Globally, it is estimated that one in four people (~2 billion) lack the essential micronutrients to grow, develop and maintain health and function(1). Micronutrients have fundamental roles in fetal weight gain, brain development, immune regulation and musculoskeletal growth and are central modulators of multi-system and organ development(2). Although micronutrient deficiencies are widespread in low-middle income countries, they are also prevalent in high-resource settings; a phenomenon termed ‘hidden hunger’(3). Due to their high requirements for vitamins and minerals, young women and children are particularly vulnerable to micronutrient malnutrition(1). Awareness of the importance of a woman's health and nutritional status prior to pregnancy is low and there is considerable scope to improve preconception health at a population level(Reference Stephenson, Heslehurst and Hall4). However, progress in resolving the burden of micronutrient malnutrition is slow, and as nutrient deficiencies are underlying factors in the development of both acute and chronic illnesses, failure to address vitamin and mineral deficiencies obstructs achievement of global development goals for health, education and social equity(Reference Högler, Aguiar and Kiely5).
The present paper focuses on three nutrients that have been identified as concerning during pregnancy: iron, iodine and vitamin D. As they share several risk factors and impact in different ways on overlapping outcomes, particularly neurological development, it is appropriate to consider how deficiency of these nutrients can have profound impacts on pregnancy outcome and fetal and infant development, health and life chances. Often discussed, micronutrient deficiency among young women prior to and during pregnancy is essentially neglected in terms of programmatic research support, policy development and implementation of measures to prevent low intakes and status.
The aim of the present paper, arising from a presentation by the first author at the Nutrition Society Winter Meeting of 2020, is to review evidence for malnutrition during pregnancy of iron, iodine and vitamin D, including non-nutritional and nutritional risk factors and health outcomes associated with deficiency. Emerging data on neurological development and challenges in defining nutritional status, linked to validated health outcomes, are discussed. The present paper is not a systematic review, but an evaluation of available evidence for the purposes of public health nutrition and clinical practice and to identify knowledge gaps.
Iron
Iron deficiency is the most common micronutrient deficiency in the world and pregnant women are vulnerable(Reference McLean, Cogswell and Egli6). Almost 1000 mg of iron must be acquired during pregnancy to support an increased maternal plasma and blood volume, the increased oxygen and iron demands of the fetus and the iron requirements of the placenta itself(Reference Fisher and Nemeth7). Worldwide, up to 38 % of pregnant women have anaemia, with an even higher prevalence in low-resource settings(8). In Europe, iron deficiency is reported in 28–85 % of pregnancies, particularly in unsupplemented women, and up to a third have iron deficiency anaemia(Reference Milman, Taylor and Merkel9).
Risk factors for iron deficiency during pregnancy
Dietary risk factors
To avoid iron deficiency while pregnant, women must enter pregnancy with sufficiently large iron stores and consume a diet abundant in bioavailable iron(Reference Lynch, Pfeiffer and Georgieff10). Unfortunately, this is not always achievable. In Europe, median serum ferritin concentrations of women of reproductive age range from 26 to 38 μg/l, suggesting that 40–50 % have depleted iron stores (indicated by ferritin ≤30 μg/l, below which no stainable bone marrow haemosiderin iron is observed) before becoming pregnant(Reference Milman, Taylor and Merkel9). To further compound this, inadequate iron intakes and poor compliance with dietary guidelines are widely reported amongst pregnant women worldwide(Reference Livock, Anderson and Lewis11–Reference Caut, Leach and Steel13); recent European data suggest that 60–100 % of women during pregnancy had iron intakes below the recommended intake(Reference Milman14). Pregnant women with poorly managed vegetarian and vegan diets(Reference Piccoli, Clari and Vigotti15) and pregnant adolescents(Reference Marvin-Dowle, Burley and Soltani16) are at particular risk.
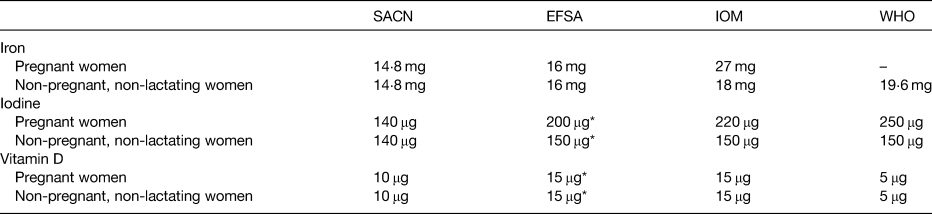
The dietary reference values for pregnant and non-pregnant, non-lactating women are presented in Table 1, with significant variability between agencies. Recommended intakes are the same for pregnant women as for non-pregnant, non-lactating women from the UK's Scientific Advisory Committee on Nutrition(17) and European Food Safety Authority(18), based on evidence that iron absorption, particularly of non-haem iron, becomes more efficient during pregnancy(Reference Barrett, Whittaker and Williams19,Reference Whittaker, Lind and Williams20) . In contrast, the US Institute of Medicine recommend higher intakes of 27 mg/d, to be met in part by supplementation, on the basis that the increased requirements of pregnancy cannot be met by diet or the body iron stores of the mother(21).
Table 1. Dietary reference values for iron, iodine and vitamin D in pregnant and non-pregnant, non-lactating women
Non-dietary risk factors
Several common pregnancy-related conditions and maternal lifestyle factors can affect maternal-fetal iron supply in utero, resulting in an increased risk of iron deficiency in the mother, her fetus or most likely both, even if dietary supply is adequate. Relative to first-time and singleton pregnancies, women who have had several pregnancies or expecting more than one baby are at increased risk of iron deficiency during pregnancy(Reference Farooq, Rauf and Hassan22,Reference Ru, Pressman and Cooper23) . Iron deficiency can also occur in the mother and fetus as a result of conditions that decrease fetal iron delivery and/or increase fetal iron demand beyond the transport capacity of the placenta(Reference Rao and Georgieff24). Gestational diabetes mellitus, hypertension and maternal smoking all induce intrauterine fetal hypoxia, limiting the oxygen supply to the fetus, resulting in increased erythropoiesis, exceeding the system's capacity and increasing the risk of iron deficiency(Reference Sweet, Savage and Tubman25–Reference Chełchowska, Ambroszkiewicz and Gajewska27). Worryingly, these conditions, particularly gestational diabetes mellitus, have been shown to have lasting consequences for the newborn infant and their development(Reference Petry, Eaton and Wobken28,Reference Siddappa, Georgieff and Wewerka29) .
Obesity in women who enter pregnancy is associated with adverse health consequences for both mother and infant(Reference Poston, Caleyachetty and Cnattingius30). However, until recently, the impact of maternal obesity on maternal and fetal iron status has been widely unacknowledged. Poorer iron status and an increased risk of iron deficiency have been reported in pregnant women who were obese prior to or during pregnancy(Reference Jones, Zhao and Jiang31–Reference Cao, Pressman and Cooper34). Although obese women are likely to have different dietary profiles to non-obese women, maternal obesity is thought to result in iron deficiency in both the mother and her infant primarily through the action of the iron regulatory hormone, hepcidin. Hepcidin controls the levels of circulating iron in the body through its influence on intestinal iron absorption and iron sequestration(Reference Fisher and Nemeth7). In healthy pregnancies, hepcidin concentrations are reduced in the second and third trimesters to increase the supply of iron into circulation to meet requirements. In pregnancies complicated by obesity, hepcidin may be overexpressed as a downstream effect of the low-grade, chronic inflammation associated with obesity, inhibiting intestinal iron absorption and the release of iron from body stores(Reference Dao, Sen and Iyer35). Elevated hepcidin and inflammatory marker concentrations have been observed in a number of studies in pregnant women(Reference Jones, Zhao and Jiang31,Reference Garcia-Valdes, Campoy and Hayes32,Reference Dao, Sen and Iyer35,Reference Flynn, Begum and White36) , although further consideration is required on the potential influence of hepcidin on iron requirements during pregnancy, particularly in complicated pregnancies(Reference Koenig, Tussing-Humphreys and Day37).
Diagnostic criteria for iron deficiency during pregnancy
Various biomarkers are available to characterise the different stages of iron status, moving from iron depletion to deficiency to iron deficiency anaemia. Hb is the most frequently used of these, due in part to the wide availability of point-of-care tests. However, Hb only assesses anaemia, the last stage of iron depletion. This is a concern, as all organs of the body become iron deficient before erythrocyte indicators are altered(Reference Petry, Eaton and Wobken28). Iron supply to the liver, heart, skeletal muscle and perhaps most crucially, the brain, is compromised prior to a reduction in Hb concentrations. Ideally, assessment of iron status amongst pregnant women should be based on a battery of biomarkers that assess iron storage, transport and functional markers. At the very least, assessment of iron status should incorporate both serum ferritin and Hb(38). Although much debate exists as to the appropriate thresholds to apply to these biomarkers in pregnant women(Reference Daru, Allotey and Peña-Rosas39,Reference Pasricha, Colman and Centeno-Tablante40) , the WHO currently recommends a threshold of <110 g/l for Hb and <15 μg/l for ferritin (accounting for inflammation as ferritin is an acute phase reactant). Although further research is needed to ensure that these thresholds are related to meaningful clinical outcomes, beyond haematological outcomes(Reference Brannon, Stover and Taylor41), application of these thresholds for both markers consistently across regions would be a start to defining the scope of the problem.
Health impacts of iron deficiency during pregnancy
Maternal and pregnancy-related
Once the oxygen carrying capacity of the blood becomes diminished, pregnant women may experience the common anaemia symptoms of paleness, weakness, fatigue and general lethargy(Reference Lynch, Pfeiffer and Georgieff10). However, the consequences of iron deficiency, particularly its end stage of anaemia, can be much more severe, resulting in adverse pregnancy and birth outcomes. Low Hb in the first trimester has been associated with an increased risk of preterm birth and low birth weight(Reference Scanlon, Yip and Schieve42–Reference Dewey and Oaks44). Such associations appear to be much weaker in the second or third trimester(Reference Dewey and Oaks44), but a threshold effect exists; moderate to severe, but not mild anaemia, was associated with an increased risk of small-for-gestational age in a meta-analysis by Kozuki and colleagues(Reference Kozuki, Lee and Katz45). Far fewer studies have explored associations with indices of iron deficiency, with conflicting evidence to date from those that have, particularly given the confounding impact of inflammation on ferritin(Reference Alwan, Cade and McArdle46,Reference Khambalia, Collins and Roberts47) . Moreover, the U-shaped curve of risk associated with iron status during pregnancy must also be considered, as elevated iron status indices, including ferritin and Hb, particularly in the third trimester, have been associated with an increased risk of some adverse birth outcomes(Reference Scanlon, Yip and Schieve42,Reference Dewey and Oaks44,Reference Rahman, Siraj and Islam48) . Excess iron intakes or elevated iron status during pregnancy is thought to increase blood viscosity, impair placental blood flow and contribute to oxidative stress, further emphasising the caution required when prescribing iron supplementation to pregnant women(Reference Dewey and Oaks44).
Fetal and infant development
Iron deficiency during pregnancy presents a triple threat for fetal development, but most critically, for fetal brain development. Iron is essential for the developing brain, due to its key role in the fundamental neuronal processes of neurotransmitter and energy metabolism and myelination, summarised in Table 2(Reference Lynch, Pfeiffer and Georgieff10,Reference Lozoff and Georgieff49) . An inadequate supply of iron, particularly during sensitive periods of critical brain development, can therefore disrupt these fundamental processes, with lasting consequences.
Table 2. Iron and fundamental neuronal processes in the developing brain

Modified from McCarthy and Kiely(Reference McCarthy and Kiely67).
Low maternal iron intake and status during pregnancy can present an immediate threat to fetal brain development. Low maternal iron intake during the third trimester resulted in altered neonatal brain structure, most notably in cortical grey matter(Reference Monk, Georgieff and Xu50) and Schmidt and colleagues reported an inverse association between maternal intake and risk of autism spectrum disorders in early childhood(Reference Schmidt, Tancredi and Krakowiak51). In a recent systematic review, Janbek et al. concluded that there was some evidence that maternal iron status during pregnancy may be associated with offspring behaviour, cognition and academic achievement, but not motor development(Reference Janbek, Sarki and Specht52). However, this research field has significant limitations due to huge variability in study design, neurological assessments performed and the timing of both the exposure and outcome assessments. The most crucial limitation is the reliance of most studies on Hb to assess maternal iron status. A large Spanish birth cohort recently reported higher scores in working memory and executive function at 7 years in children whose mothers had serum ferritin concentrations >12 μg/l in the first trimester(Reference Arija, Hernández-Martínez and Tous53).
Fetal iron accretion is compromised in mothers with moderate to severe iron deficiency. Maternal ferritin concentrations of 12–13⋅6 μg/l were identified as important thresholds below which fetal iron accretion is significantly reduced(Reference Shao, Lou and Rao54,Reference Jaime-Perez, Herrera-Garza and Gomez-Almaguer55) . Maternal iron deficiency, in addition to pregnancy complications including fetal growth restriction and prematurity, delivery by Caesarean section and the maternal and pregnancy-related factors we have already discussed, all increase the risk of neonatal iron deficiency(Reference Rao and Georgieff24,Reference McCarthy, Kenny and Hourihane56) . Neonatal iron deficiency itself is associated with abnormalities in the newborn auditory system(Reference Amin, Orlando and Wang57,Reference Choudhury, Amin and Agarwal58) , poorer recognition memory at ~15 d old(Reference Siddappa, Georgieff and Wewerka29) and poorer language ability, fine mother skills and tractability at 5 years of age(Reference Tamura, Goldenberg and Hou59). More recently, in our generally healthy, low-risk maternal–infant cohort in Ireland, we identified lasting behavioural consequences of neonatal iron deficiency in children born to mothers with obesity or delivered by Caesarean section(Reference McCarthy, Murray and Hourihane60). This is especially concerning as early cognitive and social-emotional development are strong determinants of future educational attainment, mental health, career and earning potential and overall quality of life(Reference Georgieff61,Reference Grantham-McGregor, Cheung and Cueto62) . As a final threat, infants born iron deficient or with reduced iron stores are also at increased risk of continued iron deficiency, as low iron stores at birth track through infancy and early childhood(Reference Hay, Refsum and Whitelaw63,Reference Georgieff, Wewerka and Nelson64) . Particularly during the period of critical brain development from 6 to 24 months of age, iron deficiency is associated with irreversible consequences for cognition, motor development and behaviour(Reference Lozoff, Smith and Kaciroti65).
Conclusion: iron
Inadequate iron supply in utero, due to iron malnutrition, inflammation, obesity or an underlying metabolic disease, presents a massive threat of long-term neurological consequences and represents a real cost and burden to individuals and society. Public health strategies are urgently required to improve the overall health and nutritional status of women of reproductive age, with the rising prevalence of overweight and obesity a major concern amongst this population group. Iron sufficiency during pregnancy is essential to ensure the health of the mother, improve pregnancy outcomes and protect fetal and infant brain development(Reference Georgieff66). A dual approach encompassing strategies targeting prevention and early detection is required(Reference McCarthy and Kiely67). An enhanced focus on the most effective supplementation strategies in pregnant women is also needed, with further consideration of dosing, forms of iron used and side effects. Finally, the development of screening strategies to enable early detection of pregnant women at increased risk of iron deficiency should be prioritised, with the dual purpose of protecting the health of the mother and fetal/infant brain development.
Iodine
Iodine is an essential trace element required for thyroid hormone synthesis. Critical to the regulation of BMR and macronutrient metabolism, thyroid hormones are also fundamental to normal development of the central nervous system via their regulation of cell migration, differentiation and myelination(Reference Bougma, Aboud and Harding68). Iodine deficiency disrupts the metabolism of thyroid hormones; therefore, adequate iodine status prior to and during pregnancy is critical. During pregnancy, the requirement for iodine increases by 50 %(Reference Pearce69). This is to enable increased maternal thyroxine production to ensure adequate fetal supply until the fetal thyroid can concentrate iodine and produce thyroid hormones, and to supply the placental transfer of iodine to support fetal thyroid hormone production. In addition, the increased rate of glomerular filtration during pregnancy increases renal clearance of iodine.
Determinants of iodine status
Diet and fortification
Recognition of country-specific habitual food consumption and iodised salt coverage is essential in considering dietary risk factors for low iodine status. Consumption of iodised salt is the most influential dietary determinant of iodine intake and status. Recent global estimates from UNICEF show that 88 % of households consume iodised salt(70). The highest coverage was reported for East Asia and the Pacific and South Asia (92 and 89 %, respectively) whereas in Western and Central Africa, 76 % of individuals had access to iodised salt. Although coverage has increased significantly from 70 % reported in 2012(Reference Zimmermann and Andersson71), it is estimated that 29 % of countries with data on household penetration met the international goal of at least 90 % of households consuming adequately iodised salt, whereas 30 % of countries had coverage rates of <50 %. In Europe, 27 % of households have iodised salt(72), but coverage can be as low as 5 %(Reference Lazarus and Smyth73).
In many countries, particularly in those without a salt iodisation policy, milk and dairy products make significant contributions to iodine intake and status in women of childbearing age(Reference McNulty, Nugent and Walton74–Reference Perrine, Herrick and Serdula76) and in pregnant women(Reference Perrine, Herrick and Serdula76–Reference Bath, Walter and Taylor80). However, a shift in consumption patterns away from dairy towards plant-based milks, many of which are not fortified with iodine, may reduce iodine intake and increase the risk of iodine deficiency(Reference Dineva, Rayman and Bath81). Consumption of fish and shellfish has been associated with iodine status in UK and Spanish pregnant populations(Reference Dineva, Rayman and Levie78), but consumption of seafood is typically low among women of childbearing age.
Maternal factors
Data on the relationship between maternal BMI and iodine status are mixed and subject to confounding. In a sample of Thai pregnant women, no differences in urinary iodine concentration (UIC) were observed between normal weight and overweight women; however, in this group, a BMI ≥23 kg/m2 resulted in 3⋅6-fold higher odds of low maternal-free thyroxine in the first trimester(Reference Gowachirapant, Melse-Boonstra and Winichagoon82), which may be reflective of the relationship between adiposity and hypothyroxinaemia, but not necessarily to iodine-related thyroid dysfunction. In an analysis of three European cohorts, BMI was negatively associated with a urinary iodine:creatinine ratio (UI/Creat), but not with UIC(Reference Dineva, Rayman and Levie78), which warrants further analysis as BMI is often but not always associated with adiposity. The setting is also an important factor to consider in interpreting the relationship between body weight and iodine status. In a study of Iranian women by Gargari et al.(Reference Gargari, Fateh and Bakhshali-bakhtiari83), the odds of UIC <150 μg/l decreased by 13 % for every 1 kg increase in weight during pregnancy, which the authors posit was a proxy indicator of dietary quality.
Maternal age and social factors
UIC was negatively associated with age in a sample of non-pregnant women in the National Health and Nutrition Examination Survey (NHANES (2001/2006)); however, no association of UIC with age was observed for pregnant women in the same study(Reference Perrine, Herrick and Serdula76). Dineva et al.(Reference Dineva, Rayman and Levie78) reported that there was a positive association of maternal age with UI/Creat; however, the relationship may be confounded by the association of maternal age with dietary patterns and iodine-supplement use. In the study by Gargari et al.(Reference Gargari, Fateh and Bakhshali-bakhtiari83), other factors such as whether the pregnancy was planned, the time since the most recent pregnancy, maternal education level and maternal iodine supplement use prior-to and during pregnancy were associated with better iodine status.
Assessment of iodine intake and status
Dietary reference values for iodine intake are presented in Table 1. As with all dietary assessments, the reliability of an estimate of nutrient intake is largely dependent on the quality of the food composition data(Reference Elmadfa and Meyer84). Iodine food data represent a challenge due to incomplete data and natural variability in iodine content of food, which varies with season and as a result of agricultural practices. Furthermore, the iodine content of soil varies considerably across regions(Reference Andersson, Takkouche and Egli85) due to geological factors(Reference Rohner, Zimmermann and Jooste86). Currently, food composition datasets do not provide any information on the variability of iodine content within foods. Utilisation of a probabilistic modelling approach to account for variability in iodine food composition may overcome this challenge and improve the validity and reliability of iodine intake assessments.
UIC directly reflects dietary iodine intake and is the most common indicator used worldwide to assess population iodine status. The population median UIC is assessed against the reference range for adequacy for pregnant women of 150–249 μg/l(87). However, one of the major challenges of the iodine field is the absence of an individual biomarker of iodine status. Although it is useful at a population level, UIC is heavily influenced by day-to-day variation in water and protein intakes, making it inappropriate for assessing individual iodine status(Reference Rohner, Zimmermann and Jooste86). To identify mild-to-moderate iodine deficiency, biomarkers of individual iodine status are urgently required. In recent years, thyroid hormones have been proposed as functional markers of iodine status. However, although triiodothyronine, thyroxine and thyroid-stimulating hormone are thyroid functional measures, tight homoeostatic regulation makes them unsuitable as markers of iodine status, as values can remain in the normal reference range in individuals with low iodine intake(88,Reference Ma and Skeaff89) . Thyroglobulin, a thyroid-derived protein that reflects thyroid volume in both iodine-deficient and iodine-sufficient population groups, has shown promise as a functional biomarker of iodine status(Reference Bath, Pop and Furmidge-Owen90) and further assessment of its usefulness is ongoing.
Epidemiology of iodine deficiency
Iodine deficiency disorders have been described as the leading cause of preventable impaired mental function worldwide(Reference Zimmermann, Jooste and Pandav91), estimated to affect 1⋅88 billion people(Reference Darnton-Hill92). In a global analysis of UIC in 2011, one in three school-aged children and 28⋅5 % of the general population had inadequate iodine intakes(Reference Andersson, Karumbunathan and Zimmermann93). Significant regional differences in prevalence estimates were reported, indicating that iodine deficiency is primarily an issue of geography because of variability in iodine content of soil and water as well as country- and region-specific iodisation policies and dietary patterns. Inadequate iodine intakes were estimated in 44 % of the WHO Europe region population, where iodised salt penetration is low(Reference Andersson, Karumbunathan and Zimmermann93). In 2017, pregnant women in thirty-nine of seventy-two countries with UIC data had suboptimal iodine intake where median UIC was <150 μg/l(Reference Gizak, Rogers and Gorstein94). Of the forty countries with UIC data in both school-aged children and pregnant women, twenty-nine countries reported deficiency in the women and sufficiency in the children, highlighting the limitation of using school-aged children UIC as a proxy of iodine status in other population groups, particularly in settings where child dairy intakes are high(Reference Hennessy, ní Chaoimh and McCarthy95).
Health and developmental impacts of maternal iodine deficiency
Maternal iodine deficiency can have important clinical consequences for the mother and her offspring, the severity of which are influenced by the timing and extent of the deficiency(Reference Cao, Jiang and Dou96). Prior to conception, severe iodine deficiency has been associated with reduced fecundity in women of childbearing age(Reference Mills, Buck Louis and Kannan97). During pregnancy, severe iodine deficiency may result in an increased risk of miscarriage and stillbirth(Reference Dillon and Milliez98). Neonatal consequences of severe maternal iodine deficiency are profound and most severely can manifest as cretinism, characterised by severe mental impairment with squint, deaf mutism and motor defects. Severe maternal iodine deficiency can also result in neonatal hypothyroidism, impaired growth, goitre and infant mortality(Reference Zimmermann99).
The adverse effects of mild-to-moderate iodine deficiency are less clear. Maternal iodine status has been associated with child neurological outcomes in some(Reference Abel, Caspersen and Meltzer100–Reference Murcia, Espada and Julvez105), but not all observational studies(Reference Murcia, Rebagliato and Iniguez106–Reference Ghassabian, Steenweg-de Graaff and Peeters108). Levie and colleagues(Reference Levie, Korevaar and Bath109) conducted a recent meta-analysis of individual participant data in three large European birth cohorts (n 6180)(Reference Guxens, Ballester and Espada110–Reference Boyd, Golding and Macleod112). They reported a curvilinear relationship of UI/Creat with lower verbal but not non-verbal IQ scores. Gestational age at urine sampling was identified as a significant effect modifier of the association of UI/Creat with verbal IQ scores. In stratified analyses, UI/Creat in the first 12 weeks of pregnancy was associated with an overall effect of 5 IQ points; between 12–14 weeks of gestation, UI/Creat showed a linear association with verbal IQ, with an overall effect of 3 IQ points and no association was observed after 14 weeks of gestation. Taken together, these data signpost a critical period for iodine-related (thyroid hormone-mediated) neurological development early in the first trimester. However, a recent systematic literature review and meta-analysis of the effects of iodine supplementation on thyroid function and child outcomes has shown that there is insufficient good-quality evidence to support recommendations for iodine supplementation in pregnancy in the areas of mild-to-moderate deficiency(Reference Dineva, Fishpool and Rayman113). The authors suggest that the inconsistencies in the evidence may be attributable to maternal iodine status prior to pregnancy or on study entry, the dose and form of iodine supplement, the timing of supplementation and the sensitivity of the developmental tests used.
Conclusion: iodine
There is mounting evidence that low to zero consumption of iodised salt, coupled with a shift away from dairy consumption as well as low seafood intakes among young women is contributing to low intakes of iodine. However, the nature and extent of the impact of this deficit on perinatal and child outcomes is unclear. If we are to clarify the nature of the relationship between maternal iodine status and offspring development and develop point-of-care screening to support timely intervention, we must prioritise the development of valid biomarkers of individual iodine status. Investment in food composition data and global co-operation to develop standardised approaches to using composition data with variability inherent in the estimates of nutrient exposure are also required. Well-designed and well-timed observational studies and intervention studies accounting for maternal iodine status in early gestation, or prior to pregnancy, with sensitive, clinically validated infant and child neuro-behavioural outcomes are urgently required.
Vitamin D
The classical role of vitamin D in maintaining bone health is well-described and much attention in the vitamin D field has shifted towards understanding its role in non-skeletal health outcomes, particularly in relation to immune function, non-communicable diseases and fetal and infant development(Reference Brannon and Picciano114). During early pregnancy, vitamin D metabolism changes; vitamin D binding protein concentrations increase and increases in 1-α-hydroxylase activity in the maternal kidney, placenta and decidua(Reference Zehnder, Evans and Kilby115) lead to circulating 1,25-dihydroxyvitamin D concentrations that are –two to three times higher than usual by 12 weeks of gestation, with relatively stable serum 25-hydroxyvitamin D (25(OH)D)(Reference Hollis, Wagner and Feldman116). These metabolic shifts appear to be unrelated to calcium metabolism and are not maintained during lactation. There are compelling data to support the possibility that 1,25-dihydroxyvitamin D, a potent immune modulator, is necessary for successful pregnancy implantation by adapting the immune response, creating an anti-inflammatory milieu and enabling fetal growth(Reference Hollis, Wagner and Feldman116,Reference Tamblyn, Hewison and Wagner117) . There is plenty of evidence that 25(OH)D crosses the placenta and infants are born with circulating 25(OH)D concentrations reflective of maternal status, at about 60–80 % of maternal values collected at delivery(Reference Hollis and Pittard118,Reference Viö Streym, Kristine Moller and Rejnmark119) .
Epidemiology of vitamin D deficiency
The problem of endemic low vitamin D status in many parts of the world is multifactorial. Low sunshine availability due to latitude, weather, air pollution, skin exposure or clothing practices, or a reduced ability to synthesise cholecalciferol from UVB due to skin colour, age or genetics(Reference Giovannucci and Feldman120), mean that large sectors of the global population rely on vitamin D in the food supply to maintain adequate vitamin D status. Naturally occurring sources of vitamin D, such as oily fish, are consumed irregularly and in low quantities, and global food fortification policies vary(Reference Calvo, Whiting and Barton121), contributing to low habitual vitamin D intakes among many populations(Reference Kiely and Black122). Supplementation recommendations rely on individual compliance for their effectiveness and uptake is highly variable, particularly in low- and middle-income settings(Reference Gomes, King and Dallmann123). Low vitamin D status, denoted in the present paper by a circulating 25(OH)D concentration below 50 nmol/l and vitamin D deficiency, designated as 25(OH)D <25/30 nmol/l, can also arise as a consequence of underlying health issues, frailty, inflammation, adiposity or smoking(Reference Giovannucci and Feldman120).
Vitamin D deficiency and low vitamin D status among pregnant women
In a recent systematic review, Mogire et al.(Reference Mogire, Mutua and Kimita124) reported the prevalence of serum 25(OH)D <75, 50 and 30 nmol/l across Africa. Among 133 studies including 21 591 participants from twenty-three African countries, the prevalence of 25(OH)D <50 nmol/l was 34⋅4 and 18⋅5 % were <30 nmol/l. Countries in the north of Africa and urban South Africans were most at risk, particularly women and newborn infants. Three studies of pregnant women (in Ethiopia, South Africa and Tunisia) with a total sample of 408 reported that 53 % had a serum 25(OH)D <30 nmol/l and among ten studies with 975 participants, 44 % were below 50 nmol/l. This high prevalence of deficiency and low vitamin D status among many women in Africa is concerning, particularly considering the low calcium intakes in many regions across the continent(Reference Pettifor125,Reference Prentice, Schoenmakers and Jones126) , which places children at high risk of nutritional rickets(Reference Munns, Shaw and Kiely127).
The first internationally standardised European 25(OH)D dataset, directly comparable to data from the USA and Canada, was reported in 2016(Reference Cashman, Dowling and Škrabáková128). The overall prevalence of 25(OH)D <30 nmol/l was 13 %, about twice that of the USA (5⋅9 %)(Reference Schleicher, Sternberg and Looker129) and Canada (7⋅4 %)(Reference Sarafin, Durazo-Arvizu and Tian130), but persons of ethnic minority were at much higher risk than their white counterparts (36–60 % of Black Asian and Minority Ethnic individuals in the UK and 65 % of South Asian participants in Norway). Among eighty-three low- and middle-income countries, only twenty-nine had population-based assessments of 25(OH)D(Reference Cashman, Sheehy and O'Neill131). Afghanistan, Pakistan, India, Tunisia, Syria, the West Bank and Gaza and Mongolia were classified as ‘hot spots’ for very low vitamin D status (<25–30 nmol/l) among women, pregnant women or infants on the basis of having a prevalence >20 %. Largely attributed to clothing practices, it has been noted that women and girls in low income and middle eastern countries in particular have lower 25(OH)D than their male counterparts(Reference Roth, Abrams and Aloia132,Reference Lips, Cashman and Lamberg-Allardt133) . Conclusions and strategies to address the problem are limited by the quality of the available evidence in many countries and the under-representation of minority groups in clinical research undertaken in high-income settings(Reference O'Callaghan and Kiely134).
Palacios and Gonzalez(Reference Palacios and Gonzalez135) described a high prevalence (>20 %) of 25(OH)D <30 nmol/l among pregnant women and infants in many countries, including South Asia and the Middle East. Up to 60 % of women in India and 86 % of infants in Iran had 25(OH)D <30 nmol/l. Saraf and colleagues(Reference Saraf, Morton and Camargo136) estimated the global prevalence of 25(OH)D concentrations <50 nmol/l at 54 % among pregnant women and 75 % among newborn infants, with almost one in five pregnant women and one in three newborns below 25 nmol/l. Studies among pregnant women from ethnic minorities in Wales(Reference Datta, Alfaham and Davies137), the Netherlands(Reference Van Der Meer, Karamali and Boeke138,Reference Leffelaar, Vrijkotte and Van Eijsden139) and Sweden(Reference Bärebring, Schoenmakers and Glantz140) as well as white women in the Netherlands(Reference Leffelaar, Vrijkotte and Van Eijsden139), Scotland(Reference Haggarty, Campbell and Knox141) and Sweden(Reference Bärebring, Schoenmakers and Glantz140) consistently report a high prevalence of vitamin D deficiency. Using gold standard 25(OH)D analysis of almost 1800 pregnant women in Ireland, with data comparable to non-pregnant individuals(Reference Cashman, Dowling and Škrabáková128–Reference Sarafin, Durazo-Arvizu and Tian130), we reported a prevalence of 17 % with 25(OH)D <30 nmol/l at 15 weeks of gestation; this was 49 % among women of ethnic minority(Reference Kiely, Zhang and Kinsella142). Among the infants of this cohort, 46 % of umbilical cord sera had a 25(OH)D <30 nmol/l; this was 73 % among infants born to women of ethnic minority(Reference Kiely, O'Donovan and Kenny143). Overall, these prevalence estimates elevate vitamin D deficiency among mothers and babies to the status of a serious public health problem that requires urgent action.
Implications for perinatal outcomes and fetal/infant development
Increasing mechanistic evidence that vitamin D has a role in the progression of a healthy pregnancy supports a biologically plausible hypothesis that vitamin D deficiency may, in part, contribute to adverse perinatal outcomes resulting from growth restriction, hypertension or pre-eclampsia(Reference Hollis and Pittard118,Reference O'Callaghan and Kiely144) . Neurological development is a potential consequence of vitamin D deficiency in early life. Eyles and colleagues have mapped the presence of the vitamin D receptor and 1-α-hydroxylases throughout brain tissues and have developed in vitro and animal models that describe the many ways in which maternal and offspring vitamin D deficiency exerts detrimental effects on behaviour(Reference Eyles145). Vitamin D metabolites have also been shown to cross the blood–brain barrier(Reference Eyles, Smith and Kinobe146) and it is also worth noting that growth restriction itself, also associated with vitamin D deficiency(Reference Kiely, Zhang and Kinsella142) adversely impacts neurological development, particularly in small and thin for gestational age babies(Reference O'Neill, Hannon and Khashan147).
We did not find any association with maternal or cord 25(OH)D and cognitive or behavioural outcomes at 5 years in a well-characterised low-risk prospective mother–infant cohort(Reference McCarthy, Murray and Malvisi148). Our analysis of the existing literature at the time showed conflicting signals, with significant heterogeneity in study design, including the timing and methods for exposure and outcome assessments, statistical approaches, variable adjustment for known confounders and cut-offs for 25(OH)D concentrations(Reference McCarthy, Murray and Malvisi148). Mutua and colleagues’(Reference Mutua, Mogire and Elliott149) systematic review of vitamin D and neuro-behavioural outcomes in children reached a similar conclusion; following analysis of thirty-two studies among >31 000 participants, the authors recommended standardised cut-offs for assessing vitamin D deficiency, as well as well-validated methods of assessing neuro-behavioural outcomes to allow international harmonisation and comparison of data as well as progression of the field into large well-controlled intervention studies.
Vitamin D requirements and dietary recommendations
Several systematic reviews of the role of vitamin D supplementation during pregnancy have been published lately(Reference Roth, Qamar and Watterworth150,Reference Palacios, Kostiuk and Peña-Rosas151) but there is no consensus as to whether vitamin D requirements are greater during pregnancy than in non-pregnant women and whether supplementation should be recommended. Current dietary requirements for vitamin D were established on the basis of skeletal health outcomes, and most agencies have proposed the same adequacy thresholds for 25(OH)D and dietary recommendations for vitamin D for pregnant and lactating women as non-pregnant adults(152–154), summarised in Kiely et al.(Reference Kiely, Hemmingway and O'Callaghan155) (Table 1).
The question remains therefore: on what basis should vitamin D requirements and dietary recommendations during pregnancy be targeted? Fetal and neonatal circulating 25(OH)D concentrations are dependent on maternal vitamin D status but dietary recommendations do not consider the vitamin D requirement of the developing fetus or newborn infant and assume immediate availability of an adequate vitamin D supply from early life to redress deficiency. We proposed prevention of neonatal vitamin D status <25−30 nmol/l, indicated by umbilical cord 25(OH)D, as a potential target for defining maternal vitamin D requirements during pregnancy, consistent with prevention of nutritional rickets(Reference Munns, Shaw and Kiely127,Reference O'Callaghan, Hennessy and Hull156) . The vitamin D3 intake required to maintain maternal 25(OH)D >50 nmol/l in almost all mothers, which ensured that cord sera were all >25 nmol/l, was 30 μg/d (1200 IU)(Reference O'Callaghan, Hennessy and Hull156). This was consistent with other dose–response studies from Canada(Reference March, Chen and Karakochuk157) and New Zealand(Reference Grant, Stewart and Scragg158). Further similar dose–response studies are required in different racial and ethnic groups and in various settings to generate an inclusive estimate of maternal vitamin D requirements for the prevention of neonatal deficiency, with adequate consideration of calcium intake(Reference Hemmingway, Kenny and Malvisi159). The design and protocol for such studies are available and would be a cost-effective approach to ensure safe evidence-based antenatal prevention of vitamin D deficiency among mothers and newborn infants.
Widespread maternal and neonatal vitamin D deficiency is a present danger requiring immediate public health action. Whether there is a role for additional vitamin D over and above current dietary recommendations, to support healthy pregnancy and promote optimal perinatal outcomes and infant development, is a separate question, which requires further investigation in well-designed placebo-controlled randomised trials, discussed separately(Reference Kiely, Wagner and Roth160). These issues are often conflated in the literature, leading to confusion and a narrow discussion of the core question. For example, the most recent WHO statement on vitamin D supplements during pregnancy did not address the most important issue of vitamin D deficiency prevention and the grave risk of nutritional rickets in many regions(161); by issuing a report on the Cochrane reviews of supplementation only, this statement missed an opportunity to address the most critical concerns. Public health measures, such as food fortification with vitamin D, would benefit the population as a whole, including women prior to and during pregnancy, by increasing vitamin D in the food supply, raising vitamin D intakes and status and providing a better chance of avoiding deficiency(Reference Kiely, Wagner and Roth160).
Conclusions
Deficiencies of iron, iodine and vitamin D are common, have multifactorial dietary and non-nutritional risk factors and overlap in their contribution to adverse clinical outcomes, including neurological development in children, which impacts life chances. The adverse effects of poor-quality diets, with limited availability of micronutrients, are compounded by increasing rates of obesity among young women, with consequent inflammatory and metabolic disorders, all implicated in iron and vitamin D deficiencies and interlinked in the pathways for suboptimal neural development.
Public health strategies are urgently required to improve the overall health and nutritional status of women of reproductive age. Strategies encompassing obesity prevention and early detection of malnutrition are required. Standardised screening including clinical risk factors and indices of iron status would detect pregnant women at increased risk of iron deficiency. Development of sensitive, individual biomarkers of iodine status is needed for inclusion of iodine to screening strategies, with the dual purpose of protecting the health of the mother and fetal/infant brain development. Risk assessments of vitamin D requirements during pregnancy need to be revisited from the perspective of fetal and neonatal requirements. International consensus on standardised approaches to micronutrient assessment, analysis and reporting as well as sensitive, clinically validated infant and child neuro-behavioural outcomes are required to enable useful observational and intervention studies to proceed.
Financial Support
Á. H. is supported by a Science Foundation Ireland Starting Investigator award [Functional indicators of iodine status in pregnancy – an outcome-driven, personalised nutrition approach (18/SIRG/5575)]. E. K. M. holds a Health Research Board Applying Research into Policy and Practice Fellowship [Iron deficiency assessment for protection of the newborn brain (ARPP-2020-008)].
Conflict of Interest
None.
Authorship
The authors had sole responsibility for all aspects of preparation of this paper.





