Lamotrigine is a broad-spectrum anti-epileptic known to alleviate and prevent depression in bipolar disorder. Reference Calabrese, Bowden, Sachs, Yatham, Behnke, Mehtonen, Montgomery, Ascher, Paska, Earl and DeVeaugh-Geiss1–Reference Calabrese, Goldberg, Ketter, Suppes, Frye, White, DeVeaugh-Geiss and Thompson3 Its therapeutic mechanism remains unclear but at least in healthy controls it may increase brain activity in temporal and ventral prefrontal cortical regions. Reference Li, Tenebäck, Nahas, Kozel, Large, Cohn, Bohning and George4 These regions have also been implicated in the pathophysiology of bipolar disorder. Reference Haldane and Frangou5–Reference Yurgelun-Todd and Ross7 They are also part of a distributed neural network involved in sad facial affect information processing Reference Phan, Wager, Taylor and Liberzon8,Reference Murphy, Nimmo-Smith and Lawrence9 where abnormal patterns of activation have been observed in patients with depression and bipolar disorder. Reference Lawrence, Williams, Surguladze, Giampietro, Brammer, Andrew, Frangou, Ecker and Phillips10 The aim of the present study was to explore whether lamotrigine monotherapy might redress the functional abnormalities seen in patients with bipolar disorder during sad facial affect recognition.
Method
Participants
Patients
Participants were recruited from secondary care services from the South London and Maudsley Hospital. Eligible participants fulfilled the following inclusion criteria:
-
(a) DSM–IV 11 criteria for bipolar disorder, type 1
-
(b) aged 18–50 years
-
(c) right-handed
-
(d) scoring <14 on the HRSD Reference Hamilton12 and <7 on the Young Mania Rating Scale Reference Young, Biggs, Ziegler and Meyer13
-
(e) trial of lamotrigine monotherapy was considered appropriate by the patients' treating psychiatrists.
Exclusion criteria were:
-
(a) current or past history of alcohol or substance dependence as defined by the DSM–IV;
-
(b) significant comorbid medical or neurological disorder;
-
(c) treatment-resistant bipolar disorder or history of poor medication adherence;
-
(d) history of self-harm or suicide attempts;
-
(e) current suicidal ideation;
-
(f) need of imminent hospitalisation;
-
(g) skin reaction to previous exposure to lamotrigine or other anti-epileptics;
-
(h) in female patients, pregnancy, lactation or inadequate contraception;
-
(i) current treatment with lithium or depot antipsychotic medication;
-
(j) history of rapid cycling in the past 3 years;
-
(k) any other comorbid Axis I diagnosis.
Patients were screened to confirm their suitability. Diagnosis was established using the Structured Clinical Interview for DSM–IV Axis I Disorders. Reference First, Spitzer, Gibbon and Williams14 Consenting patients were withdrawn from their existing medication over a 10-day period. They were then titrated over a 12-week period to a target dose of 200 mg/day lamotrigine (minimum dose 100 mg/day). During the study only hypnotic medication was allowed as required but not in the 48 h preceding functional magnetic resonance imaging (fMRI) data collection. Throughout the study patients were assessed on a weekly basis. Withdrawal criteria included:
-
(a) their withdrawal of consent
-
(b) deterioration in mental state
-
(c) clinically indicated changes to pharmacotherapy
-
(d) serious adverse event.
Controls
Patients were individually matched on gender and age (within a year) to healthy control participants recruited by advertisement in the local press. Healthy volunteers had no personal history of psychiatric, neurological or medical illness, or treatment with psychotropic medications and no family history of psychiatric disorders in their first-degree relatives.
The study was approved by the Ethics Committee of the Institute of Psychiatry and written consent was obtained from all participants.
Imaging paradigm and study design
Participants took part in a 5-minute experiment employing event-related fMRI, and were presented with ten different facial identities (six female, four male) (http://www.paulekman.com) depicting 150% intensity of sadness. We used 150% intensity in order to ensure optimal performance in patients and to avoid possible confounding effects of ambiguity with regards to facial emotion recognition. In addition, a neutral expression was used as a control condition. Images of sad and neutral facial expressions were interspersed with a fixation cross. Each stimulus was displayed for 2 s. In all, 60 images were displayed in a random order; the fixation cross, faces with neutral expression, and faces showing affect were each displayed 20 times. Participants were instructed to respond to sad and neutral faces by pressing the right and left button respectively on an MRI-compatible response box. No response was required when participants viewed the fixation cross. Response time and accuracy data were collected.
Healthy controls were scanned once while patients were scanned twice; at the end of the washout period (baseline) and upon completion of 12 weeks of lamotrigine monotherapy. All participants performed the same task as described above.
Image acquisition and analysis
Gradient echo echoplanar magnetic resonance images were acquired for each participant on each occasion of scanning using a 1.5 T General Electric Neurovascular Signa magnetic resonance system (General Electric, Milwaukee, Wisconsin, USA) fitted with 40 mT/m high speed gradients, with foam padding and a forehead strap to limit head motion. A quadrature birdcage head coil was used for radio frequency transmission and reception. In each of the 36 non-contiguous planes parallel to the inter-commissural (anterior commissure–posterior commissure) plane, T2*-weighted magnetic resonance images depicting blood oxygen level-dependent (BOLD) contrast were acquired with time to echo (TE) 40 ms, a repetition time (TR) 2000 ms, slice thickness 7 mm, slice skip 0.7 mm. During the 5-minute facial affect discrimination task, 150 images were collected. At the same session, a high-resolution gradient echoplanar imaging data-set was acquired for subsequent co-registration.
Image pre-processing
All images were processed and analysed using MATLAB (version 6, The Mathworks, Natick, Massachusetts, USA) and SPM2 software (Statistical Parametric Mapping, Wellcome Department of Cognitive Neurology, London, http://www.fil.ion.ucl.ac.uk/spm). Images were realigned to correct for movement. Images were normalised into Montreal Neurological Institute space (http://www.mni.mcgill.ca/) by first coregistering each participant's echoplanar images to their structural MRI image. The structural image was then normalised to a T1 template and finally the same transformation was applied to the echoplanar images. The transformed data-set for each participant was smoothed with an isotropic Gaussian filter (full-width half-maximum 8 mm) to compensate for normal variation in anatomy across participants.
Image analysis
In the first-level analysis, an event-related design was used convolved with the haemodynamic response function, global signal changes were removed and the time series were processed using a high-pass filter (128 s) to remove low-frequency artifacts. Contrast images showing the recognition of sad compared with neutral facial affect were produced.
Activation maps were obtained for each group and examined using separate one-sample t-tests for patients with bipolar disorder (at baseline and post-lamotrigine treatment) and control participants. Activations were considered significant at P<0.05, corrected for multiple comparisons at the cluster level.
A random effects, between-group comparison was utilised to compare the healthy control group with patients with bipolar disorder at baseline while performing the task. This was achieved by entering each participant's first-level contrast images into a two-sample t-test analysis using the ‘basic models’ option in SPM. Differences in activation between groups considered significant at P<0.05, corrected for multiple comparisons at the cluster level.
A random effects, within-group comparison was utilised to investigate the effect of lamotrigine on brain activation during task performance. This was achieved by entering each participant's first-level contrast images at baseline and after 12 weeks of lamotrigine monotherapy into a paired t-test analysis. The effect of lamotrigine was considered significant at P<0.05, corrected for multiple comparisons at the cluster level.
To explore the effect of potential confounders we also examined correlations between: (a) the effect of lamotrigine on brain activation during task performance with the mean difference in HRSD scores between study entry and study endpoint; and (b) baseline task-related brain activation and daily rate of reduction in the dose of sodium valproate. Activations were considered significant at P<0.001, corrected for multiple comparisons at the cluster-level.
Results
Overall, 12 patients (7 females, 5 males) with bipolar disorder, type I, fulfilling eligibility criteria were enrolled and completed the study. Patients' mean age was 42.1 years (s.d.=11.8). The mean age of the controls was 41.8 years (s.d.=10.9). All patients reached the target dose of 200 mg/day lamotrigine by the end of the 12 weeks titration period and tolerated the treatment well. No withdrawals, worsening of symptoms or serious adverse effects were noted. The patients' mean age of illness onset was 23.1 years (s.d.=5.6). They reported an average of 10.1 episodes (s.d.=6.5), the last being on average 1.1 years (s.d.=0.4) prior to study entry. At enrolment, 5 patients were being treated with a combination of an anti-epileptic (sodium valproate, n=4; carbamazepine, n=1) and a serotonin reuptake inhibitor, and 7 with sodium valproate monotherapy for an average of 8.2 months (s.d.=10.4). The mean dose of sodium valproate was 827.27 mg/day (s.d.=283.16).
Patients' mean HRSD and Young Mania Rating Scale Scores were 13.75 (s.d.=2.43) and 1 (s.d.=1.3) respectively at study entry and 10.25 (s.d.=5.65) and 0.5 (s.d.=0.75) at study endpoint. Their mean Global Assessment of Functioning scores were 64.12 (s.d.=8.52) at study entry and 70.25 (s.d.=10.44) at endpoint. Paired t-tests did not reveal any significant differences between the two time points in any of the above variables (P>0.08 for all comparisons). The mean difference between patients' HRSD score at study entry and endpoint was 3.50 (s.d.=5.07).
Functional MRI data were obtained from all 12 patients with bipolar disorder but because of to movement artifacts either at baseline or study endpoint, data from 8 individuals were used in the final analyses (5 females, 3 males).
Behavioural data
The mean accuracy of controls and patients at baseline was 38.3 (s.d.=1.18) and 33.8 (s.d.=5.27) respectively, and following lamotrigine treatment patients' mean accuracy increased to 35.2 (s.d.=4.94). There were no significant differences between patients and controls in accuracy of sad facial affect recognition (t=2.36, d.f.=14, P=0.55). Patients' accuracy did not differ compared with their baseline following lamotrigine treatment (t=7.49, d.f.=7, P=0.13).
The mean response time of controls and patients at baseline was 1.17 s (s.d.=0.19) and 1.26 s (s.d.=0.22) respectively; following lamotrigine treatment, patients' mean response time was 1.23 s (s.d.= 0.23). There were no significant differences between patients and controls in the response time (t=–1.00, d.f.=14, P=0.33). Patients' response times did not change compared with their baseline following lamotrigine treatment (t=0.76, d.f.=7, P=0.47).
Imaging data
Distribution of brain activation in control participants and patients with bipolar disorder at baseline and post lamotrigine treatment Both groups at baseline displayed activation in the middle frontal gyrus (Brodmann area; BA 46), temporal regions and cerebellum. In addition, controls activated the medial frontal gyrus (BA 6) while patients activated parietal regions. After lamotrigine treatment, patients displayed additional activation in the medial frontal gyrus (BA 6), inferior frontal gyrus (BA 44), postcentral gyrus (BA 3), medial occipital gyrus (BA 19) and the thalamus. Detailed coordinates of the regional peaks are shown in Table 1.
Table 1 Regions of brain activation during sad facial affect recognition at baseline and post-lamotrigine treatment.
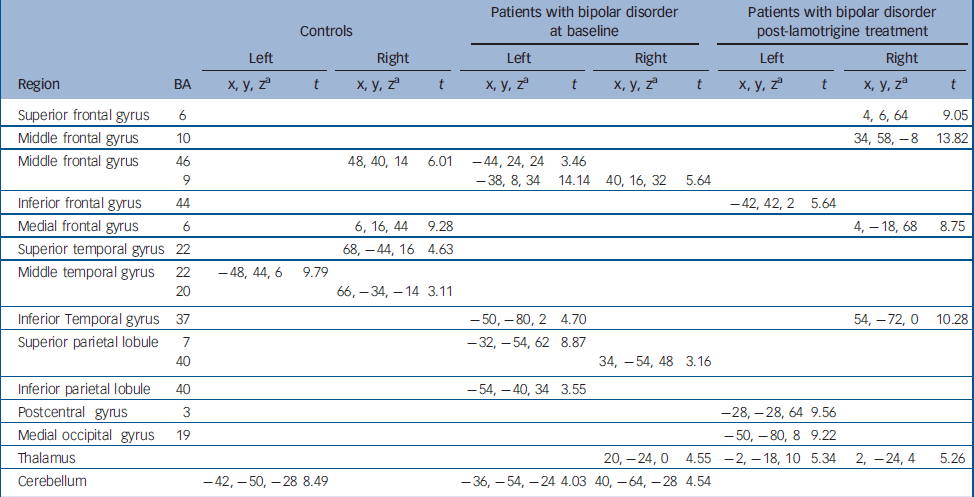
| Controls | Patients with bipolar disorder at baseline | Patients with bipolar disorder post-lamotrigine treatment | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Left | Right | Left | Right | Left | Right | ||||||||
| Region | BA | x, y, za | t | x, y, za | t | x, y, za | t | x, y, za | t | x, y, za | t | x, y, za | t |
| Superior frontal gyrus | 6 | 4, 6, 64 | 9.05 | ||||||||||
| Middle frontal gyrus | 10 | 34, 58, -8 | 13.82 | ||||||||||
| Middle frontal gyrus | 46 | 48, 40, 14 | 6.01 | -44, 24, 24 | 3.46 | ||||||||
| 9 | -38, 8, 34 | 14.14 | 40, 16, 32 | 5.64 | |||||||||
| Inferior frontal gyrus | 44 | -42, 42, 2 | 5.64 | ||||||||||
| Medial frontal gyrus | 6 | 6, 16, 44 | 9.28 | 4, -18, 68 | 8.75 | ||||||||
| Superior temporal gyrus | 22 | 68, -44, 16 | 4.63 | ||||||||||
| Middle temporal gyrus | 22 | -48, 44, 6 | 9.79 | ||||||||||
| 20 | 66, -34, -14 | 3.11 | |||||||||||
| Inferior Temporal gyrus | 37 | -50, -80, 2 | 4.70 | 54, -72, 0 | 10.28 | ||||||||
| Superior parietal lobule | 7 | -32, -54, 62 | 8.87 | ||||||||||
| 40 | 34, -54, 48 | 3.16 | |||||||||||
| Inferior parietal lobule | 40 | -54, -40, 34 | 3.55 | ||||||||||
| Postcentral gyrus | 3 | -28, -28, 64 | 9.56 | ||||||||||
| Medial occipital gyrus | 19 | -50, -80, 8 | 9.22 | ||||||||||
| Thalamus | 20, -24, 0 | 4.55 | -2, -18, 10 | 5.34 | 2, -24, 4 | 5.26 | |||||||
| Cerebellum | -42, -50, -28 | 8.49 | -36, -54, -24 | 4.03 | 40, -64, -28 | 4.54 | |||||||
Patient–control comparison at baseline
Compared with controls, greater activation was observed in patients in the parahippocampal gyrus. However, controls showed significantly more activation mostly right-sided, in the superior (BA 6) and inferior (BA 47) frontal gyri, and the precentral (BA 4) and cingulate (BA 23) gyri. Additionally, increased activation relative to patients was also seen in the left middle frontal gyrus (BA 6). Detailed coordinates of the regional peaks are shown in Table 2.
Table 2 Regional peak activations during the sad facial affect recognition task: case– control comparison at baseline.
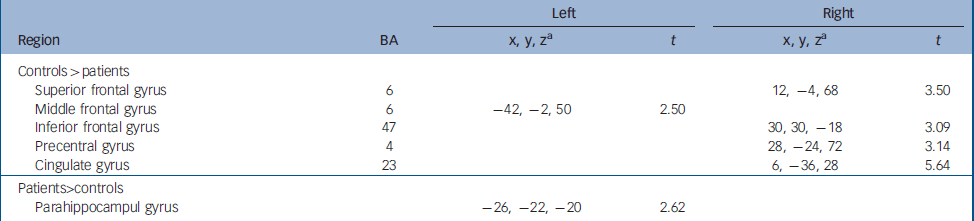
| Left | Right | ||||
|---|---|---|---|---|---|
| Region | BA | x, y, za | t | x, y, za | t |
| Controls>patients | |||||
| Superior frontal gyrus | 6 | 12, -4, 68 | 3.50 | ||
| Middle frontal gyrus | 6 | -42, -2, 50 | 2.50 | ||
| Inferior frontal gyrus | 47 | 30, 30, -18 | 3.09 | ||
| Precentral gyrus | 4 | 28, -24, 72 | 3.14 | ||
| Cingulate gyrus | 23 | 6, -36, 28 | 5.64 | ||
| Patients>controls | |||||
| Parahippocampul gyrus | -26, -22, -20 | 2.62 | |||
Differences in brain activation patterns in patients with bipolar disorder between baseline and following 12 weeks of lamotrigine monotherapy
Greater activation was observed at baseline compared with study endpoint in the right precentral gyrus (BA 4) and the left inferior temporal gyrus (BA 20).
Following 12 weeks of lamotrigine monotherapy, increased activation compared with baseline was noted bilaterally in the inferior frontal gyri (BA 44, 45) in the left precentral gyrus (BA 6) and on the right in the medial frontal gyrus (BA 6), the paracentral lobule (BA 4) and the thalamus. Detailed coordinates of the regional peaks are shown in Table 3 and illustrated in the online Fig. DS1. Figure 1 shows the mean signal intensity in the areas of treatment effect at baseline and after lamotrigine treatment, extracted with the MarsBar tool of SPM2.

Fig. 1 Mean signal intensity changes in regions of significant treatment effect at baseline and post lamotrigine treatment. GFd, medial frontal gyrus; GPrC, presentral gyrus; Lpc, paracentral lobule; GFi, inferior frontal gyrus; Th, thalamus.
Table 3 Regional peak activations during the sad facial affect recognition task.
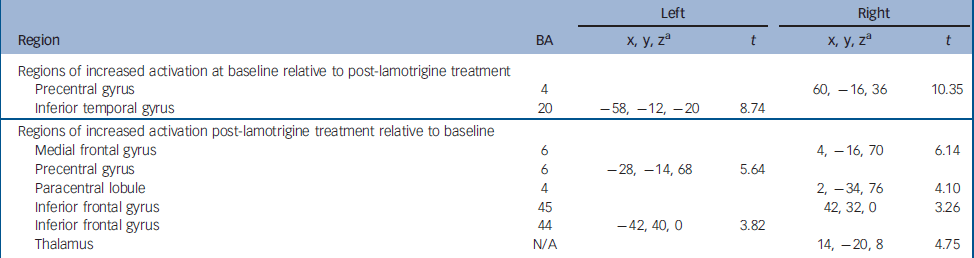
| Left | Right | ||||
|---|---|---|---|---|---|
| Region | BA | x, y, za | t | x, y, za | t |
| Regions of increased activation at baseline relative to post-lamotrigine treatment | |||||
| Precentral gyrus | 4 | 60, -16, 36 | 10.35 | ||
| Inferior temporal gyrus | 20 | -58, -12, -20 | 8.74 | ||
| Regions of increased activation post-lamotrigine treatment relative to baseline | |||||
| Medial frontal gyrus | 6 | 4, -16, 70 | 6.14 | ||
| Precentral gyrus | 6 | -28, -14, 68 | 5.64 | ||
| Paracentral lobule | 4 | 2, -34, 76 | 4.10 | ||
| Inferior frontal gyrus | 45 | 42, 32, 0 | 3.26 | ||
| Inferior frontal gyrus | 44 | -42, 40, 0 | 3.82 | ||
| Thalamus | N/A | 14, -20, 8 | 4.75 | ||
Correlations
No significant correlations, either positive or negative, were found between lamotrigine-induced changes on brain activation and the mean difference in HRSD scores between study entry and study endpoint. The rate of daily reduction in the dose of sodium valproate during the washout period correlated negatively with baseline task-related activation in the left insula (x −50, y −22, z 20).
Discussion
Effect of task
Sad facial affect recognition paradigms similar to that employed here activate a number of brain regions in healthy individuals within the dorsomedial prefrontal cortex, the cingulate and parahippocampal gyri as well as subcortical regions such as the thalamus and the caudate, although variability has been noted across studies. Reference Phan, Wager, Taylor and Liberzon8,Reference Murphy, Nimmo-Smith and Lawrence9 In this study, unmedicated patients with bipolar disorder and healthy controls showed a similar pattern of activation during the facial affect recognition task but differed in the degree of neural response in several brain regions.
Effect of medication
Dorsomedial (BA 6) and ventrolateral (BA 47) prefrontal cortical regions were more activated in controls than in patients as was the right dorsal cingulate gyrus (BA 23). Patients showed greater activation within temporal regions around the left hippocampus/parahippocampal gyrus both compared with controls at baseline, but also compared with their post-lamotrigine pattern of activation. This finding is in partial agreement with that of Lawrence et al. Reference Lawrence, Williams, Surguladze, Giampietro, Brammer, Andrew, Frangou, Ecker and Phillips10 They reported a positive correlation between the degree of depression and activation within the left hippocampus, which accords with the increased neural response in the same region observed in this study. Increased activity in temporal lobe structures is commonly seen during processing of negative, sadness-inducing material in healthy people (e.g. ‘dressing to go to their mother's funeral’) Reference Partiot, Grafman, Sadato, Wachs and Hallett15 and has also been implicated in negative affect disturbances in individuals with depression. Reference Kumari, Mitterschiffthaler, Teasdale, Malhi, Brown, Giampietro, Brammer, Poon, Simmons, Williams, Checkley and Sharma16 We observed a 25% reduction in HRSD scores at follow-up in the current sample which was not statistically significant but may still contribute to the changes in neural responses in the temporal lobe as fMRI is likely to have greater sensitivity to detect this effect.
Our results are in the opposite direction with regard to prefrontal and anterior cingulate regions where, at baseline, we found higher activation in controls and not in patients. This difference was noted for facial expressions representing high intensity of sadness and patients with bipolar disorder showed no behavioural deficits in sad affect recognition. The two studies have similar patient groups with comparable levels of residual depressive features and minimal manic symptoms. Although we employed an explicit as opposed to an implicit task, it is unlikely that this accounts for the disparity: Chen et al Reference Chen, Lennox, Jacob, Calder, Lupson, Bisbrown-Chippendale, Suckling and Bullmore17 found no difference in the pattern of activation in patients with depression and bipolar disorder using the two different types of tasks, although implicit processing was generally associated with greater neural response in both patients and controls. A significant difference with our study is the medication status of patients. Patients with bipolar disorder in the study by Lawrence et al Reference Lawrence, Williams, Surguladze, Giampietro, Brammer, Andrew, Frangou, Ecker and Phillips10 were medicated with a combination of antidepressants, atypical antipsychotics, lithium and anticonvulsants, although only one patient was on lamotrigine. However, it is difficult to argue for it leading to overactivity since similarly medicated patients with bipolar disorder in the study by Chen et al Reference Chen, Lennox, Jacob, Calder, Lupson, Bisbrown-Chippendale, Suckling and Bullmore17 were not different to controls in their neural response to sad facial expressions.
Increased neural responses following lamotrigine were seen in dorsomedial and ventrolateral prefrontal cortical regions that are known to be reciprocally connected to limbic (cingulate gyrus, amygdale–hippocampus complex) and subcortical regions (basal ganglia, thalamus, insula, brainstem) involved in generation and regulation of emotional states. Reference Cardinal, Parkinson, Hall and Everitt18 The results of this study could therefore be consistent with the idea that lamotrigine treatment may lead to a ‘normalisation’ in key prefrontal regions associated with emotional self-regulation akin to what has been observed with successful remission of depression. Reference Mayberg19
Limitations
However, it is possible that the enhanced cortical activation seen at study endpoint may relate to factors other than medication, such as learning or habituation. Patients' behavioural performance at baseline and study end was very similar and it would be difficult to argue for an effect of learning. Familiarity with the experimental set-up at follow-up may have been associated with less anticipatory anxiety or arousal. However, habituation is routinely associated with decreased rather than increased neural responses. Reference Wright, Fischer, Whalen, McInerney, Shin and Rauch20 Similarly, the level of symptoms between baseline and study endpoint were comparable and it is therefore difficult to argue that the changes in the brain activation patterns observed are consequent to significant clinical change, although symptomatic improvement may have contributed. In fact, we found no significant correlations between lamotrigine-induced changes in brain activation and differences in HRSD score between study entry and endpoint. The power of the two-by-eight data-set in this study is possibly sensitive to spurious findings and therefore the results require replication. Our findings, if replicated, may apply to clinical populations only as the effect of lamotrigine on brain function in healthy individuals was not assessed. Lamotrigine may have a unique impact on disease-related mechanisms and therefore its effect on healthy individuals may not have been necessarily informative with regards to its mechanism of action. Lamotrigine appeared to ‘normalise’ activation within the neural networks involved in facial affect processing by enhancing activation in prefrontal and reducing it in temporal regions. The clinical implications of this observation require further study.







eLetters
No eLetters have been published for this article.