Introduction
Sickle cell disease (SCD) is the most common inherited hemoglobinopathy and is estimated to affect more than 100,000 Americans [Reference Piel, Steinberg and Rees1,2]. SCD disproportionately affects Black and African Americans where approximately 1 in 360 Black newborns are diagnosed with this condition in the USA every year [Reference Kato, Piel and Reid3]. As a chronic disease, SCD causes a spectrum of micro- and macrovascular complications over the lifespan due to the rigid deformation of sickle red blood cells under hypoxic conditions [Reference Piel, Steinberg and Rees1,Reference Ware, de Montalembert, Tshilolo and Abboud4,Reference Rees, Williams and Gladwin5]. These acute and chronic sequelae contribute to the decreased life expectancy of patients with SCD to an average of 54 years as compared to 76 years in matched non-SCD patients [Reference Lubeck, Agodoa and Bhakta6]. Additionally, analysis of mortality trends in patients with SCD reveal an increased risk of death due to acute infectious disease causes [Reference Payne, Mehal and Chapman7].
In 2021, the Centers for Disease Control and Prevention reported that among Black and African Americans – who represent 12.5% of the overall US population – the COVID-19 fatality rate was notably high at 13.6% with 449,373 deaths reported that year [8]. A voluntary clinician-reported registry of US COVID-19 infections in patients with SCD reported both high hospitalization (69%) and case fatality rates (7%) but was only able to report 2 months of data from March 20 through May 21, 2020 [Reference Panepinto, Brandow and Mucalo9].
To our knowledge, few studies focused on the pre-vaccine period prior to December 2020 during the COVID-19 pandemic for the SCD population. To address this, our study expanded on existing research to provide a more comprehensive perspective on the impact of COVID-19 for patients with SCD prior to the availability of COVID-19 vaccines. We aimed to characterize hospitalized adult patients with co-occurring SCD and COVID-19, including demographic and clinical factors and associated outcomes. Characteristics and outcomes were also explored between patients who died or went to hospice versus patients who were discharged from the hospital.
Materials and Methods
This study utilized the third quarter 2020 Cerner COVID-19 database built by the Cerner Real-World Data™ (CRWD) system [Reference Ehwerhemuepha, Carlson and Moog10]. This database was released on December 12, 2020 and represents de-identified patient-level electronic health record (EHR) data extracted from participating hospitals and practices across the USA. This retrospective observational study was declared exempt from review by the ChristianaCare Institutional Review Board.
Inclusion criteria to participate in the study comprised patients aged 18 years and older, inpatient admission to US hospitals that utilized the Cerner EHR system, and records that included a dual diagnosis of SARS-CoV-2 and SCD, with SCD captured by International Classification of Diseases, Tenth Revision (ICD-10) diagnoses codes under the D57.XX group. Patients identified with sickle cell trait were excluded from the study. We did not use ICD-10 codes to determine SCD genotype as issues with validity of these codes in administrative datasets is well recognized [Reference Snyder, Lane, Zhou, Paulukonis and Hulihan11,Reference Ting, Sinha and McCavit12]. To protect patient privacy, dates for individual patient admissions to the hospital were randomly shifted plus or minus up to 1 month. Patient records in our database listed patient admissions between December 10, 2019 and October 15, 2020. Therefore, these dates represent actual hospital admission dates plus or minus 30 days from the actual admission.
Demographic and clinical variables included age in years; sex (female, male); race (Black or African American, White, other); ethnicity (Hispanic or Latinx, not Hispanic or Latinx); health insurance type (Medicaid, Medicare, other); and Elixhauser comorbidity conditions including congestive heart failure (CHF), diabetes with complications, diabetes without complications, hypertension, myocardial infarction, and peripheral vascular disease.
Outcome variables identified clinical services received and patient acuity during hospitalization. They included receipt of blood transfusion; mean hours to blood transfusion; receipt of anticoagulant; receipt of remdesivir; presence of fever (defined as temperature > 100° Fahrenheit); first, maximum, minimum, and average temperature in Celsius degrees; presence of tachypnea (defined as respiration rate > 22 breaths per minute); first, maximum, minimum, and average respiration rates; receipt of mechanical ventilation; presence of hypoxia (defined as first O2 saturation < 92%); first, maximum, minimum, and average O2 saturation; mean hospital length of stay (LOS) in days; and discharge disposition including death, transfer to hospice, transfer to another facility, returning home, or leaving the hospital against medical advice (AMA).
Characteristics were calculated for the overall sample and by discharge disposition which was grouped as “death/hospice” versus being released from the hospital and noted as “other” for patients that were transferred to another facility, returned home, or left the hospital AMA. Descriptive statistics included frequencies for categorical variables and mean [standard deviation (SD)] for continuous variables. Comparisons between discharge disposition groups were completed using chi-square or t-tests. All analyses were conducted using R statistical software version 4.0 (R Core Team).
Results
Of the 87 hospitals and practices included in the CRWD COVID-19 cohort, we identified 72,658 adult patients who were hospitalized during the study period. Of those, 171 (0.2%) patients were identified with a SCD diagnosis. We identified 11 patients who had a missing discharge disposition in their record leaving a final analytical sample of 160 patients. Nine patient records had a discharge disposition of death (N = 8) or hospice (N = 1) which approximated a 5.6% fatality rate for this sample. The remaining 94.4% (N = 151) patients had a discharge disposition classified as returned home (N = 133), transferred to another facility (N = 11), or left the hospital AMA (N = 7).
The demographic and clinical characteristics of the patient sample are presented in Table 1. A majority identified as female (55.1%), Black or African American (86.8%), and not Hispanic or Latinx (92.8%) with a mean (SD) age of 32.5 (16.9) years. Among the two discharge disposition groups, there were significant differences in mean (SD) age in years, rate of CHF, and rate of diabetes with or without complications where the death/hospice discharge disposition group of patients were older [59.6 (16.9) years vs 30.9 (15.5) years, p < 0.001] and had a higher percentage of CHF (55.6% vs 15.9%, p < 0.01) and diabetes with complications (33.3% vs 4.6%, p < 0.001) or without complications (44.4% vs 9.3%, p < 0.01). There were no other significant differences between discharge disposition groups.
Table 1. Demographic and clinical characteristics for total sample and by discharge disposition group

* Sex: N = 2 observations missing overall and in the other discharge disposition group.
Ɨ Race: N = 8 observations missing overall; N = 1 observation missing in the death/hospice discharge disposition group; N = 7 observations missing in the other discharge disposition group.
ǂ Ethnicity: N = 7 observations missing overall; N = 1 observation missing in the death/hospice discharge disposition group; N = 6 observations missing in the other discharge disposition group.
§ Health insurance type: N = 36 observations missing overall; N = 1 observation missing in the death/hospice discharge disposition group; N = 35 observations missing in the other discharge disposition group.
Table 2 presents outcomes for the overall sample and by discharge disposition group. Significant differences between groups were found for clinical variables where higher medical acuity was identified for the death/hospice disposition group and included tachypnea (71.4% vs 28.3%, p = 0.02), receipt of mechanical ventilation (33.3% vs 2.0%, p < 0.001), mean (SD) minimum O2 saturation [87.7% (9.9%) vs 93.2% (3.9%), p < 0.01], and mean (SD) LOS [13.3 (8.2) days vs 7.3 (6.4) days, p < 0.01].
Table 2. Clinical outcomes for total sample and by discharge disposition group
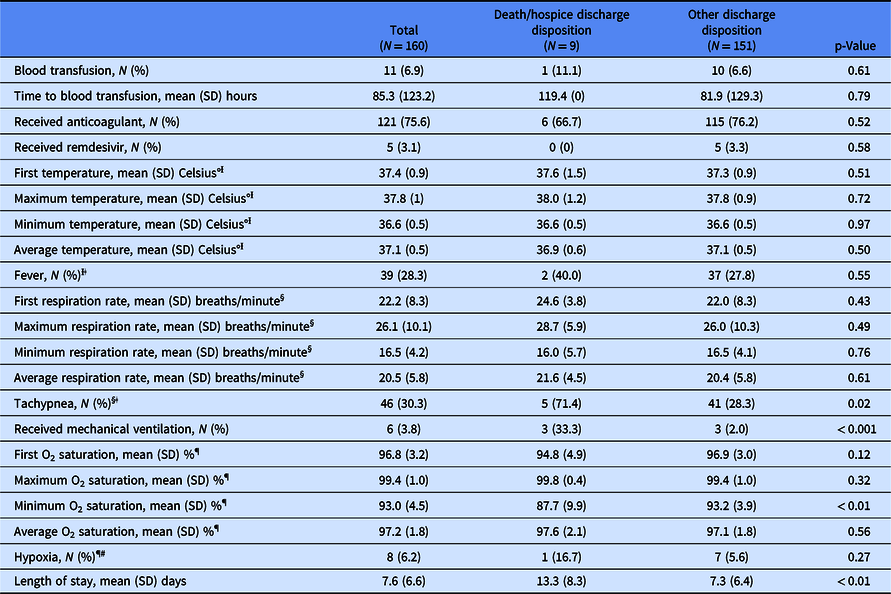
Ɨ First temperature, maximum temperature, minimum temperature, average temperature, and fever: N = 22 observations missing overall; N = 4 observations missing in the death/hospice discharge disposition group; N = 18 observations missing in the other discharge disposition group.
ǂ Fever defined as temperature > 100° Fahrenheit.
§ First respiration rate, maximum respiration rate, minimum respiration rate, average respiration rate, and tachypnea: N = 8 observations missing overall; N = 2 observations missing in the death/hospice discharge disposition group; N = 6 observations missing in the other discharge disposition group.
ǁTachypnea defined as respiration rate > 22 breaths per minute.
¶ First O2 saturation, maximum O2 saturation, minimum O2 saturation, average O2 saturation, and hypoxia: N = 30 observations missing overall; N = 3 observations missing in the death/hospice discharge disposition group; N = 27 observations missing in the other discharge disposition group.
# Hypoxia defined as first O2 saturation < 92%).
As blood transfusions are a common treatment during hospitalization for severely ill patients with SCD [Reference Howard13], we explored receipt of transfusions among our hospitalized patient sample. Overall, receipt was low with 6.9% (11/160) of patients receiving a blood transfusion. Among patients within the death/hospice discharge group (N = 9), only one (11.1%) patient was transfused with the remaining eight (88.9%) patients not receiving a transfusion. To further examine the most critically ill patients in our sample of co-occurring SCD and COVID-19, we completed a subgroup analysis to assess the use of blood transfusions and receipt of mechanical ventilation. As presented in Table 3, we found a significant difference between receipt of the two treatments (p < 0.01). Among patients who received mechanical ventilation (N = 6), only two (33.3%) patients received a transfusion.
Table 3. Subgroup analysis of patients who received a blood transfusion and/or mechanical ventilation during hospitalization

Discussion
Our study identified clinical outcomes related to high medical acuity that were significantly associated with death or hospice discharge disposition including higher rates of tachypnea, higher rates of mechanical ventilation, lower minimum O2 saturation, and longer hospital LOS in a population of adults with co-occurring SCD and COVID-19 infection. However, since our death/hospice discharge disposition group sample was relatively small, the data are somewhat skewed for tachypnea and mechanical ventilation.
In our outcomes analysis, we also noted the low rates of treatment using remdesivir (N = 5, 3.1%) with all five patients in the “Other” discharge disposition group receiving treatment and no patients in the death/hospice disposition group receiving treatment. The Food and Drug Administration (FDA) did not approve remdesivir for the treatment of COVID-19 until October 22, 2020 [14,Reference Rubin, Chan-Tack, Farley and Sherwat15]. Prior to this, the FDA released an emergency use authorization (EUA) for remdesivir in adult and pediatric patients aged 12 years and older who were hospitalized with severe cases of COVID-19 [Reference Rubin, Chan-Tack, Farley and Sherwat15,16]. However, this EUA was not issued until May 1, 2020 [Reference Rubin, Chan-Tack, Farley and Sherwat15,16], which was approximately 5 months into our 10-month study period. This may have contributed to the low remdesivir treatment rates in our study.
This study does have some limitations. We had to rely on a single ICD-10 code for SCD instead of multiple codes as the data were deidentified. There is a possibility that some people in the dataset did not have SCD [Reference Snyder, Lane, Zhou, Paulukonis and Hulihan11,Reference Ting, Sinha and McCavit12]. CRWD records only include patients hospitalized at institutions that use Cerner EHRs. While our data represent a national sample, the findings may not be generalizable to institutions that use alternative EHR systems. Use of the CRWD also prevented our ability to identify certain characteristics and services in patient records including anticoagulation dose or erythrocytapheresis. Lastly, our dataset only included hospitalized patients. Given that prior research has identified a 69% hospitalization rate for patients with SCD and COVID-19 [Reference Panepinto, Brandow and Mucalo9], we did not account for patients who received care in the outpatient setting or follow outcomes for patients transferred to other facilities which may have impacted our estimated fatality rate. Our sample was able to capture a 10-month period during the height of the COVID-19 pandemic before the FDA issued an EUA for the first COVID-19 vaccine in December 2020 [17,Reference Oliver, Gargano and Scobie18], giving us a comprehensive representation of clinical outcomes for SCD patients during this critical and unique time in the pandemic.
Current US surveillance data offer a limited perspective on the prevalence of SCD. Even less information is available regarding the impact that co-occurring infectious diseases, such as COVID-19, may have on individuals with SCD. Use of the CRWD COVID-19 cohort database allowed a unique opportunity to explore this impact at a national level in the USA. Patients with SCD and COVID-19 who died had other comorbidities including CHF and diabetes with and without complications highlighting important areas for future research to better understand risk factors for severe disease in patients with SCD. The most common complication of COVID-19 is pneumonia, a condition that if severe, should be treated with blood transfusions in patients with SCD. Only one patient in this dataset who died and only two who were mechanically ventilated were transfused, suggesting the possibility that these patients may have received care in the absence of a sickle cell expert who may have optimized management. While further research is needed to examine the effect of COVID-19 and systemic racism on health care disparities already experienced by patients with SCD [Reference Power-Hays and McGann19], this study highlights key clinical factors that may be associated with mortality for patients with co-occurring SCD and COVID-19.
Funding statement
RJC and KDW are partly supported by an Institutional Development Award from the National Institute of General Medical Sciences of the National Institutes of Health under grant U54-GM104941 (principal investigator: G Hicks). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Competing interests
The authors have no conflicts of interest to declare.