INTRODUCTION
Hepatitis C virus (HCV) was discovered in 1989. It accounted for most cases of transfusion-related hepatitis and was previously known as non-A, non-B (NANB) hepatitis [Reference Kuo1, Reference Mühlberger2]. HCV is a member of the Flaviviridae family. It is a small, enveloped, positive-sense, single-stranded RNA virus. There are six major genotypes and over 100 subtypes [Reference Smith and Simmonds3]. There are clear differences in the distribution of the genotypes around the world. Genotypes 1a and 1b account for 60% of all infections globally. It is seen in Europe, North America, and Japan. Genotype 3 is endemic in South-East Asia, genotype 4 is found in the Middle East and central Africa and genotype 5 is found in South Africa [4, Reference McOmish5]. The major risk factors for HCV infection are injecting drug use, intravenous drug use and blood or blood product transfusion [Reference Roy6–Reference Soldan8]. There is a also risk of transmission from body piercing, tattoos and medical or dental procedures when unsterilized equipment is used [Reference Haley and Fischer9]. Shared use of razors and shaving materials may also be a risk factor. Sexual transmission is a possible route with a prevalence of up to 2·5% in long-term heterosexual partners of HCV-infected individuals; but is greater than 4% in men who have sex with men [Reference Tedder10, Reference Terrault11]. Vertical transmission from an infected mother to child is seen in up to 5% of deliveries [Reference Resti12, Reference Steininger13].
Acute hepatitis C infection is often asymptomatic; however, only a small minority clear the virus spontaneously. The majority (70–85%) of patients become chronic carriers [Reference Alter14]. Of these up to 30% will progress to cirrhosis within three decades, with an associated risk of progressing to decompensated liver disease and hepatocellular carcinoma [Reference Lok15, Reference Kiyosawa16].
The infection can be cured with pegylated interferon and ribavarin therapy in up to 80% of patients depending on the genotype [Reference Manns17–Reference Hadziyannis19]. As the natural history of chronic hepatitis C is long, with the period between inoculum and development of complications measured in decades, it is difficult to identify patients as they may be asymptomatic or considered as low risk of acquiring the infection.
The World Health Organization (WHO) estimates that 180 million individuals are infected with HCV accounting for 3% of the world's population [4]. Within the UK, the estimated prevalence is 185 000 or ~0·5% of the population [20]. In Scotland ~50 000 people are estimated to be infected with 37 500–39 000 of those infected chronically. This accounts for nearly 1% of the population with the risk factor for infection in most being intravenous drug use [Reference Hutchinson7]. Migrant Pakistanis are the largest single minority group in Scotland according to the last census (2001) accounting for 2% of the total population or 31 793 individuals [21]. In Pakistan there are no population-based estimates available but studies in blood donors and other at-risk groups indicate the prevalence of hepatitis C to be 6% (range 2·5–25%) [Reference Raja22]. This is considerably higher even compared to other South Asian countries [Reference Gunasekera, Sunil-Chandra and de Silva23–Reference Labrique25]. This may be due to the infrastructure of healthcare delivery and some cultural health beliefs. Therapeutic injections have been implicated in the transmission of HCV in multiple studies from Pakistan and other countries [Reference Luby26–Reference Khan29]. Pakistani patients' preference for injection therapy in place of oral medication and healthcare providers' economic incentive to provide this is a major driver for HCV infection [Reference Khan29]. Reuse of unsterilized needles in providing injections is the leading risk factor in both genders, especially in women, because of their healthcare needs during pregnancy and childbirth [Reference Janjua30, Reference Uzma31]. Transfusion-related HCV transmission in Pakistan is another important factor which occurs due poor enforcement of regulations regarding screening [Reference Umar32–Reference Akhtar34]. Most blood banks are privately owned and use paid blood donors, who in the majority of circumstances are intravenous drug users funding their habit. Community shaving by barbers has been a reported source of HCV transmission not only in Pakistan but in studies from Morocco and Thailand [Reference Zahraoui-Mehadji35–Reference Wazir37]. Face and armpit shaving by community barbers is a common cultural practice. These barbers also circumcise male Muslim newborns in rural and semi-urban areas [Reference Wazir37]. The only community study done in England demonstrated a higher HCV prevalence in the Pakistani sub-population, but not as high as reported in Pakistan [Reference Luby26].
The aim of this project is to estimate the prevalence of HCV in the resident Pakistani population and assess the use of mosque-based outreach clinics in testing for the infection.
METHODS
In July 2009 we held discussions with Pakistani community representatives about the possibility of organizing a HCV awareness programme. It was mutually agreed that we would visit the three mosques in the city as well as a dedicated Pakistani women's centre. We spoke in the mosques following the imam's Friday teaching before afternoon prayers; a short talk was delivered about HCV in English and Urdu by two of the authors (J. F. D. and H. J.). Timing of the talks was chosen to ensure the largest congregation possible. It is estimated that more than 200 individuals were present at these mosques. The event was internally advertised for some weeks beforehand. A talk was also given at the women's centre during a social event and was attended by about 50 females. The message was a simple summary of: risk factors, prevalence, lack of symptoms, slow progressive nature of disease, complications and the existence of treatment. It concluded with an offer from us to set up short-term, outreach testing clinics. In October testing clinics were set up after Friday prayers in all four places for an hour. The imam invited people to come forward for testing, all who came forward were tested but only Pakistanis were included in this analysis. The samples were taken by standard phlebotomy. Prior to phlebotomy, participants completed a questionnaire, noting age, ethnicity, country of birth, years resident in Scotland, relevant past medical history (risk factors for HCV), family infection with HCV and travel history. Interpreters were available on site and assisted when required. Each participant received a copy of printed educational material about HCV and HBV in English and Urdu.
Venous blood samples were drawn and transported to Ninewells Hospital Virology laboratory according to standard NHS Tayside policy. Initially all samples were tested for HCV IgG and HBsAg using the Abbott Architect® system (Abbott Laboratories, USA). Patients who tested positive for anti-HCV underwent a confirmatory testing for HCV RNA by quantitative polymerase chain reaction (PCR) with a lower limit of detection of 15 IU/ml. Sera positive for HBsAg was tested for anti-Hbc, HBeAg and viral load.
All patients were contacted by mail with the results. Those who tested positive with HCV or HBV were also contacted by phone and a follow-up appointment in the HCV treatment service was arranged. These patients underwent clinical examination and investigation to rule out other causes of liver disease. Ethnicity was defined by ancestral origin. Individuals born in Pakistan were labelled as first generation and individuals born to Pakistani parents in the UK were labelled as second-generation Pakistanis. Statistical analysis was undertaken using SPSS v. 17 (SPSS Inc., USA).
RESULTS
The city of Dundee, in the east of Scotland has a large Pakistani minority population, at the last census there were 1723 Pakistani individuals registered. During this short outreach project 177 individuals completed a questionnaire and volunteered to be tested. Of these, 170 were of Pakistani origin and were included in the analysis The mean age (±s.d.) of the group was 45·11±16·7) years (Fig. 1). Overall 93% (n=159) of the individuals were born in Pakistan, in the province of Punjab. The rest were born in Scotland. The population tested was predominately male 145/170 (85·2%). Median length of residency in Scotland was 18·5 years (range 1–59 years). Only 17% (n=29) had not revisited their country of birth. Of the 25 women who volunteered for testing, four were born in UK and the rest in Pakistan. This included women born in undivided India pre-1947. They had lived in UK for an average of 32 years (range 4–59 years). Of these women 19/25 had given birth, 15/19 had children born in Scotland, while only four had children born in Pakistan. Most (86·5%) individuals had seen their General Practitioner (GP) in the preceding 2 years for illnesses or routine follow-up of unrelated conditions – only one had been tested for HCV.
The risk factors for HCV infection for all patients are shown in Table 1. It shows that at least a third of these patients were exposed to risks which could have led to the transmission of HCV. None of the individuals admitted to using intravenous drugs in the past or currently. All male patients had a history of circumcision.
Table 1. Risk-factor exposure in Pakistan
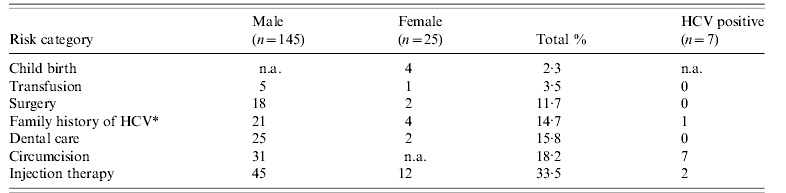
n.a., Not applicable.
* Family history=only first-degree relatives.
All patients with positive results were first-generation Pakistani males. Seven patients were anti-HCV positive, which gives a prevalence of 4·1% (95% CI 2–8%). One individual was HbsAg positive. The length of residency varied from 3 to 49 years (median 21 years). All were circumcised in Pakistan, two were exposed to injection therapy and one patient's mother had hepatitis C in Pakistan a first-degree relative with hepatitis C. Other than circumcision only two of the positive patients had a known risk factor for HCV. Five (2·9%) individuals were found to be HCV RNA positive by PCR. Their mean (±s.d.) age was 36·2±10·6 years. All were asymptomatic, had genotype 3 and elevated alanine aminotransferases (ALT). There were no cases of cirrhosis on ultrasound and fibroscan. One individual was found to be diabetic. None were co-infected with either HIV or HBV. These patients were invited to a specialist HCV clinic where the diagnosis was explained and on a subsequent visit, treatment was offered, and accepted, by the five patients.
DISCUSSION
Over the years, targeted testing of high-risk groups such as former and current intravenous drug users and the blood transfusion lookback programme has resulted in the diagnosis of up to 50% of the estimated HCV-infected population in Scotland. Ethnic minorities may have a higher rate of infection than the indigenous population and novel routes for targeted testing could be employed to engage with this group. This sizable minority population in Scotland which has never been directly approached for HCV testing, despite the high prevalence of chronic hepatitis C in Pakistan compared to other Asian countries, provided us with a unique opportunity to test this hypothesis. The prevalence of hepatitis C in Scottish Pakistanis in this outreach testing pilot project was 4·1% with 2·9% having active infection. This rate is much higher than expected from the indigenous population of about 1%. Not only this project, but also other studies performed in ethnic populations from various settings have concluded that the Pakistani sub-population has higher rates of infection that justifiy systematic interventions [Reference Luby26, Reference Mann38]. Especially as many of these communities have cultural and religious communal events that would provide effective arenas for a specifically targeted intervention.
There are several limitations that should be acknowledged. There are several selection biases; we chose to set testing clinics in mosques, which automatically excludes a proportion of Pakistanis who do not visit the mosque. Second, individuals were self-selected by volunteering for testing. Whether they volunteered because they felt they were at risk of the infection or were simply more health conscious is difficult to speculate. The sample size was small with relative underrepresentation of women. This is a weakness of an intervention directed only at mosques as most women pray at home. We did engage with a community women's group but these groups are often focused on the older generation or those not working. The majority of immigrant Pakistanis in this study came from the province of Punjab. Ali et al. [Reference Ali39] have recently reported Punjab as having the highest prevalence of HCV (4·3% weighted average, range 0·4–31·9%) compared to the rest of the country. Hence we would have extrapolated that Pakistanis living in Scotland would have had a higher prevalence of HCV. However, our lower observed rates can be explained by the ‘healthy migrant’ [Reference Gushulak40, Reference Oliver41] effect and also because most (15/19) mothers in the study had given birth in Scotland. We know most Pakistani women in rural and semi-urban areas have anaemia and multiparity, which potentially exposes them to contaminated transfusions and medical interventions during pregnancy and childbirth. This may explain why no cases of HCV were found in women in this study. Janjua et al. [Reference Janjua30] showed that among women in Pakistan the risk of HCV infection was increased with ⩾2 units of blood transfusion [adjusted odds ratio (aOR) 2·32] or >5 injections in the past 6 months (aOR 1·47).
Within this study there were only 11 second-generation Pakistanis. Seven men and four women were tested. It is possible that they did not consider themselves at risk of infection by bloodborne viruses, hence their poor participation. However, many had visited Pakistan on prolonged vacations and had undergone medical procedures there, and therefore may have an increased risk of infection. However, with the small numbers we are not able to make any statement about this risk which deserves further evaluation.
Despite the limitations of this study, discussed above, with a simple outreach approach and the investment of the time of a few members of staff for about an hour on four occasions, in facilities provided by the community, we managed to offer a HCV test that was taken up by 10% of the Dundee Pakistani population. This is based upon census data and excludes 28 individuals already diagnosed from the Pakistani community within Tayside, these figures taken together represents a lower end estimate of population prevalence of HCV of 2·03%.
When considering community-based health interventions it is logical to evaluate general medical services alongside any targeted intervention. General practice is of course the most obvious of these services. Despite most individuals in the study having recent contact with their local GPs only one was tested for HCV. This is not surprising as the patients would not have been presenting with problems due to HCV and current guidelines do not focus on ethnic origin as a risk factor that requires the offer of a HCV test [42]. As this study shows, focusing only on admitted risk factors would have missed most positive patients. Other than circumcision only two of the positive patients had a risk factor for HCV. More needs to be done in terms of raising awareness among healthcare professionals to consider offering HCV testing to Pakistanis. This is particularly true as many of these patients will have been infected in infancy and the guidelines recommend early detection so that patients can start effective antiviral therapy and reduce risk of decompensated cirrhosis and hepatocellular carcinoma [Reference D'Souza43].
Previously it has been suggested that there has been a lack of participation by ethnic groups in preventive health programmes [Reference Szczepura, Price and Gumber44]. The support we have received for this project from the Pakistani community is a testament to the contrary. It also confirms that outreach testing can be very productive with low-cost interventions.
CONCLUSION
The Pakistani population of Scotland is at higher risk of hepatitis C infection than the indigenous population because of the higher prevalence of the disease in Pakistan. The treatment of HCV infection is not only cost-effective and improves the quality of life but also reduces the risk of liver disease and hepatocellular carcinoma. This justifies the development of further community-based HCV programmes; to offer new avenues to testing, to raise awareness in health professionals and the Pakistani community who otherwise will present in the future with decompensated liver disease.
ACKNOWLEDGEMENTS
We are grateful for the help and support of the local Pakistani community in supporting this work.
DECLARATION OF INTEREST
None.



