The metabolic syndrome, which comprises a cluster of metabolic abnormalities such as hyperlipidaemia, diabetes mellitus and hypertension, is a widespread and increasingly prevalent disease in industrialised countries and contributes to an increase in cardiovascular morbidity and mortality(Reference Kissebah and Krakower1, Reference Formiguera and Canton2). Non-alcoholic fatty liver disease (NAFLD) is now recognised as the hepatic manifestation of the metabolic syndrome and is emerging as one of the most common causes of chronic liver disease worldwide(Reference Hjelkrem, Torres and Harrison3–Reference Fong, Nehra and Lindor6). NAFLD encompasses a wide disease spectrum ranging from simple hepatic steatosis to steatohepatitis, advanced fibrosis and cirrhosis(Reference Reid7–Reference Clark, Brancati and Diehl9). Liver-related morbidity and mortality due to NAFLD are observed in patients who have advanced fibrosis and cirrhosis(Reference Angulo10). Although the mechanisms that accelerate the progression of simple steatosis towards more debilitating and advanced stages of NAFLD remain poorly understood, a ‘two-hit’ hypothesis has been put forward(Reference Day11). Hepatic fat accumulation, which was initially thought to be relatively benign, represents the ‘first hit’. Studies have suggested that fat accumulation in hepatocytes is the hallmark of NAFLD and leaves them highly vulnerable to a ‘second hit’, for example, injury by oxidative stress and inflammatory cytokines, such as TNF-α and monocyte chemoattractant protein-1 (MCP-1).
At present, no pharmacotherapy is available that can fully reverse or prevent steatohepatitis(Reference Gary-Bobo, Elachouri and Gallas12). We have recognised that diet and its components contribute to the development and prevention of NAFLD(Reference Nagao, Inoue and Wang13–Reference Nagao and Yanagita15). Therefore, it is necessary to develop effective therapies for the treatment of the early stages of NAFLD and the discovery of nutrients that reduce the risk of NAFLD would be useful. In Eastern traditional therapy, many species of edible mushrooms, such as Lentinus edodes (Shiitake) and Lyophyllum decastes (Hatakeshimeji), have been used for the treatment of various diseases, including lifestyle diseases(Reference Chang16–Reference Ukawa, Izumi and Ohbuchi21). Panellus serotinus (Mukitake), which belongs to same family of mycelia such as Shiitake and Hatakeshimeji, is recognised as one of the most delicious edible mushrooms. Technology for the artificial cultivation of Mukitake in plastic greenhouses has recently been developed(Reference Nagamori22) and has contributed to the constant availability of this mushroom in the market. We previously(Reference Nagao, Inoue and Inafuku23) found that the dietary intake of powdered whole Mukitake alleviates NAFLD in db/db mice, an animal model for obesity. In the present study, we investigated the influence of Mukitake fractional extracts on the development of NAFLD in db/db mice in order to determine the physiologically active substances in Mukitake and to understand the mechanisms by which they work.
Experimental methods
Preparation of fungal extracts
Hot-air-dried and ground whole Mukitake mushrooms were extracted by incubation in 50 volumes of ethanol for 2 h under reflux. After ethanol extraction was repeated three times, the residue was incubated in 50 volumes of distilled water at 97°C three times, for 2 h each time. The filtrates in ethanol and water were lyophilised to obtain an ethanol-soluble Mukitake extract (EE) and a water-soluble Mukitake extract (WE), respectively. Approximately 18 g of EE and 32 g of WE were obtained from 100 g of dried Mukitake mushroom. The lyophilised extracts were stored at − 80°C until use.
Animals and experimental diets
All animal procedures were performed in accordance with the guidelines provided by the ethics committee on experimental animal care at Saga University, Saga, Japan. A total of six C57BL/6J mice and eighteen BKS.Cg-+Leprdb/+Leprdb/Jc1 (db/db) mice (5 weeks old, male) were purchased from CLEA Japan, Inc. (Tokyo, Japan). The mice were housed individually in plastic cages, kept at 24°C on a 12 h light–12 h dark cycle. After a week-long adaptation period, the db/db mice were randomly divided into three groups: a control (CO) diet group; a WE diet group; an EE diet group. The basal semi-synthetic diets were prepared according to the AIN-76 formulation. The WE and EE groups were fed 3 % of their respective Mukitake soluble extracts, which were substituted for sucrose in the basal diet (Table 1). The C57BL/6J mice, the progenitors of db/db mice, were fed the CO diet as the normal group. The mice consumed the diets using Rodent CAFÉ (KBT Oriental Company Limited, Saga, Japan) and were given ad libitum water for 4 weeks. At the end of the feeding period, the mice were killed by exsanguination from the heart under anaesthesia with pentobarbital sodium salt after a 9 h starvation. White adipose tissues (WAT) and liver were excised, and the serum was separated from the blood. The tissues and serum were immediately frozen in liquid N2, and were stored at − 80°C until analyses.
Table 1 Composition of the experimental diets (g/100 g)
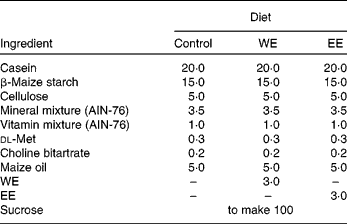
WE, water-soluble Mukitake extract; EE, ethanol-soluble Mukitake extract.
Measurement of serum parameters
TAG and cholesterol levels, as well as alanine aminotransferase activity, in the serum were measured using commercially available enzymatic kits (Wako Pure Chemical Industries, Limited, Osaka, Japan). The levels of MCP-1, total adiponectin and insulin in the serum were measured using commercial ELISA kits purchased from R&D Systems, Inc. (Minneapolis, MN, USA), Otsuka Company, Limited (Tokyo, Japan) and Shibayagi Company, Limited (Gunma, Japan), respectively.
Assay of lipid levels and lipogenic enzyme activity in the liver
Liver lipids were extracted and purified according to a method reported previously(Reference Folch, Lees and Sloane Stanley24). TAG and total cholesterol levels were determined by the methods of Fletcher(Reference Fletcher25) and Sperry & Webb(Reference Sperry and Webb26), respectively. The activities of malic enzyme, glucose-6-phosphate dehydrogenase and fatty acid synthase (FAS) in the hepatic cytosolic fraction were determined as described elsewhere(Reference Ochoa27–Reference Kelley, Nelson and Hunt29).
Histopathological study of the liver
The livers were excised and immediately fixed in 10 % buffered formalin for histological examinations. Formalin-fixed liver tissue samples were embedded in paraffin and sectioned into 4 μm thicknesses. The liver tissue sections were stained by haematoxylin–eosin to microscopically evaluate the degree of NAFLD.
Analysis of mRNA expression
Total RNA was extracted from 100 mg of perirenal WAT and liver using an RNeasy Midi Kit and an RNeasy Lipid Tissue Mini Kit from Qiagen Science (Germantown, MD, USA). A TaqMan Universal PCR Master Mix from Applied Biosystems (Bedford, CA, USA), and Assay-on-Demand, Gene Expression Products (Mm00443258_m1 for TNF-α, Mm00441242_m1 for MCP-1, Mm00440939_m1 for PPARα, Mm00440945_m1 for PPARγ, Mm00439693_m1 for insulin receptor, Mm00439720_m1 for insulin receptor substrate (IRS) 1, Mm03038438_m1 for IRS2 and Hs99999901_s1 for 18S RNA as an internal control for normalisation), purchased from Applied Biosystems, were used to measure the mRNA expression level of each gene in the quantitative real-time RT-PCR analysis. The amplifications were performed with a commercial real-time PCR system (ABI Prism 7000 Sequence Detection System; Applied Biosystems).
Liquid chromatography time-of-flight MS analysis
Whole Mukitake powder, WE, EE and whole Shiitake powder were suspended in 20 % MeCN. All the samples were analysed on a 1100 Series HPLC system, coupled with a G1969A TOF mass spectrometer system (Agilent Technologies, Santa Clara, CA, USA), operating in the positive-ion mode. A chromatographic separation was achieved on a 2·1 × 100 mm, 3·5 μm particle size Zorbax Eclipse plus C18 column (Agilent Technologies). Liquid chromatography parameters were as follows: solvent A was 15 % MeCN+0·1 % formic acid+2·5 mm-AcONH4 and solvent B was 85 % MeCN+0·1 % formic acid+2·5 mm-AcONH4. The flow rate was 0·2 ml/min, and the solvent gradient program was 15 % B at time 0, 100 % B at time 15 min and 100 % B at 30 min. The injection volume was 5 μl and the column temperature was set at 40°C. Electrospray ionization capillary voltage was set at 4000 V and the fragmentor at 120 V. The liquid nebuliser was set to 50 psig (345 kPa) and the nitrogen-drying gas was set to a flow rate of 10 litres/min. The drying gas temperature was maintained at 350°C. The stored mass range was m/z 80–1200. MassHunter Workstation Data acquisition software (Agilent Technologies) was used to operate the instrumentation. The data were processed using MassHunter Qualitative Analysis software (Agilent Technologies). The compounds were extracted from the raw data using the Molecular Feature Extraction algorithm in the MassHunter Qualitative Analysis software.
Statistical analysis
All values are expressed as means with their standard errors. The significant difference of means between C57BL/6J and db/db mice fed the CO diet was determined by Student's t test. For significance of the difference between means for the three groups of db/db mice, data were analysed by one-way ANOVA, and then the differences among the mean values were inspected using the Tukey–Kramer multiple comparison tests. Differences were considered significant at P < 0·05. Pearson's correlation coefficient test was used to assess the correlations between the variables.
Results
After experimental feeding, the dietary intake of the CO diet contributed to significant increases in body weight gain and in the weights of each WAT in db/db mice compared with those in C57BL/6J mice (Table 2). Although no significant difference was shown in dietary intake between the groups of db/db mice, body weight gain and omental WAT weight were significantly lower in the EE group than in the CO group. Hepatomegaly, macrovesicular steatosis and hepatic TAG accumulation were observed in db/db mice fed the CO diet (Fig. 1). However, hepatic TAG accumulation and macrovesicular steatosis were markedly alleviated in both the WE and the EE groups compared with the CO group. Moderate hyperlipidaemia was observed in db/db mice compared with C57BL/6J mice, and the three groups of db/db mice did not differ in their levels of serum lipid parameters (Table 3). As shown in Table 3, severe hyperinsulinaemia was also observed in control-fed db/db mice and tended to be alleviated (by 39 %) in the WE group of db/db mice. Consistent with the development of hepatic steatosis, the hepatic injury marker (alanine aminotransferase activity) in the serum was significantly increased in db/db mice fed the CO diet in comparison with the C57BL/6J mice (Fig. 2). The injury marker was significantly decreased by dietary intake of Mukitake water extract in db/db mice. The serum level of MCP-1 was drastically increased in the control-fed db/db mice compared with the C57BL/6J mice, and it was significantly decreased in the WE group compared with the CO group of db/db mice (Fig. 2). A highly positive correlation was found between the serum MCP-1 level and the hepatic injury marker in db/db mice (r 0·6943, P = 0·002, n 18). On the other hand, the serum level of adiponectin was markedly decreased in the control-fed db/db mice compared with the C57BL/6J mice, and it was significantly increased in the EE group compared with the CO group of db/db mice (Table 3).
Table 2 Effects of Mukitake extracts on growth parameters
(Mean values with their standard errors, n 6)

NO, normal; CO, control; WE, water-soluble Mukitake extract; EE, ethanol-soluble Mukitake extract.
a,b Mean values with unlike superscript letters were significantly different between each experimental diet group of db/db mice (P < 0·05).
* Mean values were significantly different between the NO and CO groups (P < 0·05).

Fig. 1 (a) Liver histology and TAG levels in C57BL/6J and db/db mice. Mice were fed the experimental diets for 4 weeks. See Table 1 for compositions of diets. (b) Haematoxylin and eosin staining of liver sections from representative mice of each experimental group (scale bar = 200 μm). Values are means, with their standard errors represented by vertical bars, n 6. * Mean values were significantly different between the normal (NO) and control (CO) groups (P < 0·05). a,b Mean values with unlike letters were significantly different between each experimental diet group of db/db mice (P < 0·05). WE, water-soluble Mukitake extract; EE, ethanol-soluble Mukitake extract.
Table 3 Effects of Mukitake extracts on serum parameters
(Mean values with their standard errors, n 6)
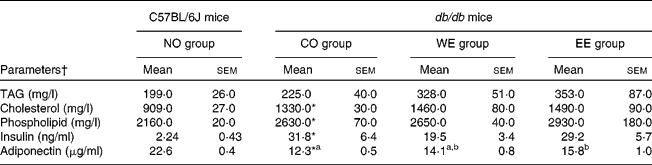
NO, normal; CO, control; WE, water-soluble Mukitake extract; EE, ethanol-soluble Mukitake extract.
a,b Mean values with unlike superscript letters were significantly different between each experimental diet group of db/db mice (P < 0·05).
* Mean values were significantly different between the NO and CO groups (P < 0·05).
† Parameters used: alanine aminotransferase and monocyte chemoattractant protein-1.

Fig. 2 (a) Hepatic injury marker activities and (b) monocyte chemoattractant protein-1 (MCP-1) levels in the sera of C57BL/6J and db/db mice. Mice were fed the experimental diets for 4 weeks. See Table 1 for compositions of diets. Values are means, with their standard errors represented by vertical bars, n 6. * Mean values were significantly different between the normal (NO) and control (CO) groups (P < 0·05). a,b Mean values with unlike letters were significantly different between each experimental diet group of db/db mice (P < 0·05). WE, water-soluble Mukitake extract; EE, ethanol-soluble Mukitake extract; ALT, alanine aminotransferase.
To examine further the effects of the Mukitake extract on the liver, the activities of key lipogenic enzymes (malic enzyme, glucose-6-phosphate dehydrogenase and FAS) were analysed (Table 4). The activities of malic enzyme and glucose-6-phosphate dehydrogenase, which supply nicotinamide adenine dinucleotide phosphate required for FAS activity, were significantly decreased in the WE group compared with the CO group of db/db mice. The WE group also showed a significant decrease in the activity of FAS, a key enzyme for the de novo synthesis of fatty acid. In the EE group, the activities of glucose-6-phosphate dehydrogenase and FAS were significantly lower than in the CO group.
Table 4 Activities of hepatic enzymes in the cytosolic fraction (nmol/min per mg protein)
(Mean values with their standard errors, n 6)
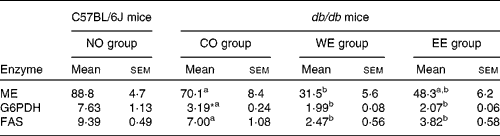
NO, normal; CO, control; WE, water-soluble Mukitake extract; EE, ethanol-soluble Mukitake extract; ME, malic enzyme; G6PDH, glucose-6-phosphate dehydrogenase; FAS, fatty acid synthase.
a,b Mean values with unlike superscript letters were significantly different between each experimental diet group of db/db mice (P < 0·05).
* Mean values were significantly different between the NO and CO groups (P < 0·05).
To gain an insight into the effect of Mukitake fractional extracts on the levels of mRNA related to insulin signalling, we examined the mRNA expression of genes in perirenal WAT and liver by real-time RT-PCR (Table 5). Compared with the C57BL/6J mice, mRNA expression of MCP-1 and TNF-α, which is known to exacerbate insulin resistance, was markedly increased, and that of PPAR, which are known to increase insulin sensitivity, was significantly decreased in the WAT of db/db mice fed the CO diet. The alterations in mRNA expression of MCP-1 and PPAR were alleviated by the dietary intake of WE in db/db mice. The EE group showed a decrease in TNF-α mRNA expression in WAT. In the liver, the mRNA expression level of IRS1, which plays a pivotal role in insulin signal transduction, was significantly lower in db/db mice fed the CO diet than in the C57BL/6J mice. The significant decrease in IRS1 mRNA expression was ameliorated in the WE group of db/db mice. mRNA expression levels of MCP-1 and TNF-α were lower in the WE group than in the CO group of db/db mice.
Table 5 Effect of experimental diets on mRNA expression of genes related to insulin signaling in the liver and perirenal white adipose tissue (arbitrary units)
(Mean values with their standard errors, n 6)
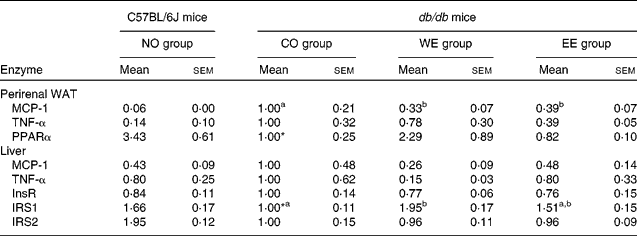
NO, normal; CO, control; WE, water-soluble Mukitake extract; EE, ethanol-soluble Mukitake extract; WAT, white adipose tissue; MCP-1, monocyte chemoattractant protein 1; InsR, insulin receptor; IRS, insulin receptor substrate.
a,b Mean values with unlike superscript letters were significantly different between each experimental diet group of db/db mice (P < 0·05).
* Mean values were significantly different between the NO and CO groups (P < 0·05).
Liquid chromatography time-of-flight MS profiles revealed that a total of 184 ions were detected in whole Mukitake powder (data not shown); 151 of these ions matched the ions detected in whole Shiitake powder (data not shown) and the other thirty-three of these ions were featured as ions peculiar to Mukitake (Fig. 3 and Table 6). Additionally, fifteen of thirty-three Mukitake featured ions were detected in Mukitake fractional extracts; seven, five and three of these ions matched for ions detected in WE, EE and both extracts, respectively (Fig. 3 and Table 6).

Fig. 3 Differential analysis between Mukitake and Shiitake using liquid chromatography time-of-flight MS. Whole Mukitake powder, water-soluble Mukitake extract, ethanol-soluble Mukitake extract and whole Shiitake powder were analysed on a 1100 Series HPLC system coupled with a G1969A TOF mass spectrometer system. See Table 6 for presumptive formula of thirty-three Mukitake featured ions. A, E, H, K, L: detected in ethanol-soluble extract; C, D, F, J, M, N, O: detected in water-soluble extract; B, G, I: detected in both extracts.
Table 6 Presumptive formula of Mukitake featured ions in Fig. 3

WMP, whole Mukitake powder; EE, ethanol-soluble Mukitake extract; WE, water-soluble Mukitake extract.
Discussion
We investigated the effects of Mukitake fractional extracts on the development of NAFLD in db/db mice. The results suggested that dietary intake of WE prevents the development of NAFLD partly through the suppression of hepatic lipogenesis and the reduction of MCP-1 production in db/db mice. Additionally, the results also suggested that hepatic steatosis was prevented by the suppression of fatty acid synthesis in the liver and the enhancement of serum adiponectin levels in EE-fed db/db mice.
The db/db mice have a functional defect in the leptin receptor, which causes them to suffer from hyperphagia and develop a syndrome involving multiple metabolic and hormonal disorders, which shares many features with the human metabolic syndrome(Reference Hummel, Dickie and Coleman30–Reference Lee, Proenca and Montez32). In the present study, hepatic TAG mass measurement and histopathological evidence clearly show that NAFLD developed in db/db mice fed a CO diet (Fig. 1). A significant reduction in hepatic TAG content, macrovesicular hepatocytes and activities of key enzymes for de novo synthesis of fatty acids was observed in both the WE and EE groups of db/db mice. Therefore, it was suggested that dietary intakes of both Mukitake fractional extracts can alleviate hepatic fat accumulation, a ‘first hit’ in the ‘two-hit’ hypothesis described above.
Various studies(Reference Marceau, Biron and Hould33–Reference Marchesini, Bugianesi and Forlani35) have indicated that insulin resistance is the essential first pathological step in the development of NAFLD. In fact, hepatic steatosis is now proposed to be a feature of the insulin resistance syndrome along with type 2 diabetes, visceral obesity and hyperlipidaemia(Reference Marceau, Biron and Hould33–Reference Marchesini, Bugianesi and Forlani35). After the experimental feeding period, db/db mice fed the CO diet had severe hyperinsulinaemia as one feature of type 2 diabetes (Table 3). Dietary intake of WE tended to decrease the serum insulin level (by 40 %) and the hepatic TNF-α mRNA expression (by 85 %), whereas IRS1 mRNA expression in the liver was significantly increased in the WE group (Tables 3 and 5). Given the fact that TNF-α impairs insulin signalling through the inhibition of the IRS1 function(Reference Hotamisligil36), improvement in insulin sensitivity by the intake of WE contributed to the prevention of hepatic steatosis in db/db mice. Dietary intake of WE also prevented the elevation of serum alanine aminotransferase activity (Fig. 2), which is the most common presentation of NAFLD at any stage(Reference Cave, Deaciuc and Mendez37). Adipose tissue not only stores excess energy in the form of fat but also secretes physiologically active substances called adipocytokines, such as TNF-α and MCP-1. MCP-1 is a member of the CC chemokine family and induces inflammatory responses through the recruitment of inflammatory cells. It is up-regulated by inflammatory stimuli such as TNF-α(Reference Baggiolini38, Reference Sartipy and Loskutoff39). Recent findings(Reference Kanda, Tateya and Tamori40) have also shown that MCP-1 is a key molecule in insulin resistance and NAFLD as a ‘second hit’ in the ‘two-hit’ hypothesis. In the present study, serum MCP-1 levels and MCP-1 mRNA expression in the liver and WAT were markedly increased in control-fed db/db mice compared with C57BL/6J mice, and the drastic increases in db/db mice were ameliorated by dietary intake of WE (Fig. 2 and Table 5). The highly positive correlation between serum MCP-1 level and the hepatic injury marker was observed in both the present study and a previous study(Reference Nagao, Inoue and Inafuku23). MCP-1 mRNA expression has been known to be regulated by the activation of transcription factor NF-κB, and the phosphorylation of the inhibitor of κB kinase-β (IKKβ) triggers the activation of NF-κB in response to pro-inflammatory stimuli(Reference Maeda, Shimomura and Kishida41–Reference Ghosh and Karin43). Our previous study(Reference Nagao, Inoue and Inafuku23) indicated that the dietary intake of whole Mukitake powder prevents the development of NAFLD through the suppression of MCP-1 production in db/db mice, and that WE inhibits IKKβ activity in vitro. Given the fact that an inhibitor of IKKβ prevented insulin resistance in diabetic mice(Reference Kim, Kim and Fillmore44), the results led us to speculate that WE prevented the development and progression of NAFLD by the reduction in MCP-1 production through interference in the IKKβ-NF-κB signalling pathway in db/db mice.
On the other hand, a significant reduction in omental (visceral) fat weight and a significant increase in serum adiponectin levels were observed in db/db mice fed EE (Tables 2 and 3). Visceral fat is more strongly related to metabolic risk factors than any other fat compartment(Reference Grundy45) and adiponectin, one of the most abundant adipocytokines, has been strongly suggested to play a protective role against the metabolic syndrome(Reference Matsuzawa46–Reference Bajaj, Suraamornkul and Piper49). Although the apparent amelioration of hyperinsulinaemia and hepatic injury was not observed in the EE group, we consider that EE has a preventive function for visceral obesity and hepatic steatosis through the enhancement of the serum adiponectin level.
In addition, differential analysis between Mukitake and Shiitake, mycelia from the same family, using liquid chromatography time-of-flight MS technology revealed that there are seven and five compounds that only exist in WE and EE, respectively (Fig. 3 and Table 6). Further structural identification and evaluation of physiological properties of these compounds would be necessary in future study.
In conclusion, the present study demonstrated that Mukitake contains at least two different physiological substances that alleviate NAFLD: one through the reduction in MCP-1 production by its interference in the IKKβ-NF-κB signalling pathway and the other through an increase in the serum adiponectin level and the prevention of visceral fat accumulation.
Acknowledgements
We thank Tomomi Yokota and Kazuyoshi Morinaga for their technical assistance. The authors declare no conflicts of interest. M. I. and K. N. made substantial contributions to the conception and design of the study, performing the experiment, assembly, analysis and interpretation of data and drafting the manuscript. S. N., B. S., N. I., N. N. and H. N. participated in the experimental work and collection, assembly and analysis of data. T. T. and T. Y. contributed to planning of the experiment and in discussion of the results. The present study was supported by a research grant from the Japanese Ministry of Education, Culture, Sports, Science and Technology.











