Mn, Fe, Zn and Se are well-known essential elements that act as cofactors for a variety of important proteins and enzymes(1). Many toxicological and human evidence have revealed that a deficiency of these elements was associated with many chronic diseases (e.g. CVD, diabetes and chronic liver disease)(Reference Mohammadifard, Humphries and Gotay2–Reference Himoto and Masaki5). However, excessive exposure to these elements, either voluntarily via supplementation or involuntarily via intake of contaminated food and water, has also been linked to increased risks of diabetes(Reference Shan, Chen and Sun4), the metabolic syndrome(Reference Yuan, Xu and Ye6), kidney(Reference Shil and Pal7) and gut diseases(Reference Fang, Zhuo and Yu8), and cancer(Reference Kok, Van Duijn and Hofman9). Cr is also postulated to be involved in regulating the metabolism of carbohydrates and lipids by enhancing insulin’s efficacy(Reference Vincent10), though the European Food Safety Authority found no convincing evidence that Cr is an essential element(11).
The collection of spot samples is non-invasive and convenient to implement(Reference Esteban and Castano12,Reference Sun, Bertrand and Franke13) . Therefore, many epidemiological studies, especially large-scale biomonitoring investigations, such as the Canadian Health Measures Survey (CHMS)(14), the German Environmental Specimen Bank (ESB)(Reference Wiesmuller, Eckard and Dobler15) and the French National Nutrition and Health Survey (ENNS)(Reference Fréry, Saoudi and Garnier16), all have utilised single spot urine sample to determine individuals’ exposure status of Cr, Mn, Fe, Zn and Se. However, the concentrations of these elements in spot samples may vary greatly over time due to the variation in external exposure (driven by the changing lifestyle or dietary habits)(Reference Smolders, Koch and Moos17,Reference Wang, Feng and Zeng18) and urinary excretion rate (UER) (driven by changing salt intake and physiological status)(Reference Aylward, Hays and Smolders19). Using creatinine to adjust for urine dilution may also introduce variation that does not reflect urine dilution(Reference Barr, Wilder and Caudill20).
Very few studies to date have explored the reproducibility of urinary concentrations of Cr, Mn, Fe, Zn and Se. In a study conducted in the USA, Gargas et al. (Reference Gargas, Norton and Paustenbach21) found that Cr concentrations in spot urine samples varied by orders of magnitude in ten volunteers. In a Belgium study, Smolders et al. (Reference Smolders, Koch and Moos17) reported poor reproducibility in urinary Mn concentrations over a 6-d period in four couples (creatinine-adjusted intraclass correlation coefficient (ICC) = 0·28). In an Italy study, Paglia et al. (Reference Paglia, Miedico and Tarallo22) found high seasonal variations in Mn, Zn and Se in first-morning voids (all ICC < 0·50) in seven healthy volunteers. However, no studies have comprehensively assessed the within-day, between-day and between-month variability of Cr, Mn, Fe, Zn and Se concentrations in different sample types (e.g. spot samples, first-morning voids or 24-h collections). The impact of different concentration correction methods (i.e. creatinine adjustment, creatinine as a covariate and UER) on the reproducibility of these elements in urine was also unclear.
To fill the data gap in this study, we characterised the within-day, between-day and between-month variability of serial measurements of Cr, Mn, Fe, Zn and Se in spot samples, first-morning voids and 24-h collections from eleven Chinese adult men, using different correction models. Additionally, we mimicked the exposure classification process in epidemiological studies to calculate the sensitivity and specificity for classifying individuals’ high (top 33 %) 3-month average exposures with single or repeated specimens.
Methods
Study population
From 22 October 2012 to 21 January 2013, eleven healthy adult men were recruited to participate in the study designed to explore the variability in urinary heavy metals and haloacetic acids(Reference Wang, Feng and Zeng18,Reference Wang, Zeng and Wang23) . Their basic demographics have been shown in our previous study(Reference Wang, Feng and Zeng18). To reduce the uncertainty related to demographic characteristic and lifestyle factors that may contribute to the variability in urinary element concentrations, we included only volunteers who were non-smokers and did not have any occupational exposure to metals. Their age and BMI ranged from 21 to 28 years (mean: 23·64 ± 1·96) and 18·5 to 25·1 kg/m2 (mean: 21·67 ± 2·13), respectively. On average, participants urinated six times per d (range: 3–11 times), and the volume of spot samples was 195 ml (range: 20–741 ml). Participants had no restriction on their daily dietary intake but were requested to record the types of food consumed throughout the eight sampling days. Three men reported eating fish only once in the eight sampling days during the 3-month sampling period, and none of the participants took dietary supplementation or ate seafood other than fish on the sampling days. This study was conducted according to the guidelines laid down in the Declaration of Helsinki and approved by the Ethics Committee of Tongji Medical College. All participants signed informed consent forms before participation.
Urine sample collection
During the 3-month sampling period, all spot samples were collected from the eleven adult men on days 0, 1, 2, 3 and 4 (days apart) and on days 30, 60 and 90 (including day 0, months apart). To reduce the effect of changing dietary patterns on the weekend(Reference Gibson, Gibson and Kitching24), all the samples were collected on weekdays. Detailed sampling procedure has been described in our prior publications(Reference Wang, Feng and Zeng18,Reference Wang, Zeng and Wang23) . Briefly, participants were asked to collect all their spot samples in element-free plastic containers throughout the sampling days. Research staff recorded the total volume and collection time of each void, decanted the samples into a trace element-free polyethylene cup and then stored at −40°C refrigerator until analysis. A total of 529 spot samples (six of 535 possible samples were missing) were finally gathered, including eighty-eight first-morning voids and 24-h collections.
Spot samples were defined as any individual urine void collected throughout a given day. A first-morning void was the first sample gathered from an individual starting at 05.00 hours. We reconstructed 24 h collections using the volume-weighted average of all voids gathered from an individual at or after 00.00 hours(Reference Wang, Feng and Zeng18,Reference Wang, Zeng and Wang23) .
Determination of chromium, manganese, iron, zinc and selenium
Urinary concentrations of Cr, Mn, Fe, Zn and Se were quantified using our established method(Reference Feng, He and Chen25,Reference Wan, Chen and Lu26) . Briefly, 3·0 ml of urine sample was nitrified with 15 μl HNO3 67 % v/v (OptimaTM grade; Fisher) at 5°C overnight, of which 1·0 ml was diluted five times using HNO3 1·2 % v/v (OptimaTM grade; Fisher). The target analytes were then quantified using an Agilent 7700x inductively coupled plasma-MS (ICP-MS; Agilent Technologies). Standard reference materials (SRM) (2670a and 1640a) and spiked pool urine samples were used as quality controls. Detected values of Cr, Mn, Fe, Zn and Se were within the range of SRM 2670a and 1640a. The spiked recoveries of these elements ranged from 90 % to 110 %, and the within-day and between-day variations were <10 %. To control for possible contamination, a reagent blank sample (i.e. 3 ml deionised water) was processed together with every twenty samples. Analyte values in all blank samples were lower than the limits of quantification (LOQ). When the detected concentrations in urine are below LOQ, it was substituted by LOQ/(21/2). Urinary creatinine concentrations were determined using an automated clinical chemistry analyser(Reference Wang, Sun and Huang27).
Statistical analyses
All analyses were performed using Stata (version 13.1; Stata Corp) and R software (version 3.5.1, https://www.r-project.org/). Descriptive statistics were used to assess the normality of urinary Cr, Mn, Fe, Zn and Se concentrations. Because of their skewed distribution, log-transformed values were applied for subsequent analyses.
Urinary measurements of Cr, Mn, Fe, Zn and Se were expressed in the following four ways: (a) as unadjusted concentrations (μg/l), (b) as creatinine-adjusted concentrations (μg/g creatinine) calculated by dividing the unadjusted concentrations by urinary creatinine(Reference Wiesmuller, Eckard and Dobler15), (c) by adding creatinine as a covariate(Reference Kim, Steuerwald and Parsons28), and (d) as the UER of each element (μg/h), computed by dividing the total mass by the time since prior void(Reference Akerstrom, Sallsten and Lundh29). Akaike information criterion values were used to assess the fit of the above-mentioned models (lower value indicates a better model fit).
A line chart was constructed to visually assess the within-individual variability in Cr, Mn, Fe, Zn and Se concentrations in spot samples during the study period. Mixed random-effect models were utilised to estimate the between-individual, within-individual inter-day and within-individual intra-day variances in spot samples. For the first-morning and 24-h specimens, only the between-individual and within-individual variances were estimated. The variance apportionment of these elements was further separately reported for samples that were collected days apart (on days 0, 1, 2, 3 and 4) and months apart (on days 0, 30, 60 and 90). In addition, a stratified analysis was conducted to compare the variance apportionment for samples collected in the morning (0.00–11.59 hours), afternoon (12.00–18.00 hours) and evening (18.01–23.59 hours)(Reference Preau, Wong and Silva30). ICC, the proportion of between-individual variance and the total variance, was used to identify the degree of reproducibility. An ICC value <0·40 is regarded as poor reproducibility, 0·40–0·75 as fair to good reproducibility and ≥0·75 as excellent reproducibility(Reference Rosner31).
Mixed regression models were constructed to evaluate how well spot or first-morning specimens predicted the same-day 24-h collections. The predictive ability of the models was evaluated using coefficients of determination (R 2). Marginal R 2 and conditional R 2 were separately computed to characterise the proportion of variance explained by fixed or random effects(Reference Nakagawa and Schielzeth32).
To evaluate how well single or repeated randomly selected specimens identified the highest tertile (top 33 %) of 3-month average exposures(Reference Bradman, Kogut and Eisen33), the arithmetic mean of log-transformed Cr, Mn, Fe, Zn and Se concentrations were computed for each participant using all their spot samples gathered over 3 months; the men who were truly in the highest (top 33 %) exposure group were then identified. Afterwards, ten data sets were created, and each containing a single randomly selected spot sample (or first-morning void) from each participant; the ‘predicted’ highest (top 33 %) exposure group was also identified. The average sensitivity and specificity across ten separate data sets were reported. The analyses were replicated to evaluate whether collecting repeated specimens (two or three) from each participant improved the classification.
Statistical power
The sample size of this study was estimated using the R ‘ICC.Sample. Size-package’ based on the method developed by Zou(Reference Zou34). Assuming that the ICC of creatinine-adjusted Cr, Mn, Fe, Zn and Se were higher than 0·15, according to previously published data(Reference Smolders, Koch and Moos17,Reference Paglia, Miedico and Tarallo22) , 461 measurements would be sufficient to achieve a power of 90 % for our reliability analysis.
Results
Table 1 presents the unadjusted (μg/l), creatinine-adjusted (μg/g creatinine) and UER (μg/h) of urinary Cr, Mn, Fe, Zn and Se in three different sample types. Detection rates of Cr, Mn, Fe, Zn and Se in spot samples were 99·6 %, 99·8 %, 100 %, 100 % and 100 %, respectively. Line chart showed that creatinine-adjusted concentrations of Cr, Mn, Fe, Se and Zn changed up to two or three orders of magnitude (online Supplementary Fig. S1). However, no apparent rhythmic pattern was observed.
Table 1. Unadjusted (μg/l), creatinine-adjusted (μg/g creatinine) and urinary excretion rate (μg/h) of chromium, manganese, iron, zinc and selenium concentrations in spot samples, first-morning voids and 24-h collections from eleven men*
(Mean values, medians and interquartile ranges (IQR))

* Percentage of samples > limits of quantification for Cr, Mn, Fe, Zn and Se was 99·6, 99·8, 100, 100 and 100, respectively.
Table 2 shows the variance apportionment of Cr, Mn, Fe, Zn and Se in spot samples based on unadjusted, creatinine-adjusted, creatinine as a covariate and UER models. The lowest Akaike information criterion value was achieved with creatinine-adjusted models for Zn, creatinine as a covariate model and UER model for Se. However, the between-individual and within-individual variance components of these elements were similar based on different correction models. Given that modelling creatinine as a covariate cannot be implemented for 24-h collections and that the UER model is not practical for large population studies since sampling volume and time are required for calculation, only creatinine-adjusted concentrations were used for all consequent analyses.
Table 2. Variance apportionment of log-transformed concentrations of chromium, manganese, iron, zinc and selenium in spot samples collected from eleven men (n 529)
(Akaike information criteria (AIC); variances (σ 2) and percentages)
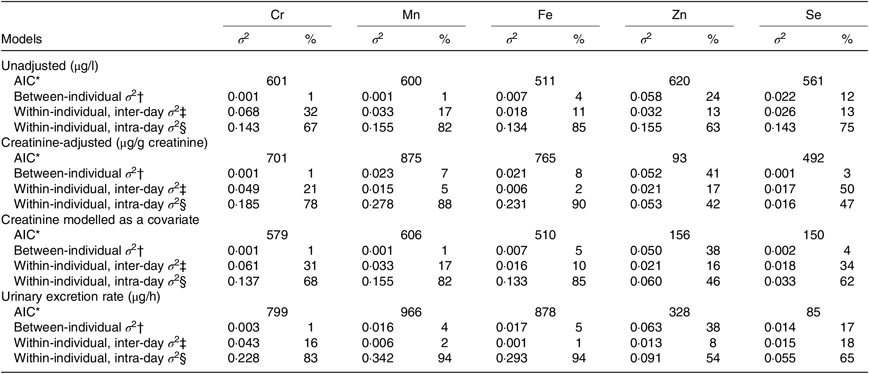
* AIC values were used to compare the model fits.
† Proportion of between-person variance to total variance.
‡ Proportion of within-person inter-day variance to total variance.
§ Proportion of within-person intra-day variance to total variance.
Table 3 shows the variance apportionment of creatinine-adjusted Cr, Mn, Fe, Zn and Se concentrations in three different sample types. Fair to good reproducibility was obtained for serial measurements of Zn (creatinine-adjusted ICC = 0·41) in spot samples over 3 months, whereas Cr, Mn, Fe and Se showed poor reproducibility (creatinine-adjusted ICC = 0·01–0·08). For Cr, Mn, Fe and Zn, the within-individual intra-day variance was the largest component of the total variance (range: 42–90 %). For Se, however, the within-individual inter-day variance was predominant (50 %). Compared with spot samples, apparent increases in ICC were obtained for Mn and Fe in first-morning voids, and Mn, Fe and Zn in 24-h collections. However, the within-individual variance remained larger than the between-individual variance, except for Zn in 24-h collection. The results were largely unchanged when additionally including ‘time interval since the prior urination’ as a covariate (n 529; data not shown).
Table 3. Variance of log-transformed creatinine-adjusted chromium, manganese, iron, zinc and selenium concentrations in three sample types*
(Intraclass correlation coefficients (ICC); variances (σ 2) and percentages)
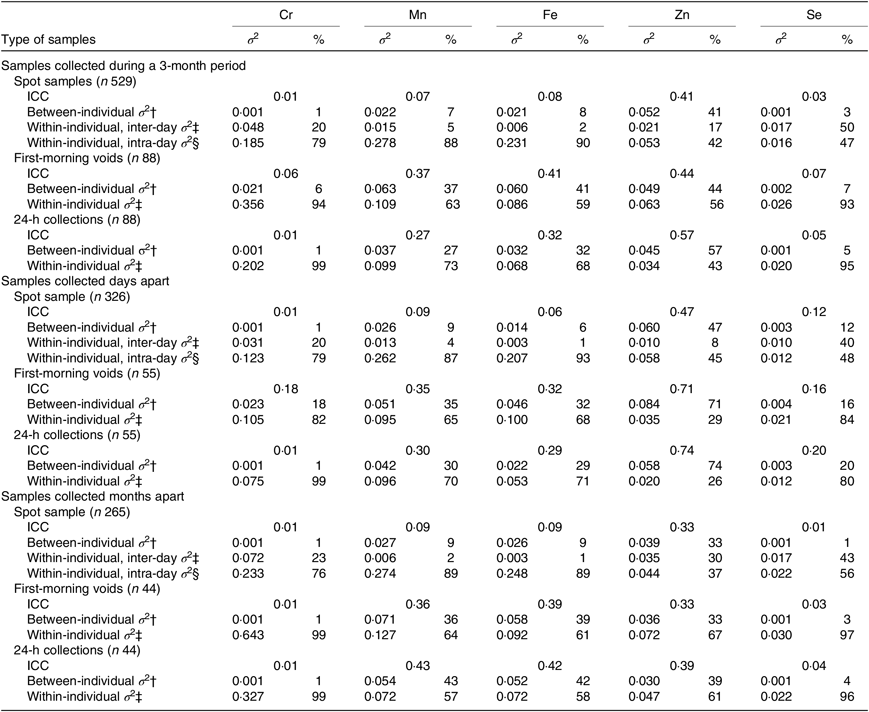
* Days apart: days 0, 1, 2, 3, and 4; months apart: days 0, 30, 60, and 90.
† Proportion of between-individual variance to total variance.
‡ Proportion of within-individual inter-day variance to total variance.
§ Proportion of within-individual intra-day variance to total variance.
Stratified analyses showed that the reproducibility of serial measurements of Zn in spot samples collected days apart was fair to good (creatinine-adjusted ICC = 0·47), which became poor when the samples were gathered months apart (creatinine-adjusted ICC = 0·33). Poor reproducibility ranging from 0·01 to 0·12 was observed for Cr, Mn, Fe and Se, irrespective of whether the spot samples were gathered days apart or months apart. Compared with spot samples, apparently higher ICC (>0·20) was obtained for Mn, Fe and Zn in first-morning and 24-h specimens gathered days apart, and Mn and Fe in first-morning and 24-h specimens gathered months apart (Table 3). The variance apportionment of Cr, Mn, Fe, Zn and Se in the samples collected at different times of the day was also estimated. The reproducibility of Cr, Mn, Fe and Se was poor irrespective of whether the samples were collected in the morning, afternoon or evening (ICC = 0·01–0·13). Fair to good reproducibility was observed for Zn in the afternoon and evening samples (ICC = 0·55 and 0·48, respectively) (online Supplementary Table S1).
Table 4 shows how well spot or first-morning specimens predicate the same-day 24-h collections. Low to moderate predictive power was achieved for Cr, Mn, Fe, Zn and Se based on spot samples (conditional R 2: 0·27–0·65). Compared with spot samples, using first-morning voids as predictors provided apparent increases in conditional R 2 for Cr (0·73 v. 0·27) and slight increases for Zn (0·81 v. 0·65) and Se (0·75 v. 0·59).
Table 4. Creatinine-adjusted models of 24-h concentrations of chromium, manganese, iron, zinc and selenium using the same-day spot samples or first-morning voids as predictors†
(β-Coefficients, 95 % confidence intervals, intercepts, marginal coefficients of determination (R 2) and conditional R 2)
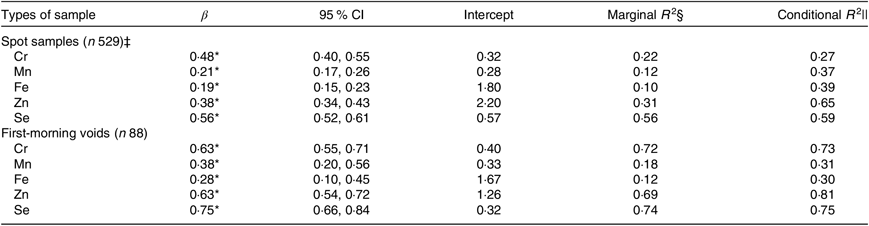
* P < 0·001.
† Spot samples and first-morning voids were analysed on a log-transformed scale.
‡ Spot samples included first-morning voids.
§ Proportion of variance explained by fixed effects.
|| Amount of variance explained by fixed and random effects.
Table 5 shows the results of sensitivity and specificity analyses. When single spot sample or first-morning void was used to identify the highest tertile of 3-month average concentrations, relatively low sensitivities in a range of 0·23–0·50 were observed for Cr, Mn, Fe, Zn and Se. Compared with a single measurement in spot samples, ≥10 % increases in sensitivities were observed for all five elements when two or three spot samples were randomly selected from each men days apart. However, moderate sensitivity was obtained only for Zn (0·67). Similarly, using two or three first-morning voids as predictors showed higher sensitivity for Cr, Mn, Fe, Zn and Se compared with a single first-morning void. Collection of specimens months apart did not offer an apparent improvement in exposure classification over the specimens collected days apart.
Table 5. Sensitivity and specificity of one, two or three urine samples as a predictor to identify the highly exposed men (top 33 %) based on their 3-month average measures*
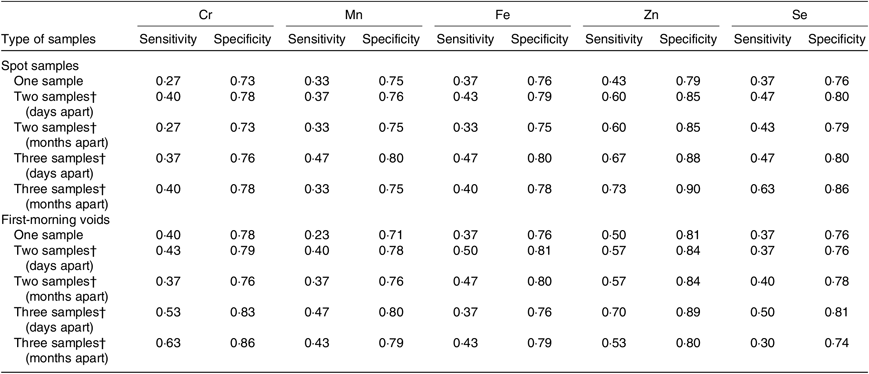
* Days apart: days 0, 1, 2, 3, and 4; months apart: days 0, 30, 60, and 90. Calculations use creatinine-adjusted Cr, Mn, Fe, Zn and Se concentrations (µg/g creatinine) on a log10 scale.
† Specimens were collected on different days.
Discussion
Among eleven non-occupationally exposed healthy adult men, high within-individual variability in the concentrations of Cr, Mn, Fe and Se was exhibited in spot samples over periods ranging from days to months (creatinine-adjusted ICC = 0·01–0·12). The reproducibility of Zn concentrations in spot samples was fair to good over five consecutive days (creatinine-adjusted ICC = 0·47), which became poor when the specimens were gathered months apart (creatinine-adjusted ICC = 0·33). Similar to the trend of ICC, our classification analysis showed that using one, two or even three spot samples to identify the highest tertile of 3-month average concentrations of Cr, Mn, Fe and Se could result in high degree of classification error (i.e. low sensitivities); by contrast, two and three randomly selected spot samples appeared to offer moderate to high sensitivities (≥ 0·6) for Zn.
Cr, Mn, Fe, Zn and Se are proposed to be essential elements that are naturally present in food and water and are also available as dietary supplements(1,Reference Sengupta35) . Additional intake may benefit individuals who have insufficient intake. However, excessive exposures have demonstrated adverse health effects both in animal and in human studies(1,Reference Sengupta35) . In this study, we found high within-individual variability in urinary concentration of Cr, Mn, Fe and Se over both short and long periods of time, which is not unexpected, given the changing exposure status due to the variation in the daily dietary consumption(Reference Aylward, Hays and Smolders19). The changing urinary flow rate that is related to salt intake and physiological status may also affect the reproducibility of these elements(Reference Aylward, Hays and Smolders19). The reproducibility of Zn concentrations in spot samples was fair to good over five consecutive days (creatinine-adjusted ICC = 0·47). Within-individual variation in biomarker concentrations is affected by various factors, especially the elimination half-life. Zn is mainly stored in muscle and bones with a half-life of 244 d(Reference Solomons36–Reference Chasapis, Loutsidou and Spiliopoulou38), which is longer than that of Cr, Mn, Fe and Se (range from <30 h to <116 d)(Reference Paustenbach, Panko and Fredrick39–Reference Friberg and Elinder41). Consistent with our findings, previous studies have conformably revealed that the within-individual variance exceeds the between-individual variance for urinary concentration of Cr, Mn, Zn and Se(Reference Smolders, Koch and Moos17,Reference Paglia, Miedico and Tarallo22) , though the reported ICC vary slightly across studies, probably because of the differences in study design (e.g. study population, sample types and sampling duration). For instance, Paglia et al. (Reference Paglia, Miedico and Tarallo22) reported a poor reproducibility for Zn in first-morning voids across seasons (creatinine-adjusted ICC = 0·37), which was comparable to our first-morning voids that were collected months apart (creatinine-adjusted ICC = 0·33) but was much lower than our samples that were collected days apart (creatinine-adjusted ICC = 0·71).
Making collections over 24 h is proposed as the ‘gold standard’ to evaluate an individuals’ daily exposures to chemicals that are excreted mainly through urine(42). In the present study, only low to moderate predictive ability (conditional R 2 0·27–0·65) was obtained when using spot samples as predictors of the same-day 24-h concentrations of Cr, Mn, Fe, Zn and Se. A first-morning void is recommended as a better sample type than spot sample since it is more concentrated and reflects accumulative exposures(Reference Bradman, Kogut and Eisen33,Reference Kissel, Curl and Kedan43) . However, compared with spot samples, using first-morning voids to predict the same-day 24-h average exposures showed an apparent advantage only for Cr (conditional R 2: 0·73 v. 0·27). Moreover, although serial measurements of Mn, Fe and Zn in first-morning voids or 24-h collections showed higher reproducibility than spot samples, their within-individual variance remained larger than the between-individual variance, suggesting that improvements in exposure estimation over weeks or months cannot offset the additional effort needed to gather these two sample types. In support of this speculation, our classification analysis revealed that a single measurement of Cr, Mn, Fe, Zn and Se in first-morning voids only correctly identified ≤50 % of the men who truly had high (top 33 %) 3-month average exposures.
Our findings highlight the importance of characterising analyte-specific patterns of variability to improve exposure estimation in epidemiological studies. In our classification analysis, we revealed a tendency similar to that of the ICC. For instance, two spot samples are sufficient to correctly classify 60 % of the men who had the highest (top 33 %) 3-month average Zn concentrations; for Cr, Mn, Fe and Se, however, at least three specimens were required to achieve a similar sensitivity. In our stratified analyses, we compared the variance apportionment for samples that were collected at different time points and found that the sensitivity and specificity of specimens collected months apart did not offer apparent improvements in exposure classification over the specimens collected days apart. The reproducibility of Cr, Mn, Fe and Se was uniformly poor irrespective of whether the samples were collected in the morning, afternoon or evening, which indicates that changing the collection time of day will not substantially improve the exposure estimation. For Zn, however, specimens collected in the afternoon and evening showed slightly higher reproducibility than that the morning samples, which means changing the collection time of day will optimise exposure assessment when multiple specimens are collected over a period of time.
The geometric mean concentrations of Cr, Mn, Fe, Zn and Se in this study were comparable with those reported among 2004 adult residents of Wuhan, China(Reference Feng, He and Chen25). However, our geometric mean urinary concentrations of Mn (1·5 μg/l) and Se (7·5 μg/l) were different from those reported for the males from the CHMS of Canada (0·078 μg/l and 57 μg/l for Mn and Se, respectively)(14) and adults from Germany (0·063 μg/l for Mn)(Reference Heitland and Koster44), implying regional variability in the extent and source of exposure. The main strength of this study was that we comprehensively assessed the within-day, between-day and between-month variability in Cr, Mn, Fe, Zn and Se concentrations in spot, first-morning and 24-h urine specimens. The impact of different concentration correction methods (i.e. creatinine-adjusted, creatinine as a covariate and UER models) on the reproducibility of these elements was also assessed. Our findings support the feasibility of using the creatinine-adjusted model to control for urinary dilution for Cr, Mn, Fe, Zn and Se in healthy adult men. Nevertheless, our study has several limitations. First, our recruited study volunteers were relatively homogeneous (young men aged between 21 and 28 years), living in a restricted district during the study period, which may have resulted in an underestimation of the between-individual variability compared with the general population who have more heterogeneous demographic characteristics (e.g. sex, and age). Second, our study population was relatively small, which limits our ability to explore the effects of various sources of uncertainties (e.g. exposure uncertainties that were related to diet and lifestyle) on the within-individual and between-individual variances. Third, duplicate aliquots were not carried out to evaluate uncertainty due to analytical factors, which may have also biased our estimation of ICC.
In conclusion, our findings revealed that serial measurements of Cr, Mn, Fe, Zn and Se in spot samples, first-morning voids and 24-h collections uniformly showed strong within-individual variability, and a single measurement is not enough to efficiently characterise individuals’ long-term exposures. Collection of repeated specimens is a feasible approach to reduce exposure misclassification. If multiple specimens were collected from each individual over a period of time, changing the collection time of day may optimise exposure assessment for Zn; whereas this may not be necessary for Cr, Mn, Fe and Se.
Acknowledgements
The authors thank all the individuals who volunteered to participate in this study as well as those who gave the technical assistance.
The project was supported by the National Key Research and Development Program of China (2017YFC0907500 and 2017YFC0907504), Hubei Province Science Fund for Distinguished Young Scholars (2018CFA033) and the National Postdoctoral Program for Innovative Talents (No. BX201700087). None of the funding sponsors was involved in the design and conduct of the study; collection, management, analysis and interpretation of the data; and preparation, review or approval of the manuscript.
H. G. C. performed the statistical analysis, contributed to the discussion, wrote the manuscript, and reviewed and edited the manuscript; Y. J. C, C. C., Z. Z. T., Q. L., P. W., J. X. C. and Y. X. W. collected the data and conducted laboratory analyses; Y. X. W. and A. P. planned and designed the study; H. G. C., Y. X. W. and A. P. have full access to the data in this study and take complete responsibility for the integrity of the data and the accuracy of the data analysis; and all authors contributed to the research conception and design or interpretation and analysis of the data, and drafted, read and approved the final manuscript.
The authors declare that there are no conflicts of interest.
Supplementary material
For supplementary material/s referred to in this article, please visit https://doi.org/10.1017/S0007114519001193








