Asthma, one of the most common chronic diseases in the world, is estimated to affect more than 350 million people(Reference Vos, Allen and Arora1). Asthma is a major public health concern, and identifying modifiable risks factors to improve asthma prevention is of major importance. Recent worldwide modifications in dietary habits – resulting in a decrease in diet quality, especially in western world, characterised by higher intakes of refined and pre-packaged foods with a poor nutritional quality and a low intake of fruits and vegetables – have been associated with increased prevalence of asthma(Reference Garcia-Larsen, Del Giacco and Moreira2).
In public health strategies aiming to tackle the deleterious consequences of poor diet, front-of-pack (FOP) nutrition labels have received growing attention to help consumers to make healthier choices at the point of purchase. Most of the FOP nutrition labelling relies on the nutritional quality of foods using a nutrient profiling system (NPS). Among the available nutrient profiling systems, NPS developed by the UK Food Standard Agency (named FSA-NPS) is one of the most scientifically validated systems in the European context(Reference Julia, Kesse-Guyot and Ducrot3–Reference Julia and Hercberg5). It has been developed and validated initially in the British food environment(Reference Julia, Touvier and Méjean6), but previous studies have demonstrated its applicability to the French context after some modifications by the French High Council for Public Health (Haut Conseil de la Santé Publique; HCSP)(Reference Julia, Kesse-Guyot and Ducrot3, Reference Julia, Ducrot and Péneau4, 7, Reference Julia, Kesse-Guyot and Touvier8).
For these reasons the modified Food Standards Agency Nutrient Profiling System (FSAm-NPS/HCSP) was used in France to underlie a FOP nutrition label, the Nutri-Score, which was implemented in 2017. A dietary index (DI) based on the FSAm-NPS (FSAm-NPS DI) has been developed, reflecting the overall nutritional quality of the diet at the individual level, and employed to validate the algorithm used for the computation of the Nutri-Score(Reference Julia, Touvier and Méjean6, Reference Julia, Méjean and Touvier9). Less healthy diets, as expressed by higher FSAm-NPS DI, have been associated with a higher risk of several chronic diseases, such as overall cancer(Reference Donnenfeld, Julia and Kesse-Guyot10), breast cancer(Reference Deschasaux, Julia and Kesse-Guyot11), CVD(Reference Adriouch, Julia and Kesse-Guyot12, Reference Adriouch, Julia and Kesse-Guyot13), the metabolic syndrome(Reference Julia, Fézeu and Ducrot14) or weight gain and obesity(Reference Julia, Ducrot and Lassale15).
To the best of our knowledge, no study has investigated the association between the overall nutritional quality of diet, based on a nutrient profiling system of food consumed, and asthma.
Thus, our aim was to investigate the association between the FSAm-NPS DI and the asthma symptom score in a large cohort of French adults.
Methods
Study population
Participants were a selection of volunteers from the NutriNet-Santé study(Reference Hercberg, Castetbon and Czernichow16), a prospective observational cohort study launched in May 2009 to evaluate the determinants of eating behaviours and the relationships between nutrition and chronic disease risk. Details of the NutriNet-Santé study are extensively described elsewhere(Reference Hercberg, Castetbon and Czernichow16). Participants of the NutriNet-Santé study, all aged ≥18 years, gave electronic informed consent. All procedures have been approved by the institutional review board of the French Institute for Health and Medical Research (0000388FWA00005831) and the French Institutional Ethics Committee (CNIL numbers 908450 and 909216). The NutriNet-Santé Study is registered in ClinicalTrials.gov (NCT03335644).
Dietary data collection
At inclusion and twice a year thereafter, participants were invited to complete three non-consecutive, self-administered, web-based 24-h dietary records randomly allocated over a 2-week period, including 2 week-days and 1 weekend day. Self-administered, web-based, 24-h dietary records have been validated against urinary(Reference Lassale, Castetbon and Laporte17) and plasma biomarkers(Reference Lassale, Castetbon and Laporte18) and interview by a trained dietitian(Reference Touvier, Kesse-Guyot and Méjean19). For the present study, to have a better estimate of dietary habits, we included participants who completed at least three 24-h dietary records since their inclusion till 2 years of follow-up. They reported all foods and beverages consumed at each eating occasion. Portion sizes for each food and beverage were estimated using validated photographs(Reference Le Moullec, Deheeger and Preziosi20) or by indicating the exact quantity in grams or the volume in millilitres. Mean daily dietary alcohol and nutrient intakes were estimated using the NutriNet-Santé food composition table, which includes more than 3000 different items(21).
We also excluded underreporting participants identified on the basis of the method proposed by Black(Reference Black22) using Schofield equations(Reference Schofield23) and taking into account sex, age, height and weight, as well as physical activity level (PAL), number of 24-h records, intra-individual variabilities of reported energy intake and BMR, and intra-/inter-variabilities of PAL. However, it is important to note that the exclusion of subjects (online Supplementary Fig. S1) following the Goldberg cut-off point was not optimal due to its low sensitivity(Reference Black22).
Modified Food Standards Agency Nutrient Profiling System dietary index computation
The FSAm-NPS score for all foods and beverages was computed based on their nutrient content for 100 g. Positive points (0–10) were allocated for the content of energy (kJ), total sugar (g), SFA (g), and Na (mg). Negative points (0–5) were allocated for the content of fruits, vegetables and nuts, fibres and proteins. FSAm-NPS scores for foods and non-alcoholic beverages were based on a discrete continuous scale ranging from −15 (most healthy) to +40 (less healthy). Thus, an increase in the score reflects a decreasing nutritional quality of the food or beverage item.
Specific modifications of the score for cheese, added fats and beverages were made to maintain a high consistency with the French nutritional recommendations, as proposed by the French HCSP, leading to the FSAm/HCSP algorithm(7).
The FSAm-NPS DI was computed at the individual level using arithmetic energy-weighted means. The corresponding equation has been described elsewhere(Reference Julia, Fézeu and Ducrot14). Increasing FSAm-NPS DI reflects decreasing overall diet quality.
Respiratory data
To improve the respiratory characterisation in the cohort, a non-mandatory detailed questionnaire on respiratory health based on international standardised recommendations(Reference Burney, Luczynska and Chinn24) was proposed in April 2016 to all the active participants (n 121 568). As June 2017, the survey was completed by 40 152 adults (online Supplementary Fig. S1).
We used the asthma symptom score(Reference Pekkanen25, Reference Sunyer, Pekkanen and Garcia-Esteban26), which has been previously proposed as a continuous measure of asthma in epidemiological studies. It is a validated score that has shown good predictive ability against outcomes related with asthma. The asthma symptom score is assessed on a scale from 0 to 5, with higher scores indicating a higher number of symptoms. It is based on the number of respiratory symptoms during the past 12 months: (1) breathless while wheezing, (2) woken up with chest tightness, (3) attack of shortness of breath at rest, (4) attack of shortness of breath after exercise and (5) woken by attack of shortness of breath.
‘Ever asthma’ was defined by at least one positive answer to the question ‘Have you ever had asthma?’ in main questionnaires, or by a positive answer to ‘Have you ever had an asthma attack?’ or ‘Have you ever had an attack of shortness of breath at rest with wheezing’ in the respiratory survey. Information about family history of asthma was also collected.
Allergic rhinitis was defined as a positive answer to the following questions: ‘Have you ever had allergic rhinitis?’ or ‘Have you ever had hay fever?’
Covariate assessment
Baseline questionnaires provided information on sociodemographic(Reference Vergnaud, Touvier and Méjean27) and anthropometric measurements,(Reference Lassale, Péneau and Touvier28, Reference Touvier, Méjean and Kesse-Guyot29) including age and sex. Educational level was classified into four groups (<13, 14, 15–16 and ≥17 years), and smoking status into three groups (never smokers, ex-smokers, current smokers). Among ever smokers, pack-years were calculated to estimate the amount of tobacco smoke. BMI was calculated as weight (kg)/height2 (in m2) and categorised according to the WHO classification (<18·5, 18·5–24, 25–29, ≥30 kg/m2)(30). Physical activity was assessed using the short form of the French version of the International Physical Activity Questionnaire(Reference Hagströmer, Oja and Sjöström31). The latter allows estimating three levels of physical activity: vigorous (≥60 min/d), moderate (30–59 min/d), low (<30 min/d).
Statistical analysis
Analyses were conducted separately among men and women to take into account sex differences in the diet–asthma association(Reference Fuseini and Newcomb32).
Baseline characteristics of participants are reported as means and standard deviations or numbers and percentages according to sex-specific quintiles of the FSAm-NPS DI.
The asthma symptom score was considered as a continuous variable, and a negative binomial regression was performed to evaluate the association between quintiles of the FSAm-NPS DI and the asthma symptom score.
The following potential confounders were included in the main model: age, smoking, pack-years, educational level, leisure time physical activity, daily energy intake, alcohol intake (g/d, continuous), presence of allergic rhinitis and family history of asthma. Tests for linear trends were performed using quintiles of FSAm-NPS DI score as an ordinal variable.
The asthma symptom score has the potential to reveal asthma in individuals not previously identified as thus. Hence, we carried out a sensitivity analysis to highlight the strength of the score by inclusion of participants who had never previously reported symptoms of asthma since their inclusion in the NutriNet-Santé cohort till answered the respiratory survey. Furthermore, since diet quality is often associated with smoking habit, and to take into account potential residual confounding by cigarette smoking, we also conducted a sensitivity analysis stratified by smoking status. Finally, as diet affects BMI, and obesity is likely a risk factor for asthma, BMI might be a potential mediator in the diet–asthma association; thus we also performed a stratified analysis based on BMI.
To handle missing data, we used multiple imputations methods (n 10) according to a Markov chain–Monte Carlo approach(Reference Yuan33). Data were analysed using SAS version 9.4 (SAS Institute). All tests were two-sided, and a significance level of 0·05 was used.
Results
Participant characteristics
Among the 40 152 participants who filled in the non-mandatory, web-based questionnaire on respiratory health, we excluded those with less than three dietary records till their 2 years of follow-up (n 2122). The final sample included 34 323 participants (25 823 women and 8500 men) for which the FSAm-NPS DI could have been computed (online Supplementary Fig. S1). Overall, the average FSAm-NPS DI score was 6·1 (sd 2·2) in women and 6·0 (sd 2·1) in men. Mean participant age was 54 (sd 14) years (53 (sd 14) years for women and 59 (sd 13) years for men).
Participant characteristics are shown in Table 1 for women and Table 2 for men according to quintiles of the FSAm-NPS DI.
Table 1. Characteristics of the participants, before imputation, according to the quintiles (Q) of the Food Standards Agency Nutrient Profiling System dietary index (FSA-NPS DI), among women (n 25 823) from the NutriNet-Santé study
(Mean values and standard deviations; numbers and percentages)

* To convert kcal to kJ, multiply by 4·184.
† Number of respiratory symptoms during the past 12 months: (1) breathless while wheezing, (2) woken up with chest tightness, (3) attack of shortness of breath at rest, (4) attack of shortness of breath after exercise, and (5) woken by attack of shortness of breath. Each item is scored from 0 to 1, and the total asthma symptom score ranges from 0 to 5.
‡ Defined by at least one positive answer to the question ‘Have you ever had an asthma attack?’ in main questionnaires (baseline or follow-up), and by a positive answer to ‘Have you ever had an asthma attack?’ or ‘Have you ever had an attack of shortness of breath at rest with wheezing’ in the respiratory survey (2016).
Table 2. Characteristics of the participants, before imputation, according to the quintiles (Q) of the Food Standards Agency Nutrient Profiling System dietary index (FSA-NPS DI), among men (n 8500) from the NutriNet-Santé study
(Mean values and standard deviations; numbers and percentages)
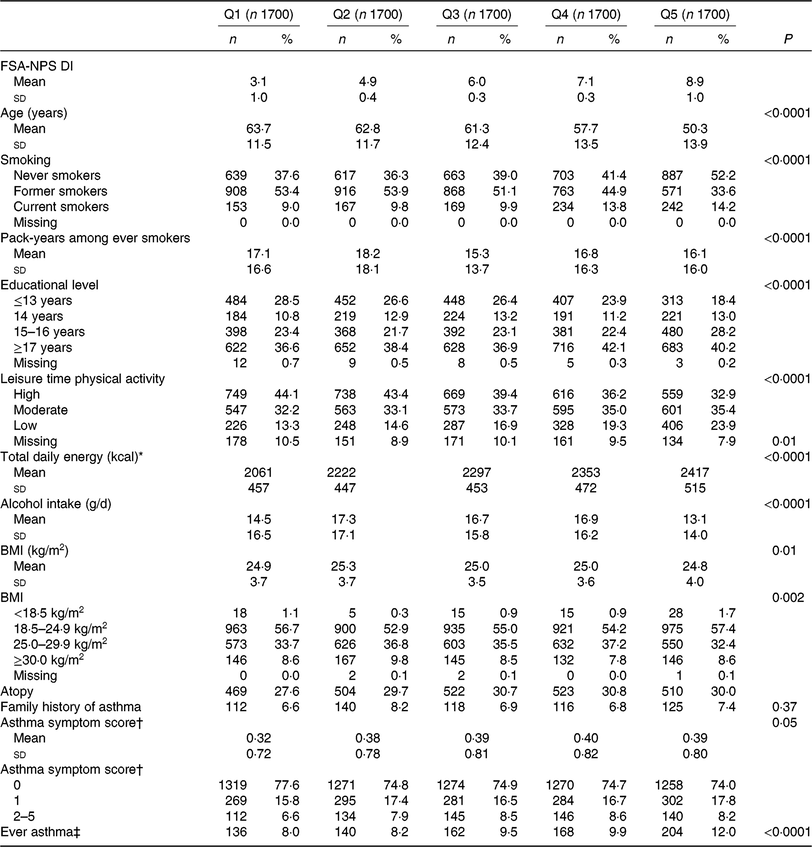
* To convert kcal to kJ, multiply by 4·184.
† Number of respiratory symptoms during the past 12 months: (1) breathless while wheezing, (2) woken up with chest tightness, (3) attack of shortness of breath at rest, (4) attack of shortness of breath after exercise, and (5) woken by attack of shortness of breath. Each item is scored from 0 to 1, and the total asthma symptom score ranges from 0 to 5.
‡ Defined by at least one positive answer to the question ‘Have you ever had an asthma attack?’ in main questionnaires (baseline or follow-up), and by a positive answer to ‘Have you ever had an asthma attack?’ or ‘Have you ever had an attack of shortness of breath at rest with wheezing’ in the respiratory survey (2016).
Table 3. Associations between quintiles (Q) of the Food Standards Agency Nutrient Profiling System dietary index (FSA-NPS DI) and asthma symptom score among women and men from the NutriNet-Santé study (n 34 323)
(Numbers of participants; mean values and standard deviations; odds ratios and 95 % confidence intervals)
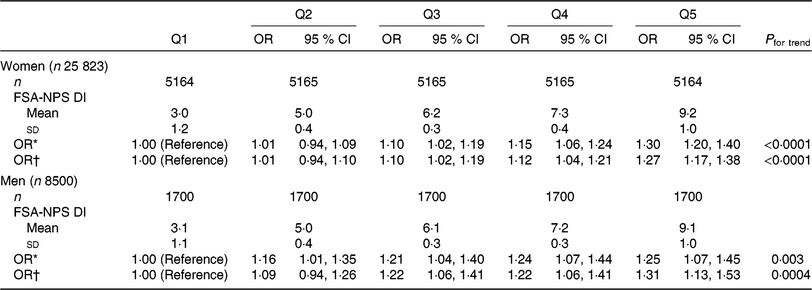
* Models were adjusted for age.
† Models were further adjusted for smoking, pack-years (among ever smokers), educational level, leisure time physical activity, total daily energy, alcohol intake, allergic rhinitis and family history of asthma.
Both among women (Table 1) and men (Table 2), compared with participants with the lowest FSAm-NPS DI (quintiles 1, healthier diet), participants with the highest FSAm-NPS DI (quintiles 5, less healthy diet) were significantly younger, more likely to be current smokers, had higher educational level, practiced less intense physical activity, had higher energy intakes and reported more allergic rhinitis and less ever asthma. Among women only, participants with the lowest FSAm-NPS DI were more likely to be overweight or obese (Table 1).
Associations between the modified British Food Standards Agency Nutrient Profiling System dietary index and asthma symptom score
Associations between FSAm-NPS DI and the asthma symptom score are presented in Table 3 for women and men. Accordingly, 28 % of women and 25 % of men reported at least one asthma symptom. After adjusting for several potential confounders, we observed that a higher FSAm-NPS DI was positively and significantly associated with greater asthma symptoms both among women and men. OR for the highest FSAm-NPS DI (quintile 5) v. the lowest FSAm-NPS DI (quintile 1) was 1·27 (95 % CI 1·17, 1·38) in women and 1·31 (95 % CI 1·13, 1·53) in men.
Restricting analysis to participants without ever asthma (n 23 435 women and 7853 men) did not modify the observed associations, and similar associations were reported between the asthma symptom and the FSAm-NPS DI both among women (online Supplementary Table S1) and men (online Supplementary Table S2). After stratification by smoking status, associations remained significant and of similar magnitudes within each stratum among women (online Supplementary Table S3). Among men, the associations were significant only in never and former smokers (online Supplementary Table S4). Lastly, after stratification based on BMI, associations remained significant within each stratum among women (online Supplementary Table S5). Associations were still positive but were statistically significant only for participants with BMI < 25 kg/m2 among men (online Supplementary Table S6).
Discussion
In this large cohort of French adults, a higher FSAm-NPS DI score, reflecting poorer food choices in the diet, was associated with a higher asthma symptom score. The association remained significant after adjusting for a wide range of potentially confounding variables and was also significant in participants without ever asthma.
To our knowledge, no other study has investigated the association between asthma and a dietary score based on a nutrient profiling system of the foods consumed. Indeed, the FSA score was initially developed to account for the nutrients for which a major concern has been raised regarding public health significance, but not specifically asthma. In this context, in France, several studies have reported significant associations between the FSAm-NPS DI and increased risks of cancer(Reference Donnenfeld, Julia and Kesse-Guyot10, Reference Deschasaux, Julia and Kesse-Guyot11), CVD(Reference Adriouch, Julia and Kesse-Guyot12, Reference Adriouch, Julia and Kesse-Guyot13), the metabolic syndrome(Reference Julia, Fézeu and Ducrot14) and obesity(Reference Julia, Ducrot and Lassale15).
The FSAm-NPS DI, based on the FSAm-NPS of the foods consumed, has been shown to reflect the nutritional quality of the diet(Reference Julia, Touvier and Méjean6, Reference Julia, Méjean and Touvier9). Still, few studies have been conducted to assess the association between overall nutrition diet quality and asthma. A study conducted in the USA in a large cohort of women reported no association between overall nutritional diet quality assessed by the Alternative Healthy Eating Index (AHEI-2010) and the risk of adult-onset asthma(Reference Varraso, Chiuve and Fung34). However, the authors used a dichotomous definition of asthma, which, compared with the asthma symptom score, may not correctly reflect phenotypic variability in asthma(Reference Pekkanen25, Reference Sunyer, Pekkanen and Garcia-Esteban26). In contrast, using data from the French prospective Epidemiological study on the Genetics and Environment of Asthma study, but using the asthma symptom score as a continuous definition of asthma, Li et al. reported a significant association between a higher overall nutritional diet quality measured by the AHEI-2010 and improvement in asthma symptoms in never smokers(Reference Li, Kesse-Guyot and Dumas35). We also described on the NutriNet-Santé cohort that scores reflecting a healthier diet (AHEI-2010, MED-LITE and Programme National Nutrition Santé Guideline Score (PNNS-GS)) were associated with a statistically significant decreased risk of asthma symptoms(Reference Andrianasolo, Kesse-Guyot and Adjibade36). Our results are consistent with mechanistic studies regarding the associations between nutritional factors and asthma. First, studies have been conducted on the role of fruits and vegetables and dietary fibres(Reference Julia, Touvier and Méjean6), which are major components of the FSAm-NPS DI, in explaining the diet–asthma association, at least partly. Secondly, studies have also been conducted on the potential role of salt, another component of the FSAm-NPS DI, on asthma and other chronic lung disease, such as chronic bronchitis(Reference Suadicani, Hein and Gyntelberg37). In fact, high salt intake was reported to be risky for lung inflammation through a specific activation state in the macrophages, termed M(Na)(Reference Zhang, Zheng and Du38). In line with these findings, a case–control study conducted in Australia using an a priori score, which reflected the inflammatory potential of overall diet, the Dietary Inflammatory Index (DII), reported greater DII score in participants with ever asthma(Reference Wood, Shivappa and Berthon39). Finally, a recent study reported that lower SCFA production, end-products of fermentation of dietary fibres (mainly from fruits, vegetables or legumes), which can lead to an imbalanced gut microbiota(Reference McKenzie, Tan and Macia40), was associated with increased airway inflammation(Reference Halnes, Baines and Berthon41).
This study has some limitations that need to be pointed out. First, our results should be extrapolated with caution since participants from the NutriNet-Santé cohort were all volunteers involved in a long-term study that investigated the association between nutrition and health, with overall more health-conscious behaviours and higher socio-professional and educational levels(Reference Andreeva, Deschamps and Salanave42). Moreover, it has been observed that participants from the NutriNet-Santé cohort reported higher intake of healthy foods compared with participants from a representative French population-based study(Reference Andreeva, Deschamps and Salanave42). As unhealthy dietary behaviours are underrepresented in our study, the strength of the diet–asthma association is likely weakened in our sample compared with the general population. Second, the respiratory data used in the analysis were collected cross-sectionally and limit the conclusions that could be drawn with regard to causality. However, the association was also significant in participants without ever asthma. Third, we used self-reported questionnaires to gather data, which are inherently prone to biases(Reference Rosenman, Tennekoon and Hill43) and might have led to misclassification and possibly weakening of associations. However, self-reported questionnaires are frequently used in population studies for epidemiological purposes, and objective validation studies performed in the NutriNet-Santé cohort supported the accuracy of self-reports as a measure of diet(Reference Lassale, Castetbon and Laporte17, Reference Lassale, Castetbon and Laporte18) and anthropometrics(Reference Lassale, Péneau and Touvier28). In addition, the FSAm-NPS DI was also validated against food consumption, nutrient intake and biomarkers of nutritional status in several studies(Reference Julia, Touvier and Méjean6, Reference Julia, Méjean and Touvier9, Reference Deschamps, Julia and Salanave44). In addition, the observational data may also be subject to residual confounding although we adjusted for several potential confounders. Fourth, since the overall nutritional quality of the diet has been associated with chronic obstructive pulmonary disease (COPD), potential overlaps between COPD and asthma might contribute to the association. However, since similar results were observed among never smokers, overlap between COPD and asthma was less likely in our study.
The key strengths of our study are the large sample size that allowed us to account for several potential confounders and to have sufficient statistical power to investigate stratified associations. Furthermore, we used validated tools to assess asthma symptoms(Reference Pekkanen25, Reference Sunyer, Pekkanen and Garcia-Esteban26), and dietary data were assessed by repeated 24-h dietary records (at least three) that reflect usual dietary behaviors.
In conclusion, our results suggest that unhealthy food choices, as reflected by a higher FSAm-NPS DI, were associated with greater asthma symptoms. Thus, these results reinforce the relevance of a public health approach to help consumers make healthier food choices by using a clear and easy-to-understand FOP nutrition label based on the FSAm-NPS, such as the Nutri-Score, which has been recently implemented in France.
Acknowledgements
The authors thank Nathalie Druesne-Pecollo and Thi Duong Van (Equipe de Recherche en Epidémiologie Nutritionnelle, Centre d’Epidémiologie et Statistiques Sorbonne Paris Cité, Inserm (U1153), Inra (U1125), Bobigny, France) for their contribution to the development and implementation of the respiratory survey in the Nutrinet-Santé study. We also thank Younes Esseddik, Frédéric Coffinieres, Régis Gatibelza and Paul Flanzy (computer scientists); and Nathalie Arnault, Véronique Gourlet, Dr Fabien Szabo, Julien Allegre, Laurent Bourhis (data managers/biostatisticians) and Dr Fatoumata Diallo (physician) for their technical contribution to the NutriNet-Santé study. We thank all the volunteers of the NutriNet-Santé cohort.
The NutriNet-Santé study was supported by the following public institutions: Ministère de la Santé, Santé Publique France, Institut National de la Santé et de la Recherche Médicale, Institut National de la Recherche Agronomique, Conservatoire National des Arts et Métiers and Université Paris 13.
Author contributions were as follows. R. M. A., C. J., R. V., M. T., E. K. G., S. H. and P. G. designed and conducted the research; C. J., R. V., M. E., M. T., E. K. G., S. H. and P. G. provided essential reagents or materials; R. M. A., C. J. and P. G. analysed data or performed statistical analyses; R. M. A., C. J., S. H. and P. G. wrote the manuscript and had primary responsibility for final content; C. J., R. V., M. E., M. T., E. K. G., S. H. and P. G. were involved in interpreting the results and editing the manuscript for important intellectual content. All authors read, edited and approved the final manuscript.
We have no conflict of interest to declare.
Supplementary material
For supplementary materials referred to in this article, please visit https://doi.org/10.1017/S0007114519000655






