An increasing number of children and adolescents are being diagnosed with the metabolic syndrome (MetS), a cluster of risk factors including abdominal fatness, hypertension, adverse lipid profile and insulin resistance, which may increase the later risk of lifestyle diseases( Reference Duncan, Li and Zhou 1 , Reference Eisenmann 2 ). Healthy eating practices during childhood may help prevent the development of the MetS and type 2 diabetes, CVD and mortality( 3 ).
Children from most Western populations consume too low amounts of fish, fibre, fruits and vegetables and too high amounts of sugar and saturated fat relative to dietary guidelines( 4 – 6 ). Public schools are a highly relevant setting for nutritional interventions with the potential to reach children from various socio-economic backgrounds. Danish children consume 40–45 % of their daily energy during school hours and after-school activities, mainly in the form of cold lunches and snacks brought from home( Reference Hoppe, Biltoft-Jensen and Trolle 7 ). The Danish school setting thus offers the opportunity to determine the health-promoting potential of meal provision at schools without existing school meal programmes. Most of the randomised school trials have used multi-component strategies to modify parts of the existing school menus and lunches in combination with physical activity and lifestyle education and few have measured cardiometabolic risk markers( Reference Arbeit, Johnson and Mott 8 – Reference Trevino, Yin and Hernandez 17 ). To our knowledge, no randomised controlled trial has assessed the impact of introducing a nutritionally balanced full school meal programme on the overall cardiometabolic profile.
The composition of the New Nordic Diet (NND) is in accordance with the Nordic Nutrition Recommendations and the Danish food-based guidelines( Reference Mithril, Dragsted and Meyer 18 ). It is rich in foods with strong health-promoting potential such as fish, whole grains, fibre and coarse vegetables including cabbage, root vegetables and legumes( Reference Mithril, Dragsted and Meyer 19 ). In parallel with the Mediterranean diet, consumption of such a diet has recently been reported to be associated with reduced mortality( Reference Olsen, Egeberg and Halkjaer 20 ) and improvements in CVD risk markers( Reference Adamsson, Reumark and Fredriksson 21 , Reference de Mello, Schwab and Kolehmainen 22 ) in adults, but there is a lack of randomised controlled trials in children.
Thus, in the present study, the effect of school lunch and snacks based on the NND on the MetS score (primary outcome) and on individual cardiometabolic markers and body composition (secondary outcomes) was investigated in 8–11-year-olds at nine Danish schools without existing school meal programmes.
Materials and methods
Study design and participants
The Optimal Well-Being, Development and Health for Danish Children through a Healthy New Nordic Diet (OPUS) School Meal Study is a cluster-randomised, controlled, non-blinded, cross-over trial that was carried out in children in forty-six classes at nine Danish schools (four to eight participating classes per school). For two consecutive 3-month periods, children received school meals based on the NND and their usual packed lunch from home (control) in random order. A cluster-randomised design was chosen for practical reasons and to avoid dietary contamination between peers, but the outcomes were evaluated at the individual level, taking into account the correlation within schools, year groups, classes and siblings. Children were recruited from May to October 2011, and data collection was performed from August 2011 to June 2012. A comprehensive description of the study design and recruitment has been published previously( Reference Damsgaard, Dalskov and Petersen 23 ). The study was registered at www.clinicaltrials.gov as NCT01457794.
Schools were recruited by telephone and e-mail (Fig. 1). Inclusion criteria for schools were as follows: (1) location in the eastern part of Denmark (Zealand and Lolland-Falster); (2) at least four classes at the third- and fourth-grade levels; (3) suitable kitchen facilities available for food preparation; (4) high motivation for participation as determined by the study team( Reference Damsgaard, Dalskov and Petersen 23 ). All the 1021 third- and fourth-grade children at the nine included schools were invited to participate in the study. Written information about the study was given to the parents, and oral information about the study was given to both parents and children. Exclusion criteria for children were as follows: (1) diseases or conditions that might obstruct the measurements or put the child at risk if eating the NND school meals (e.g. severe food allergies)( Reference Damsgaard, Dalskov and Petersen 23 ) and (2) concomitant participation in other scientific studies involving radiation or blood sampling. The guardians of 82 % of the invited children gave written informed consent for participation (Fig. 1). The study was conducted according to the guidelines laid down in the Declaration of Helsinki, and all procedures involving human subjects were approved by the Committees on Biomedical Research Ethics for the Capital Region of Denmark (H-1-2010-124).

Fig. 1 Flow diagram depicting the progress of schools and children through the study. NND, New Nordic Diet; MetS, metabolic syndrome.
Intervention
During the 3-month NND period, the children were served a mid-morning snack, an ad libitum hot lunch meal and an afternoon snack. The lunch meals and snacks were designed according to the NND guidelines, which are based on seasonal, local Nordic ingredients( Reference Mithril, Dragsted and Meyer 19 ). The intention was that the NND should contain less meat and more berries, cabbage, root vegetables, legumes, potatoes, wild plants, whole grains, nuts, fish and seaweed than the average Danish diet( Reference Mithril, Dragsted and Meyer 19 ). The school meals were designed to cover 40–45 % of the daily energy requirement of an 11-year-old boy( Reference Hoppe, Biltoft-Jensen and Trolle 7 , 24 ). The NND guidelines as well as the existing knowledge on the dietary intake of Danish children were taken into account when designing the school meals. Thus, for some food groups not commonly eaten by Danish children (e.g. cabbage), the recipes were aimed at covering 100 % of the dietary guidelines through the school meals, while for other more commonly eaten food groups (e.g. fruits), a lower covering percentage by the school meals was aimed for. School lunches were served buffet style, and neither total energy intake nor the intakes of specific food groups were strictly controlled. However, children were encouraged to taste everything and to keep a reasonable plate distribution where vegetables and potatoes/grains constituted most of the plate. More than one lunch serving was permitted when available, but most snacks were portioned. The weekly menu schedule consisted of a soup day, a fish day, a meat day, a vegetarian day and a buffet day( Reference Damsgaard, Dalskov and Petersen 23 ). The NND school meals were free of charge, children cooked, tasted and served the food, and the 15 min usually set aside for lunch were increased to 20–25 min. Non-participating children from the included school classes were also offered the meals and allowed to participate in the cooking sessions. The control diet consisted of the children's habitual lunch packs from home, typically consisting of cold open-faced rye bread sandwiches with meat topping and some fresh fruits, which were consumed during the usual lunch break.
Study procedures and outcomes
Baseline interview and dietary intake and physical activity measurements
At baseline, each family was given instructions on recording dietary intake and physical activity and was interviewed about demographics and socio-economic status. Pubertal status was self-evaluated by the child based on breast development in girls and pubic hair in boys (Tanner stages). As very few children were in stages 3–5, the variable was recoded to ‘did not enter puberty’ (stage 1) or ‘entered puberty’ (stages 2–5). The household educational level was defined as the level of education of the parent with the highest level in the household, and families were categorised into six groups as described by Statistics Denmark( 25 ). Children were defined as immigrants/descendants if all grandparents and one or both parents were born outside of Denmark.
Dietary intake and physical activity were measured for seven consecutive days at baseline and at 3 months and 6 months. With parental assistance, children recorded their daily intake of food and beverages using web-based dietary assessment software developed for and validated in Danish 8–11-year-olds( Reference Biltoft-Jensen, Bysted and Trolle 26 – Reference Biltoft-Jensen, Hjorth and Trolle 28 ). Portion sizes were estimated based on photos, and individual intakes were calculated with the General Intake Estimation System software (National Food Institute, Technical University of Denmark) using data from the Danish Food Composition Databank( 29 ). Dietary intake data of only children who maintained records for at least 4 d were included in the dietary intake analyses. Moreover, based on the reported energy intake and estimated BMR( Reference Henry 30 ), under-reporters (energy intake: BMR ≤ 1·05) and over-reporters (energy intake: BMR>2·29) were excluded from the dietary intake analyses( Reference Black 31 ). Physical activity was measured using ActiGraph™ tri-axis accelerometer monitors (GT3X+ or GT3X, ActiGraph, Pensacola) as described previously( Reference Hjorth, Chaput and Michaelsen 32 ). Moderate-to-vigorous physical activity (min/d) defined as ≥ 2296 vertical counts/min( Reference Trost, Loprinzi and Moore 33 ) was assessed, as this has been shown to have stronger associations with cardiometabolic risk factors( Reference Ekelund, Luan and Sherar 34 ) compared with total physical activity (counts/min) in children. The intervention did not affect physical activity( Reference Hjorth, Chaput and Michaelsen 32 ).
Clinical examinations
Clinical examinations and blood sampling were performed at baseline and at 3 and 6 months in a mobile laboratory truck parked at the schools. Except for body composition, all measurements were performed in the morning following an overnight fast: intake of one to two glasses of water was permitted. Height was measured three times to the nearest 0·1 cm using a portable stadiometer (CMS Weighing Equipment), with the children holding their heads in the Frankfort horizontal plane. The mean of three measurements was used. Body weight was measured to the nearest 0·1 kg on a digital scale (Tanita 800S; Tanita). Children wore light clothing and were asked to empty their bladders before measurement. Sex- and age-adjusted z-scores for BMI were calculated using AnthroPlus software (WHO)( 35 ). The prevalence of underweight, overweight and obesity was assessed based on age- and sex-specific cut-offs defined by centiles passing through BMI of 18·5, 25 and 30 kg/m2 at 18 years( Reference Cole, Bellizzi and Flegal 36 , Reference Cole, Flegal and Nicholls 37 ). Children's whole-body composition was measured by dual-energy X-ray absorptiometry (Lunar Prodigy; GE Medical Systems) using Encore software version 13.5. Most of the children had a standardised breakfast before the scanning. Fat mass index and fat-free mass index were calculated as follows( Reference VanItallie, Yang and Heymsfield 38 ):
The standard settings of the scanner were used for calculating abdominal fat mass. Abdominal fat mass was defined as the amount of truncal fat in kg within the lower 20 % of the area between the iliac crest and the lower edge of the mandible, and android fat mass:total fat mass ratio was calculated. Blood pressure and heart rate were measured with an automated device (UA-787 Plus; A&D Medical) using two different cuff sizes (18–22 and 22–32 cm) after a 10 min rest. Another device (ProBP 3400 Sure BP; Welch Allyn, Inc.) with three different cuff sizes (12–16, 15–21, and 20–26 cm) was used for children with arm circumferences < 22 cm. The same device was used for each child at all the three examinations. Measurements were performed three times, and the mean of the last two measurements was used. Mean arterial pressure was calculated as 1/3 × systolic blood pressure+2/3 × diastolic blood pressure.
Blood sampling and blood analyses
A 35 ml fasting venous blood sample was drawn from the forearm of each child. Local anaesthetic patches (EMLA; AstraZeneca) were provided to the families before the visit. Whole-blood glucose concentrations were determined immediately after sampling using a Glucose 201 analyser (Hemocue Danmark). Heparinised blood and blood with EDTA were centrifuged at 2500 g for 10 min at room temperature, and aliquoted plasma was stored at − 80°C for batch analysis of cholesterols, TAG and C-reactive protein (CRP) and of IL-6 and total adiponectin. Blood collected in serum tubes with gel was centrifuged after 30 min of coagulation at room temperature, and aliquoted serum was stored at − 80°C for batch analysis of insulin. Plasma total cholesterol and HDL-cholesterol, TAG and CRP (high-sensitive) were quantified on the Vitros 5.1 FS analyser (Ortho-Clinical Diagnostics) with a lower CRP detection limit of 0·1 mg/l. LDL-cholesterol was quantified using Friedewald's equation( Reference Friedewald, Levy and Fredrickson 39 ). Serum insulin was quantified on the ADVIA Centaur XP device (Siemens Healthcare), and homeostasis model of assessment-insulin resistance (HOMA-IR) was calculated as follows:
Insulin concentrations were converted from pmol/l to mIU/l by dividing by 6·945. Plasma IL-6 (high-sensitive) and adiponectin were quantified in duplicate using ELISA (R&D Systems). A total of thirty-five children were excluded from the CRP and IL-6 analyses as they had CRP concentrations >10 mg/l, indicating acute inflammation. The inter-assay and intra-assay CV were as follows: 1·4 and 1·2 % (total cholesterol); 2·0 and 1·2 % (HDL-cholesterol); 1·5 and 0·8 % (TAG); 2·5 and 3·1 % (insulin); 1·3 and 0·8 % (CRP); 6·7 and 2·9 % (IL-6); 11 and 3·8 % (adiponectin). The inter-assay variation was 4·0 % for glucose.
Primary outcome
The OPUS School Meal Study had two separate primary outcomes in two scientific fields: (1) the continuous MetS score reported herein and (2) concentration performance, to be reported separately( Reference Damsgaard, Dalskov and Petersen 23 ). The MetS score was obtained by combining the z-scores derived from separate analyses of the following five outcomes: mean arterial pressure; plasma HDL-cholesterol concentrations; plasma TAG concentrations; HOMA-IR; waist circumference. This was based on the International Diabetes Federation's definition of the MetS for children aged ≥ 10 years( Reference Zimmet, Alberti and Kaufman 40 ), although HOMA-IR rather than blood glucose concentration was included because the latter is highly stable in non-diabetic children, even among obese adolescents with MetS features( Reference Gobel, Jensen and Frokiaer 41 ).
Sample size
Power calculations were conservative and based on a linear mixed model as described previously( Reference Damsgaard, Dalskov and Petersen 23 ). The resulting standard error of the estimated difference between intervention and control periods was used for sample size calculation as in a two-sample t test, with 80 % power and a 5 % significance level. Assuming nine schools with four classes per year group, twenty-two children per class, and an intra-child correlation coefficient of 0·5 resulted in a detectable difference of 0·11 sd in the primary outcome. The pilot study showed a sd of 2·943 in the MetS score( Reference Damsgaard, Dalskov and Petersen 23 ), corresponding to a 0·32 (0·11 × 2·943) difference in the MetS score between NND and control periods, which translates into, for example, a 2 % reduction in waist circumference in combination with a 0·05 mmol/l increase in HDL-cholesterol concentrations, assuming no differences in mean arterial pressure, TAG concentrations or HOMA-IR. These differences were judged to be attainable and relevant and, in addition, inclusion of nine schools was considered to be logistically manageable. Accordingly, a total of 673 children would have to complete the study. Allowing for an estimated 75 % inclusion rate and 15 % dropout, about 1055 children had to be invited to participate in the study.
Randomisation
Cluster randomisation by year group within schools was employed as the unit of randomisation because school activities are typically co-ordinated by year group in Denmark. First, schools were randomly assigned to two blocks of five and four schools, respectively, to minimise seasonal effects as the schools needed to be visited sequentially. Then, within each block, a simple randomisation was applied to the schools to the allocation order of NND and control for the year groups. Randomisation was done by a statistician not involved in data collection or analysis and, for logistical reasons, before the children were invited to participate in the study (Fig. 1). Investigators, schools and participants were not blinded to the allocation order.
Statistical analysis
The primary outcome was defined using residuals from separate linear mixed models fitted to the five secondary outcomes that were included in the primary outcome; standardisation was carried out as in ordinary regression using the leverage for each observation( Reference Demidenko and Stukel 42 ). Analysis of the primary outcome was based on an ANCOVA-type linear mixed model with the intervention as the fixed effect and school, year group within school, class, sibling and child as the random effects, but without any additional adjustment. Apart from that at baseline, most children had two repeated measurements over time and some children also had their siblings participating. This clustering as well as the nesting of children in classes, classes in year groups and year groups in schools necessitated the inclusion of child, sibling, class, year group and school as the random effects( Reference Carlin, Gurrin and Sterne 43 ).
Dietary intake and secondary outcomes were analysed using ANCOVA-type hierarchical linear mixed models with the same random effects that were included for the primary outcome. CRP was analysed using a linear mixed model for left-censored data and fish intake was analysed using a linear mixed-effects hurdle model( Reference Min and Agresti 44 ), with child being the only random effect in both analyses. The hurdle model resulted in an unconditional analysis of intake (yes/no) and a separate analysis of the amount consumed by those children reporting fish intake. As large units of randomisation (year group within school) were used, the randomisation was not expected to level out all differences at the class or child level, and therefore all analyses were adjusted for sex, visit (3 or 6 months), allocation order of the NND and control interventions baseline value for the variable of interest and baseline age. Height at all the three time points was included as a covariate, except in the analyses of dietary intake, height, BMI z-score, fat mass index and fat-free mass index. Analyses of blood pressure and heart rate also included the blood pressure device (small/large) used. Pearson's correlation coefficients were used to evaluate dependencies between secondary outcomes.
A conservative analysis strategy based on the intention-to-treat concept was used. Multiple imputation of missing outcome values was performed using the corresponding available-case linear mixed models. In case both outcome values (at 3 and 6 months) were missing, imputation was based on only predicted values using the fixed-effects estimates. In case only one outcome value was missing (at 3 or 6 months), the imputed values were obtained using both estimated fixed-effects estimates and predicted random-effects estimates at all levels. Missing baseline values were imputed using joint modelling of baseline and outcome values using a linear mixed model approach( Reference White and Thompson 45 ) including age, sex, and allocation order of the NND and control interventions as well as the random effects stated above. Missing values for height were imputed using linear regression including age, sex and baseline height.
Sensitivity analyses based on the complete-case population and analyses adjusted for time spent on moderate-to-vigorous activity at baseline, household educational level and pubertal status were carried out to assess whether the effects of the intervention may have been confounded by these factors. Furthermore, it was investigated whether the estimated differences between NND and control periods depended on the BMI of the children at baseline by including in the models the effect modification of linear and quadratic terms in baseline BMI. This was done for the primary outcome and for those secondary outcomes that were significantly affected by the NND school meals in complete-case analyses. The complete-case population included all children with the outcome value of interest and covariate information measured at baseline and at 3 and 6 months. Baseline comparisons of boys v. girls and of completers v. non-completers were performed using two-sample t tests and χ2 tests.
Model checking was based on visual inspection of residual and normal probability plots. Logarithm, square-root and log–log transformations were used as appropriate. Power calculations, randomisation and statistical analysis were performed using R (R Development Core Team). In particular, the extension packages lme4, HLMdiag and multcomp were used for the mixed-model analyses. Customised R scripts were written to fit the hurdle model.
Results
A total of 834 children, including twenty-eight pairs of siblings, were allocated to receive the NND school meals and control diet (Fig. 1). During the study, sixty-nine children (8·3 %) withdrew, mainly due to change of schools or class (n 29), dislike of or too time-consuming measurements (n 15), or dislike of the NND school meals (n 13) (Fig. 1). Of the 834 children, eleven withdrew before the first clinical examination. Thus, intention-to-treat analyses were based on the 823 children (98·7 %) who had some clinical data available from at least one time point (Fig. 1). A total of 631 children (76·7 % of the intention-to-treat population) who had the MetS markers measured at baseline and at 3 and 6 months constituted the complete-case population for the primary outcome. The difference (n 192) between the size of the intention-to-treat population and that of the complete-case population was mainly due to cases with insufficient blood sample volumes, who refused blood sampling or withdrew from the study.
At baseline, most of the children were of normal weight, 14 % were overweight/obese and 10 % were underweight (Table 1). About one-third of the children had entered puberty and, as expected, this proportion was higher among girls than among boys (46 % compared with 23 %; P< 0·001). Non-completers did not differ from completers with regard to sex or year group distribution, age, anthropometry or pubertal stage, but were less likely to be of high educational background (P= 0·001) and more likely to be immigrants/descendants (15 % compared with 10 %; P= 0·02). Data from 162 of the 646 children who had recorded their dietary intake for at least 4 d at baseline and at 3 and 6 months were excluded due to unreliable energy intakes (130 energy under-reporters and thirty-two energy over-reporters). The remaining 484 children had higher intakes of protein, fibre, vegetables and fish and a lower intake of fat (mainly saturated) during the NND period than during the control period (Table 2).
Table 1 Baseline characteristics of the total study population (Mean values and standard deviations, n 795–823)
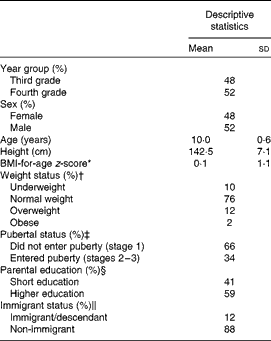
* As defined by the WHO( 35 ).
† Based on the definitions proposed by Cole et al. ( Reference Cole, Bellizzi and Flegal 36 , Reference Cole, Flegal and Nicholls 37 ).
‡ Based on self-evaluated Tanner stages.
§ Based on the highest educational level achieved in the household. Short education includes upper and lower secondary education and vocational education; higher education includes short higher education, bachelor's degree and higher.
∥ Children defined as immigrants/descendants if all grandparents and one or both parents were born outside of Denmark.
Table 2 Dietary intakes at baseline and after the control and New Nordic Diet (NND) periods and differences between NND and control periods in children with valid dietary recordings from baseline and 3 and 6 months (Medians and interquartile ranges (IQR); mean values and 95 % confidence intervals, n 484)

* Adjusted for visit, allocation order of the NND and control interventions and sex (as fixed effects); baseline values and baseline age (as covariates); and school, class, year group within school, sibling and child (as random effects), n 484.
† Percentage point increase in fish consumers.
‡ n 460 as children reporting no fish intake at both 3 and 6 months were not included in this analysis.
The NND school meals did not affect the MetS score compared with the control diet (Table 3). Among the secondary outcomes, diastolic blood pressure, mean arterial pressure, plasma total cholesterol and HDL-cholesterol concentrations, plasma TAG concentrations and HOMA-IR were slightly lower after the NND period, whereas waist circumference and android fat mass:total fat mass ratio were slightly higher than those recorded after the control period. Total cholesterol:HDL-cholesterol ratio, plasma glucose, CRP, IL-6, and adiponectin concentrations, BMI z-scores and other measures of body composition did not differ during the NND and control periods (Table 3 and data not shown). Overall, complete-case analyses confirmed these results, although small reductions in heart rate and increases in fat mass index were found to be significant in these analyses (online supplementary Table S1). Further adjustment for physical activity, pubertal status and household educational level did not alter the results (data not shown), and none of the differences between NND and control periods were dependent on the BMI of the children at baseline (online supplementary Table S2). As expected, HOMA-IR and waist circumference were positively correlated at baseline (r 0·44, P< 0·001). The other cardiometabolic markers included in the MetS score were positively correlated with HOMA-IR (r 0·27 to 0·44, all P< 0·001) and waist circumference (r 0·22 to 0·23, all P< 0·001), except for HDL-cholesterol concentration, which was negatively correlated with HOMA-IR and waist circumference (both r − 0·21, P< 0·01).
Table 3 Metabolic syndrome score, cardiometabolic markers, anthropometry and body composition at baseline and after the control and New Nordic Diet (NND) periods and differences between NND and control periods(Mean values and standard deviations; medians and interquartile ranges (IQR); mean values and 95 % confidence intervals)

MetS, metabolic syndrome; HOMA-IR, homeostasis model of assessment-insulin resistance.
* Adjusted for visit, allocation order of the NND and control interventions, sex, baseline values, baseline age, height if appropriate and also school, class, year group within school, sibling and child as the random effects, n 823. Blood pressure and heart rate were also adjusted for the blood pressure device used.
† The MetS score was based on mean arterial pressure, plasma HDL-cholesterol concentrations, plasma TAG concentrations, HOMA-IR and waist circumference. As baseline values were included in the calculation of the score, it had no value at baseline.
Discussion
The results of the present study showed that the introduction of a full school meal programme rich in fish, vegetables and dietary fibre and low in total and saturated fat did not improve the primary outcome, the MetS score, in a population of mainly normal-weight Danish children. The NND school meals improved the secondary outcomes blood pressure, plasma TAG concentrations, insulin resistance and total cholesterol concentrations, but reduced HDL-cholesterol concentrations and increased waist circumference, which was also reflected in the body fat distribution. The results of the intention-to-treat analyses were confirmed by complete-case analyses and by adjusted analyses, indicating that the findings are related to the school meals and not confounded by physical activity or pubertal development.
To our knowledge, no previous randomised trial has investigated the impact of diet on MetS scores in children. However, some randomised school meal intervention trials have investigated the effects of diet on the cardiometabolic markers included in the primary outcome of the present study. In contrast with the present study, these trials were mostly designed for obesity prevention and based on modest dietary modifications of existing school menus, typically aimed at reducing the intakes of fat and salt and increasing the intakes of fruits, vegetables and/or fibre combined with lifestyle education and physical activity intervention( Reference Arbeit, Johnson and Mott 8 – Reference Trevino, Yin and Hernandez 17 ), and the results are mixed. In the school-based randomised trial conducted by Arbeit et al. ( Reference Arbeit, Johnson and Mott 8 ) (Heart Smart), no effects were found on anthropometry or blood pressure, but an increase in HDL-cholesterol concentrations was observed. In another randomised trial in which the total fat intake from school lunches was reduced, no changes in body size, cholesterol concentrations or blood pressure were found among 3714 children after 3 years of intervention( Reference Luepker, Perry and McKinlay 9 ). In contrast with this and with the findings of the present study, Hollar et al. ( Reference Hollar, Lombardo and Lopez-Mitnik 14 ) showed reductions in BMI z-score, but no effects on blood pressure after 2 years of intervention. Improvements in blood glucose or insulin concentrations were reported by three relatively new, large school-based randomised nutrition trials( Reference Foster, Linder and Baranowski 15 – Reference Trevino, Yin and Hernandez 17 ), and indices of body fatness were also found to be reduced in two of these trials( Reference Foster, Linder and Baranowski 15 , Reference Singhal, Misra and Shah 16 ). However, all these studies were multi-component trials and included a physical activity intervention, which could have been responsible for the effects. A cross-sectional study carried out in Australian 14-year-olds found that a habitual ‘healthy dietary pattern’ characterised by high intakes of fruits, vegetables, fish and legumes is inversely associated with fasting glucose concentrations and positively associated with HDL-cholesterol concentrations in only boys( Reference Ambrosini, Huang and Mori 46 ).
The small improvements in three of the outcomes included in the MetS score were counterbalanced by the small increase in waist circumference and reduction in HDL-cholesterol concentrations, resulting in no effect on the MetS score. However, although it has been demonstrated that children diagnosed with the MetS in childhood are more likely to have the MetS as adults( Reference Morrison, Friedman and Wang 47 ), the long-term clinical significance of MetS scores in relatively healthy, mainly normal-weight children has, to our knowledge, not been investigated. The Bogalusa Heart Study( Reference Webber, Srinivasan and Wattigney 48 ) and the Cardiovascular Risk in Young Finns Study( Reference Juhola, Magnussen and Viikari 49 ) demonstrated that blood lipid profile and blood pressure exhibit tracking from childhood and adolescence into adulthood and that in adults insulin resistance, blood pressure and TAG concentrations are positively associated with CVD mortality( Reference Eschwege, Richard and Thibult 50 – Reference Criqui, Heiss and Cohn 52 ). Cook et al. ( Reference Cook, Cohen and Hebert 53 ) has estimated that a 2 mmHg reduction in diastolic blood pressure in the adult population would result in a 15 % reduction in stroke incidence. Based on this indirect evidence and viewed from a population perspective, a 0·4 mmHg reduction in blood pressure or a 0·02 mmol/l reduction in fasting TAG concentrations in children may have implications for long-term cardiometabolic risk, if it is sustained over years.
In the present study, the NND school meals were found to reduce HDL-cholesterol concentration rather than increasing it, but the total cholesterol:HDL-cholesterol ratio, which has been suggested to be strongly associated with IHD in adults( Reference Lewington, Whitlock and Clarke 54 ), remained unaffected. Thus, whether the slightly reduced HDL concentration has negative implications for longer-term cardiometabolic health is unclear. Furthermore, although the results of the present study do not indicate that children with a higher BMI gained more abdominal fat than their thinner peers, the increase in waist circumference might be detrimental in the longer term, as waist circumference has been shown to be a good predictor of visceral adipose tissue in children( Reference Brambilla, Bedogni and Moreno 55 ) and associated with CHD in adults( Reference Canoy, Boekholdt and Wareham 56 ).
The small effects on the individual cardiometabolic outcomes probably reflect that the dietary changes were small. As a whole-diet intervention was used, disentanglement of the individual effects of each food is difficult, and the results probably reflect a combined effect of subtle changes in the intakes of fish, rapeseed oil, nuts and coarse vegetables as well as a reduction in total fat intake. However, based on existing evidence, it is speculated that mainly the slightly increased intake of fish, contributing n-3 long-chain PUFA (LCPUFA), and the reduction in total fat intake may have been responsible for some of the effects. In the Dietary Intervention Study in Children including 8–10-year-olds with elevated LDL-cholesterol concentrations, a lowering of total fat intake was found to evoke reductions in total cholesterol, LDL-cholesterol and HDL-cholesterol concentrations corresponding to 0·04, 0·03 and 0·01 mmol/l per energy% fat( Reference Lauer, Obarzanek and Hunsberger 57 ), i.e. comparable to the reductions observed in the present study. The school meals contained a mixture of lean and fatty fish. Assuming a mean content of 1 g of n-3 LCPUFA per 100 g fish in the school meals, a 10 g/d increase in fish intake concomitantly with a reduction in blood pressure of 0·4 mmHg would translate into about 4 mmHg for 1 g of n-3 LCPUFA per d. In accordance with this, we have previously shown a blood pressure reduction of about 3–4 mmHg for 1 g of n-3 LCPUFA per d in slightly overweight teenage boys who were supplemented with fish oil( Reference Pedersen, Molgaard and Hellgren 58 ). Also, according to Harris( Reference Harris 59 ), reductions in TAG concentrations of about 25 % can be achieved with 3·5–3·9 g of n-3 LCPUFA per d. This would correspond to a 6–7 % reduction for 1 g of n-3 LCPUFA per d. In the present study, TAG concentrations were lowered approximately 3 % during the NND period compared with the control period, which would translate into about a 30 % reduction for 1 g of n-3 LCPUFA per d, i.e. a much larger effect. This may be a result of the combined effects of not only n-3 LCPUFA from fish, but potentially also small increases in the intakes of rapeseed oil, nuts and other healthy foods. These potential food- and nutrient-specific effects of the school meals should be investigated further.
The health-promoting potential of this full school meal programme might have been greater in groups of children with less beneficial risk profiles and consuming less healthy habitual diets, but this would require that actions be taken so as to avoid the increase in waist circumference. Furthermore, although the results indicate no overall clinical benefits of the intervention, the improved blood pressure, insulin resistance and plasma TAG concentrations despite small increases in relation to abdominal fat mass are surprising and may indicate that the metabolic disadvantages in relation to waist circumference and HDL-cholesterol concentrations were outweighed by other metabolically protective effects of this dietary regimen.
The dietary records showed no differences in energy intake between NND and control periods and thus provided no explanation for the slight increase in waist circumference. As the study population was mainly normal weight, included 10 % underweight children and was not focused on overweight and obesity, specific restrictions were not imposed on the eating of, for example, overweight children. Still, the dietary records may have failed to capture minor differences in energy intake. Thus, despite thorough measurements of energy intake and physical activity, it is not known whether the small increases in waist circumference and abdominal fat were due to higher intake during school hours or at home or due to small differences in physical activity, which could also have contributed to the reduction in HDL-cholesterol concentrations.
The results of the present study are strengthened by a considerable sample size and a high participation rate (82 %) compared with the 59–74 % in other school intervention trials( Reference Luepker, Perry and McKinlay 9 , Reference Foster, Linder and Baranowski 15 , Reference Trevino, Yin and Hernandez 17 ). The baseline diet of the children in the present study was in line with the representative Danish National Dietary Surveys( 4 ), and the prevalence of overweight and obesity corresponded to the level found in a recent Danish cohort( Reference Pearson, Hansen and Sorensen 60 ). All educational groups were represented, and the proportion of immigrants/descendants matched the numbers in the total Danish population( 61 ). This indicates that the study population was highly representative of Danish children. Furthermore, the study had a very low dropout rate, mainly due to reasons not related to the study and the completion rate of 76 % for the primary outcome for all the three visits was in line with the 72–86 % reported in earlier trials( Reference Luepker, Perry and McKinlay 9 , Reference Foster, Linder and Baranowski 15 , Reference Trevino, Yin and Hernandez 17 ). The observation that dropouts were more likely to be from households with low educational background compared with children who completed the study might reflect lower personal resources, problems in understanding the study instructions or lesser familiarity with the NND school meals( Reference Damsgaard, Dalskov and Petersen 23 ). However, dropouts did not differ with regard to age, sex, ethnicity or anthropometry( Reference Damsgaard, Dalskov and Petersen 23 ), which reduces the likelihood of bias.
The present study is limited by its relatively short intervention period; therefore, the potential long-term effects of adherence to and effects of diet on cardiometabolic health are unknown. Assessment of dietary intake is difficult, especially in children, and the presented dietary data may not be extrapolated to the total study population. However, the registration tool that was used was developed for and validated specifically in this age group. These validations showed that the overall energy intake was underestimated by < 1 % compared with the total energy expenditure measured by 7 d accelerometers( Reference Biltoft-Jensen, Hjorth and Trolle 28 ) and that the children's reported intakes of fruits, juices and vegetables correlated with plasma carotenoid concentrations (r 0·58, P< 0·01)( Reference Biltoft-Jensen, Bysted and Trolle 26 ). Full description and discussion of the effect of the intervention on dietary intake have been published previously( Reference Andersen, Biltoft-Jensen and Christensen 62 ). Although the children were encouraged to taste everything that was served and guided regarding a desirable distribution of the different food groups on the plate, the lunch meals were served ad libitum. Therefore, although the school meals lived up to the NND guidelines, the children's intakes during school hours may not have done so. Moreover, the intervention was designed to influence only about 40–45 % of the children's daily intake for 5 of the 7 d, so large effects on total intake and consequently on cardiometabolic markers may not be expected.
In conclusion, the results of the present study showed that the provision of school meals rich in fish, vegetables and fibre for 3 months did not affect the MetS score in 8–11-year-old Danish children. The school meals resulted in small improvements in the secondary outcomes blood pressure, plasma TAG concentrations and insulin resistance and in also small, undesirable changes in HDL-cholesterol concentrations and waist circumference. In future trials and school meal programmes, actions such as portion size restriction should be taken so as to avoid unnecessary fat gain. Moreover, the food- and nutrient-specific mechanisms behind the opposing effects on the cardiometabolic markers as well as the potential long-term effects of school meal interventions on cooking skills and preferences for healthy foods should be explored further.
Supplementary material
To view supplementary material for this article, please visit http://dx.doi.org/10.1017/S0007114514003043
Acknowledgements
The authors thank the participating children, their families, the school managements, teachers and other staff as well as the entire study team for their contribution to the study.
The OPUS study was supported by the Nordea Foundation (grant no. 02-2010-0389). Danæg A/S, Naturmælk, Lantmännen A/S, Skærtoft Mølle A/S, Kartoffelpartnerskabet, AkzoNobel Danmark, Gloria Mundi and Rose Poultry A/S provided foods in kind for the study. The Nordea Foundation and the food sponsors had no role in the design and analysis of the study or in the writing of this article.
The authors’ contributions are as follows: C. T. D. and S. D. designed and conducted the research and wrote the article; R. P. L. conducted the research and performed the statistical analysis; C. R. supervised the statistical analysis and wrote the ‘Statistical analysis’ section; M. F. H. conducted the research and processed the physical activity data; L. L. helped write the article; L. B. S. and R. A. P. designed and conducted the research; M. R. A. and S. S. were responsible for the blood analyses; R. A. conducted the research and processed the dietary intake data; I. T. designed the research and was responsible for the dietary intake data; C. M., A. A. and K. F. M. designed the research and supervised the data collection and manuscript writing; C. T. D. had primary responsibility for the final content. All authors read and approved the final version of the manuscript.
A. A. has received royalties from the sale of New Nordic Diet cookbooks from FDB/Coop. C. T. D., S.-M. D., R. P. L., C. R., M. F. H., L. L., L. B. S., R. A. P., M. R. A., S. S., R. A., I. T., C. M. and K. F. M. declare no conflicts of interest.






