associated with increased risk of S. aureus colonization in children [Reference Lamaro-Cardoso29]. However, it can be assumed that use of kindergarten was not a major confounder in our analysis, as there have been places in kindergarten for all children in Tromsø since 2005 [30] and 89·4% of female non-HCWs residing with children were part-time or full-time workers. Thus, we hypothesize that the environmental pressure caused by high rates of contact transmission of S. aureus from both patients and children may exceed the ability of female HCWs (i.e through hand hygiene, immune responses) to defend themselves against colonization.
The population structure of S. aureus colonizing HCWs and non-HCWs was similar, as expected from others [Reference Elie-Turenne13] and our previous comparative study of clinical and nasal carrier S. aureus isolates in Tromsø [Reference Sangvik17]. However, we observed that work in healthcare services was associated with an increased risk of spa types t012 and t015, which both ranked in the top five in our study and are also frequent worldwide [Reference Fenner31, Reference Grundmann32]. Thus, we may expect spa types t012 and t015 to be frequent causes of autoinfections in patients in the community and in hospitals, with an increased potential for transmission, that may also partly explain our findings.
Among men, HCW status and residing with children predicted S. aureus carriage by spa types and not overall carriage rates. Even though there is considerable degree of uncertainty in our estimates, we can hypothesize that in the male human population, common S. aureus spa types have specific main niches defined by environmental factors. The female preference of the common spa type t012 in our study population, may possibly confound the results in women [Reference Sangvik17].
The main limitation of our study is the lack of information on profession, workplace and patient contact. The cross-sectional design demands caution in interpretation regarding any causal relationship. Thus, future prospective population-based studies including work and home exposures are needed to improve our knowledge about high-risk groups and environmental targets for more effective prevention of S. aureus carriage in HCWs. As the prevalence of S. aureus carriage in younger adults is relatively high, particularly in men [Reference Gorwitz33, Reference Olsen34], the role of work in healthcare services for carriage should also be explored in the working population aged <30 years. Furthermore, the S. aureus epidemiology in HCWs having contact with the elderly requires increased focus given the fact that nursing-home residents are a population at risk for carrying S. aureus and MRSA [Reference Pfingsten-Wurzburg35]. Recent antibiotic use not registered at the screening (i.e. ceased >24 h before visit) and lack of enrichment during culturing may have caused false negatives independently of HCW status. Thus, non-differential misclassification of S. aureus nasal carrier status may have biased our odds ratio estimates towards null.
The major strengths of our study are the population-based design, high participation rate, large set of observations, validated outcome variable including spa type, gender-stratified analysis, and the adjustment for major confounders (i.e. age, BMI, smoking, etc.). Furthermore, representative data were achieved as demonstrated by the age distribution within the different age groups included.
The absence of MRSA in this study is in line with the low prevalence of MRSA in clinical S. aureus samples registered in the Norwegian surveillance programme during 2007–2008 [36]. Importantly, the surveillance data do not reflect a healthy population, and therefore cannot be directly compared to MRSA/MSSA colonization rates in our study, including healthy persons only (i.e not hospitalized or institutionalized).
In conclusion, our study indicates that work in healthcare services in combination with residing with children is a high-risk cluster for S. aureus carriage in women. The synergism of work and home environmental risk factors should be addressed in future studies to increase our understanding of S. aureus epidemiology in HCWs and suggest novel targets for improved infection control strategies against exogenous MSSA and MRSA infections in patients.
ACKNOWLEDGEMENTS
We thank Bjørg C. Haldorsen, Trine Tessem, Bettina Aasnæs and Tonje Holan for excellent technical assistance. This work was supported by grants from the Research Council of Norway (grant no. 191264); Northern Norway Regional Health Authority (project no. 7150.00003, grant SFP877-09 and SFP920-10); and the Odd Berg Group Medical Research fund 2007.
DECLARATION OF INTEREST
None.








