TAG-rich lipoproteins and their remnants are strongly associated with CVD and all-cause mortality(Reference Nordestgaard1–Reference Toth6). The postprandial TAG response after a high-fat meal has been shown to be larger in men compared with women(Reference Lairon, Lopez-Miranda and Williams7, Reference Wojczynski, Glasser and Oberman8), which may be a contributing factor to why men have earlier first-time event, and higher mortality rate, of CHD(Reference Bots, Peters and Woodward9, Reference Leening, Ferket and Steyerberg10). TAG-rich lipoproteins include all lipoproteins of various sizes that are enriched with TAG, that is, chylomicrons and VLDL(Reference Pirillo, Norata and Catapano11, Reference Nakajima, Nakano and Tokita12), and men have also been shown to have higher postprandial response of TAG-rich VLDL particles compared with women(Reference Wojczynski, Glasser and Oberman8).
Lipoproteins can be divided into subclasses based on their altered particle size as they undergo lipolysis. In epidemiological studies, all VLDL subclasses have been associated with increased CVD risk, and in particular smaller VLDL particles(Reference Wurtz, Havulinna and Soininen13–Reference Fischer, Kettunen and Wurtz17). LDL-cholesterol is a causal CVD risk factor(Reference Ference, Ginsberg and Graham18), and all LDL subclasses are associated with increased CVD risk(Reference Wurtz, Havulinna and Soininen13, Reference Holmes, Millwood and Kartsonaki14, Reference Mora, Caulfield and Wohlgemuth19). Plasma TAG concentration is inversely correlated with HDL-cholesterol (HDL-C) concentration(Reference Nordestgaard, Benn and Schnohr2, Reference Kolovou, Mikhailidis and Kovar5, Reference Bansal, Buring and Rifai20), and there is epidemiological evidence that HDL-C is protective against CVD(Reference Madsen, Varbo and Tybjaerg-Hansen21–Reference Di Angelantonio, Sarwar and Perry23). There is a well-known sex difference in fasting HDL-C concentration, with women having higher HDL-C than men(Reference Koutsari, Zagana and Tzoras24). HDL particle concentration (HDL-P) has lately been shown to be a better marker for residual CVD and mortality risk than HDL-C(Reference Mora, Glynn and Ridker25, Reference McGarrah, Craig and Haynes26), but it is unclear to what extent HDL particle size matters as literature shows inconsistent results(Reference Wurtz, Havulinna and Soininen13, Reference Holmes, Millwood and Kartsonaki14, Reference McGarrah, Craig and Haynes26, Reference Wu, Fan and Tian27).
Both long-term interventional studies and epidemiological data indicate that dairy products are a heterogeneous food group with various effects on blood lipids(Reference de Goede, Geleijnse and Ding28) and all-cause mortality(Reference Guo, Astrup and Lovegrove29, Reference Tognon, Nilsson and Shungin30). For the first time, we recently demonstrated divergent postprandial TAG and HDL-C responses (measured as the 0–6 h incremental AUC (iAUC0–6h)) between different dairy products in healthy adults(Reference Hansson, Holven and Øyri31), with a borderline significant effect of sex on serum TAG-iAUC0–6h. We have also shown that dietary fat quality affects the peak time of larger VLDL subclasses postprandially in healthy adults and adults with familial hypercholesterolemia(Reference Oyri, Hansson and Bogsrud32). How intake of different dairy products affects postprandial lipoprotein subclass concentrations in women and men has, to the best of our knowledge, not been reported. Thus, the aim of this sub-study was to investigate if men and women respond differently to high-fat dairy meals, with the same amount of fat from different dairy products, with respect to postprandial lipoprotein subclass concentrations. The primary outcomes for this exploratory sub-study were the total 6 h postprandial particle concentration responses of six VLDL subclasses and four HDL subclasses, and secondary outcomes were the corresponding particle concentration responses of intermediate-density lipoprotein (IDL) and LDL subclasses, all measured as the iAUC0–6h.
Subjects and methods
Subjects
A total of forty-seven healthy subjects (thirty-three women and fourteen men) who accomplished at least one visit in a postprandial study at the University of Oslo between September 2016 and April 2017 were included in this exploratory sub-study. A total of twenty-one women and ten men completed all four study visits (Fig. 1). As abdominal obesity is associated with an elevated postprandial TAG response(Reference Jackson, Walden and Murray33), subjects between 18 and 70 years of age with BMI 18·5–25 kg/m2 and waist circumference <80 cm for women and <94 cm for men, or BMI ≥25 kg/m2 and waist circumference ≥80 cm for women and ≥94 cm for men were recruited to the postprandial study. Complete inclusion and exclusion criteria have been described previously(Reference Hansson, Holven and Øyri31).
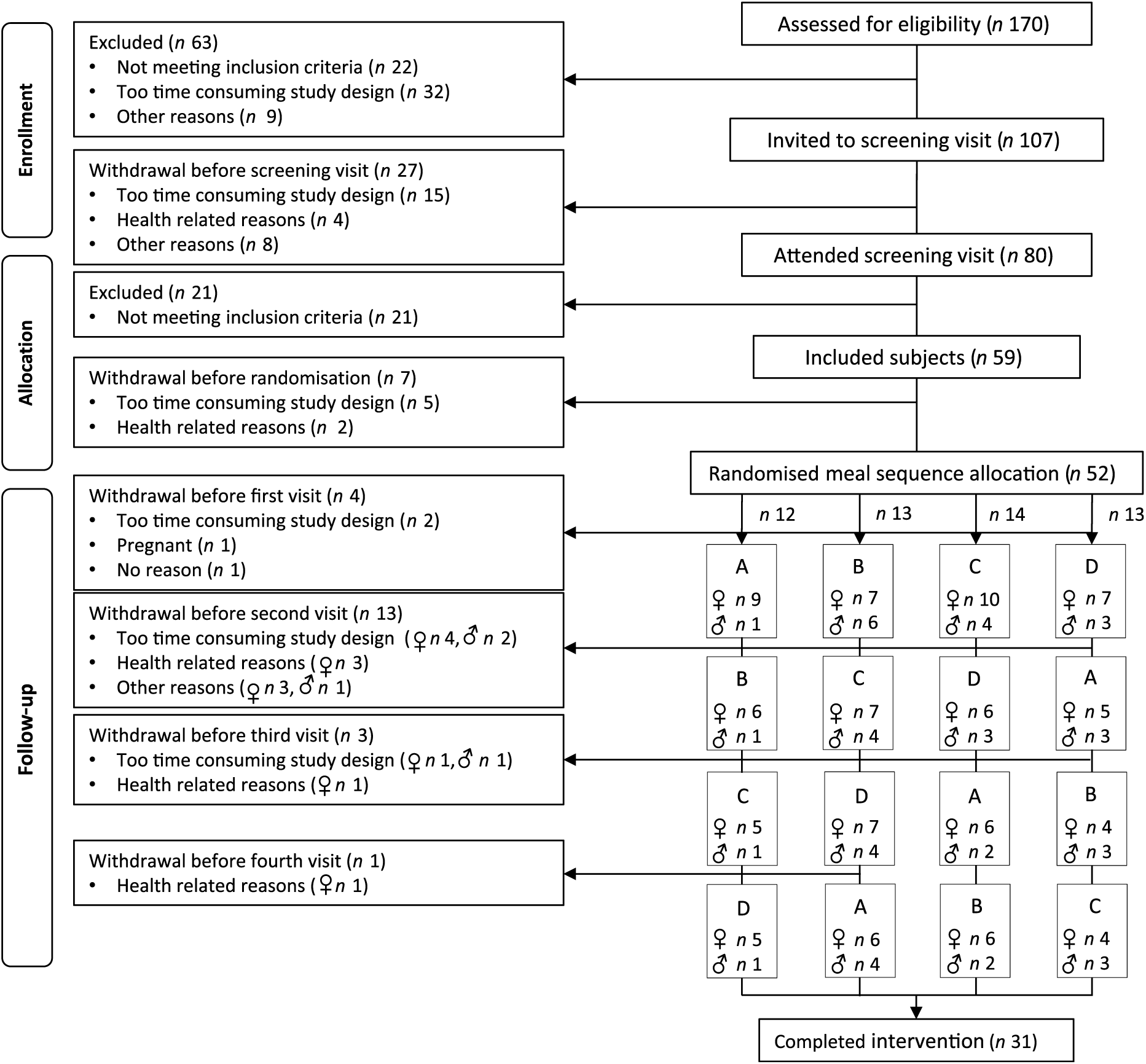
Fig. 1. Flow chart of the study participants. (A) Meal rich in fat from butter; (B) meal rich in fat from medium-hard cheese; (C) meal rich in fat from whipped cream; (D) meal rich in fat from sour cream.
Study design
A randomised controlled cross-over study was conducted with four high-fat dairy meals as intervention, as has been described earlier (Reference Hansson, Holven and Øyri31). Briefly, each meal consisted of three toasted slices of white bread (Pågen Rosta), raspberry jam (Nora Bringebærsyltetøy) and either butter (TINE Smør), medium-hard cheese (TINE Gräddost), whipped cream (TINE Kremfløte) or sour cream (TINE Seterrømme), corresponding to 45 g of fat and approximately 60 energy percent of fat with similar fatty acid profiles. The energy content of each meal was 629 kcal (2632 kJ) for the butter meal, 715 kcal (2992 kJ) for the cheese meal, 652 kcal (2728 kJ) for the whipped cream meal and 655 kcal (2741 kJ) for the sour cream meal. The subjects were randomly allocated to one of the four test meal orders (order 1: A_B_C_D, order 2: B_C_D_A, order 3: C_D_A_B and order 4: D_A_B_C; A = butter, B = cheese, C = whipped cream and D = sour cream) by block randomisation performed by the principal investigator. Allocation ratio was 1:1:1:1. Each test day was separated by a 3- to 5-week-long washout period for premenopausal women not taking contraceptives, and a minimum washout period of 2 weeks for other participants. Before each test day, subjects received a reminder to fast for 12 h (with no fatty food for 14 h) and not perform any strenuous physical activity or drink alcohol the last 24 h before visit. Blood samples were drawn fasting, and 2, 4 and 6 h after the meal. Subjects were encouraged to be physically inactive during the 6 h period of blood sampling.
Clinical measurements
Weight was measured by the Medical Body Composition Analyzer seca 515/514 (seca, software version 1.1). Blood pressure was measured three consecutive times in a sitting position in the subjects’ non-dominant arm by a Dinamap Carescape v100 (GE Medical System) at a screening visit.
Blood sampling and lipoprotein subclass measurement
Serum was collected in silica gel tubes (Becton Dickinson Vacutainer Systems) and kept in room temperature for 30–60 min to ensure complete blood coagulation before 15 min centrifugation at 1500 g (Thermo Fischer Scientific). Serum samples were then stored in a refrigerator until being analysed. Standard blood biochemical measurements were performed at an accredited medical laboratory (Fürst Medical Laboratory)(Reference Hansson, Holven and Øyri31). Plasma was collected in EDTA tubes (Becton Dickinson Vacutainer Systems) and kept on ice for less than 15 min before being centrifuged at 2000 g for 15 min at 4°C (Thermo Fischer Scientific). The samples were distributed into smaller tubes and frozen at −80°C for lipoprotein subclass analysis. Lipoprotein subclass profiling was achieved using a commercial proton NMR metabolomics platform (Nightingale Health Ltd) with the following classifications: extremely large (XXL) VLDL with particle diameters of at least 75 nm (including chylomicrons), five VLDL subclasses (very large (XL), large (L), medium (M), small (S) and very small (XS), with average particle diameters of 64·0, 53·6, 44·5, 36·8 and 31·3 nm, respectively), one IDL subclass with an average particle diameter of 28·6 nm, three LDL subclasses (L, M and S, with average particle diameters of 25·5, 23·0, and 18·7 nm, respectively) and four HDL subclasses (XL, L, M and S, with average particle diameters of 14·3, 12·1, 10·9 and 8·7 nm, respectively). Details about the NMR metabolomics platform have been described previously(Reference Soininen, Kangas and Wurtz34, Reference Wurtz, Kangas and Soininen35).
Ethics
The study was approved by the Regional Committees for Medical and Health Research Ethics (2016/418/REK sør-øst B) and conducted according to the principles of the Declaration of Helsinki. Written informed consents were obtained from all subjects. The study was registered at www.clinicaltrials.gov as NCT02836106.
Statistics
The primary outcome of the original study was the serum TAG-iAUC0–6h, and the sample size calculation has been described previously(Reference Hansson, Holven and Øyri31). In this exploratory sub-study, differences in characteristics between women and men at baseline were analysed by the Mann–Whitney U test using IBM SPSS Statistics for Windows 24.0 (IBM Corp.). Baseline characteristics are presented as medians (25th–75th percentiles). Data from the postprandial measurements were analysed with a linear mixed model using Stata Special Edition 15.1 (StataCorp LLC). All subjects who completed at least one test day were included in the analysis. The response variable was the iAUC0–6h, calculated from the different time points using the trapezoid method(Reference Carstensen, Thomsen and Hermansen36, Reference Matthews, Altman and Campbell37), and the model included the variables meal, visit number, age, BMI, sex and a meal×sex interaction as fixed effects in addition to a random intercept at subject level. Response differences between meals were stratified by sex when the meal×sex interaction was significant. Non-significant meal×sex interactions were excluded from the model, and as the meal×sex interaction represents sex-specific meal differences, response differences between meals were calculated for the whole study group independent of sex in these cases. All results beyond baseline characteristics originate from this model. The significance level was set to α=0·05. To adjust for multiple testing when performing comparisons on each lipoprotein subclass, the Bonferroni correction was applied (i.e. all P values for differences between meals were multiplied by the number of meal comparisons, and all P values for meal response differences between sexes were multiplied by the number of meals). Only Bonferroni-corrected P values are presented in the text and the figures. Pairwise meal comparisons within each sex were performed by combining the appropriate regression coefficients from the linear mixed model. All data that were analysed by the linear mixed model are shown as mean values with standard errors in the figures.
Results
Baseline characteristics
The median age was 30 years for women and 33·5 years for men, with no significant difference between sexes (P=0·50). Women had higher baseline fasting serum HDL-C (P<0·001) and apo A-1 (P=0·005), and higher fasting plasma XL-HDL-P (P<0·001), L-HDL-P (P<0·001) and M-HDL-P (P=0·04) compared with men (Table 1). Women also had lower BMI (P=0·007) and systolic blood pressure (P=0·001). Men, on the contrary, had higher fasting plasma XXL-VLDL-P (P=0·05), XL-VLDL-P (P=0·03), L-VLDL-P (P=0·01), M-VLDL-P (P=0·01) and S-VLDL-P (P=0·03) compared with women (Table 1).
Table 1. Baseline characteristics of the fasting study subjects*
(Medians and interquartile ranges (IQR))
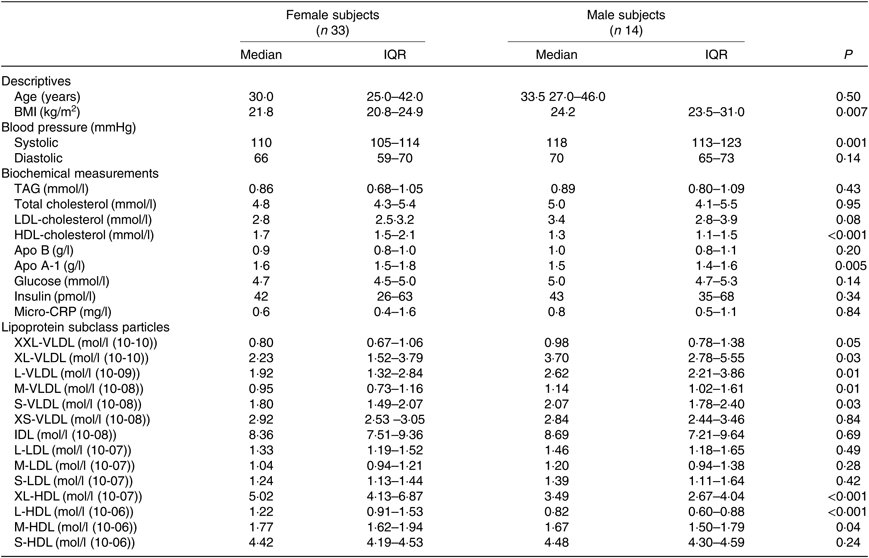
CRP, C-reactive protein; XXL, extremely large; XL, very large; L, large; M, medium; S, small; XS, very small.
* Statistical analyses were performed with the Mann–Whitney U test.
Postprandial differences between sexes
The meal by sex interaction, representing sex-specific meal differences, was only statistically significant for XS-VLDL-P (P=0·008). Significant sex effects on other lipoprotein subclass responses were independent of meal.
VLDL subclasses. After intake of each of the four test meals, men tended to have larger iAUC0–6h particle concentrations for all VLDL subclasses, except XS-VLDL, compared with women. However, a significant sex effect, independent of meal, was only found for M-VLDL (P=0·04), whereas a borderline significant sex effect was found for XXL-VLDL (P=0·05) and S-VLDL (P=0·06) (Fig. 2, online Supplementary Table 1). In contrast, men showed a decrease in XS-VLDL-P, whereas women showed a small increase after intake of sour cream (P=0·01) (Fig. 2, online Supplementary Tables 1 and 2).
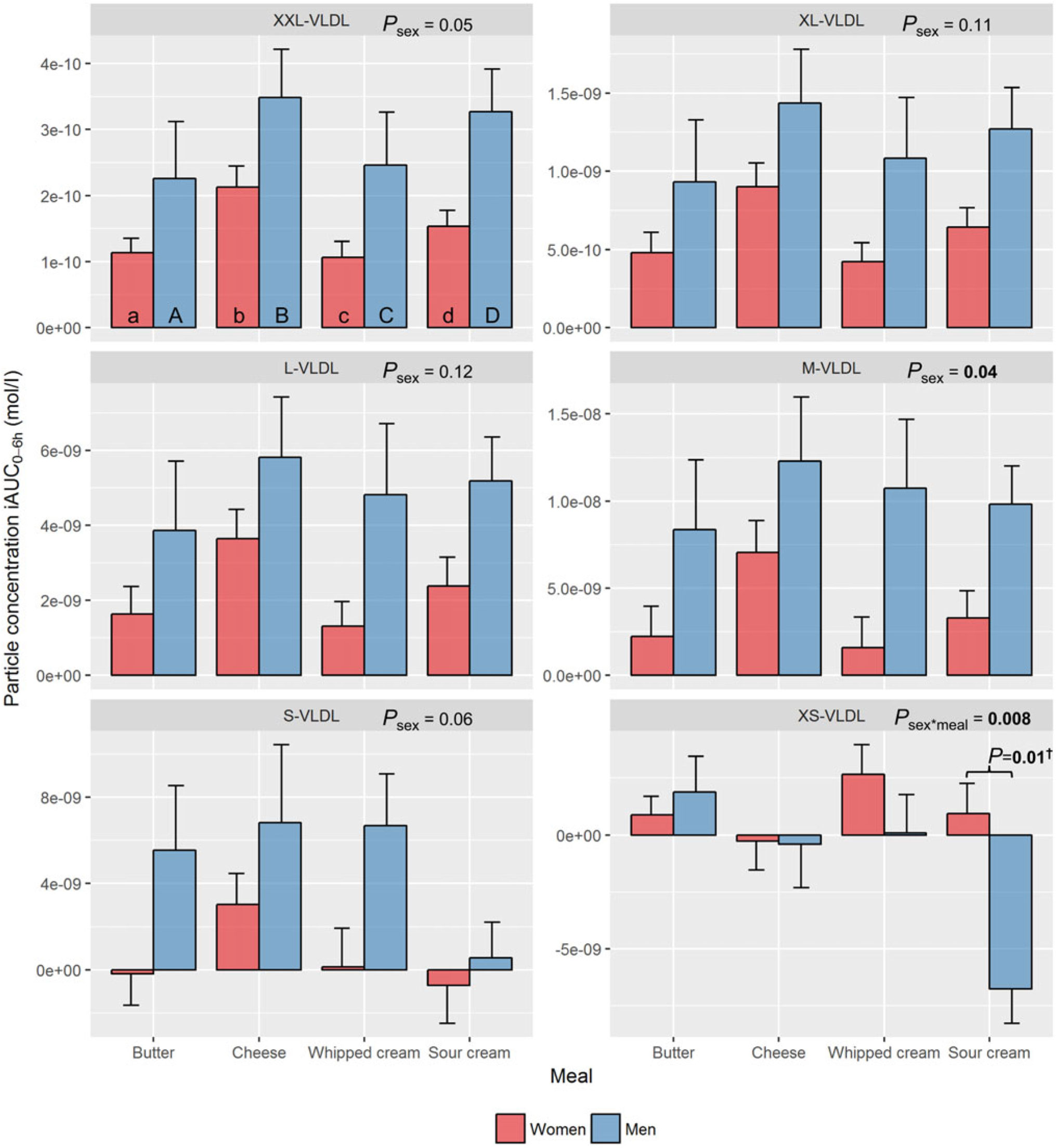
Fig. 2. Plasma 0–6 h incremental AUC (iAUC0–6h) particle concentrations of VLDL subclasses after intake of meals with butter, cheese, whipped cream and sour cream in healthy men and women. Values are means, with standard errors represented by vertical bars. The original linear mixed model included meal, age, BMI, sex, visit number and a sex × meal interaction as fixed effects. All subclasses presenting a P value for sex as a main effect (P sex) had no significant sex×meal interaction, and the interaction was consequently excluded from the model for those subclass analyses. † Bonferroni-corrected P value. (a) n 26, (A) n 10, (b) n 23, (B) n 12, (c) n 26, (C) n 12, (d) n 25, (D) n 11. (a) Response in women after intake of meal rich in fat from butter; (A) response in men after intake of meal rich in fat from butter; (b) response in women after intake of meal rich in fat from cheese; (B) response in men after intake of meal rich in fat from cheese; (c) response in women after intake of meal rich in fat from whipped cream; (C) response in men after intake of meal rich in fat from whipped cream; (d) response in women after intake of meal rich in fat from sour cream; (D) response in men after intake of meal rich in fat from sour cream. L, large; M, medium; S, small; XL, very large; XS, very small; XXL, extremely large.
HDL subclasses. Sex had a significant impact on the increase in XL-HDL-P (P=0·009) and L-HDL-P (P=0·001) with larger increases observed in women compared with men, independent of meal (Fig. 3, online Supplementary Table 1).
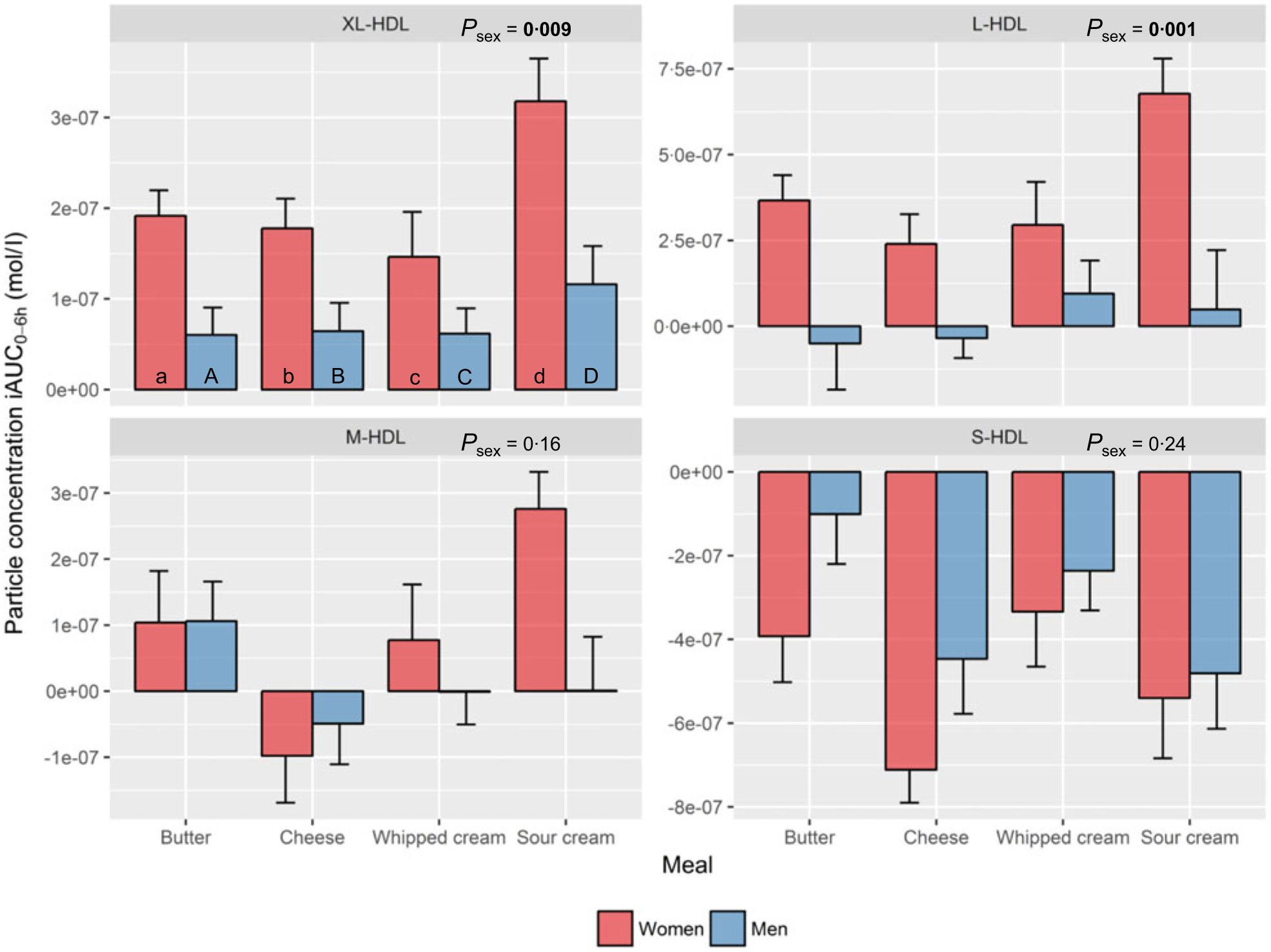
Fig. 3. Plasma 0–6 h incremental AUC (iAUC0–6h) particle concentrations of HDL subclasses after intake of meals with butter, cheese, whipped cream and sour cream in healthy men and women. Values are means, with standard errors represented by vertical bars. The original linear mixed model included meal, age, BMI, sex, a sex × meal interaction and visit number as fixed effects. All subclasses presenting a P value for sex as a main effect (P sex) had no significant sex×meal interaction, and the interaction was consequently excluded from the model for those subclass analyses. (a) n 26, (A) n 10, (b) n 23, (B) n 12, (c) n 26, (C) n 12, (d) n 25, (D) n 11. (a) Response in women after intake of meal rich in fat from butter; (A) response in men after intake of meal rich in fat from butter; (b) response in women after intake of meal rich in fat from cheese; (B) response in men after intake of meal rich in fat from cheese; (c) response in women after intake of meal rich in fat from whipped cream; (C) response in men after intake of meal rich in fat from whipped cream; (d) response in women after intake of meal rich in fat from sour cream; (D) response in men after intake of meal rich in fat from sour cream. L, large; M, medium; S, small; XL, very large.
Intermediate-density lipoprotein and LDL subclasses. Sex had a significant impact on the decrease in S-LDL-P (P=0·05) with overall larger decreases observed in women compared with men, independent of meal (Fig. 4, online Supplementary Table 1).
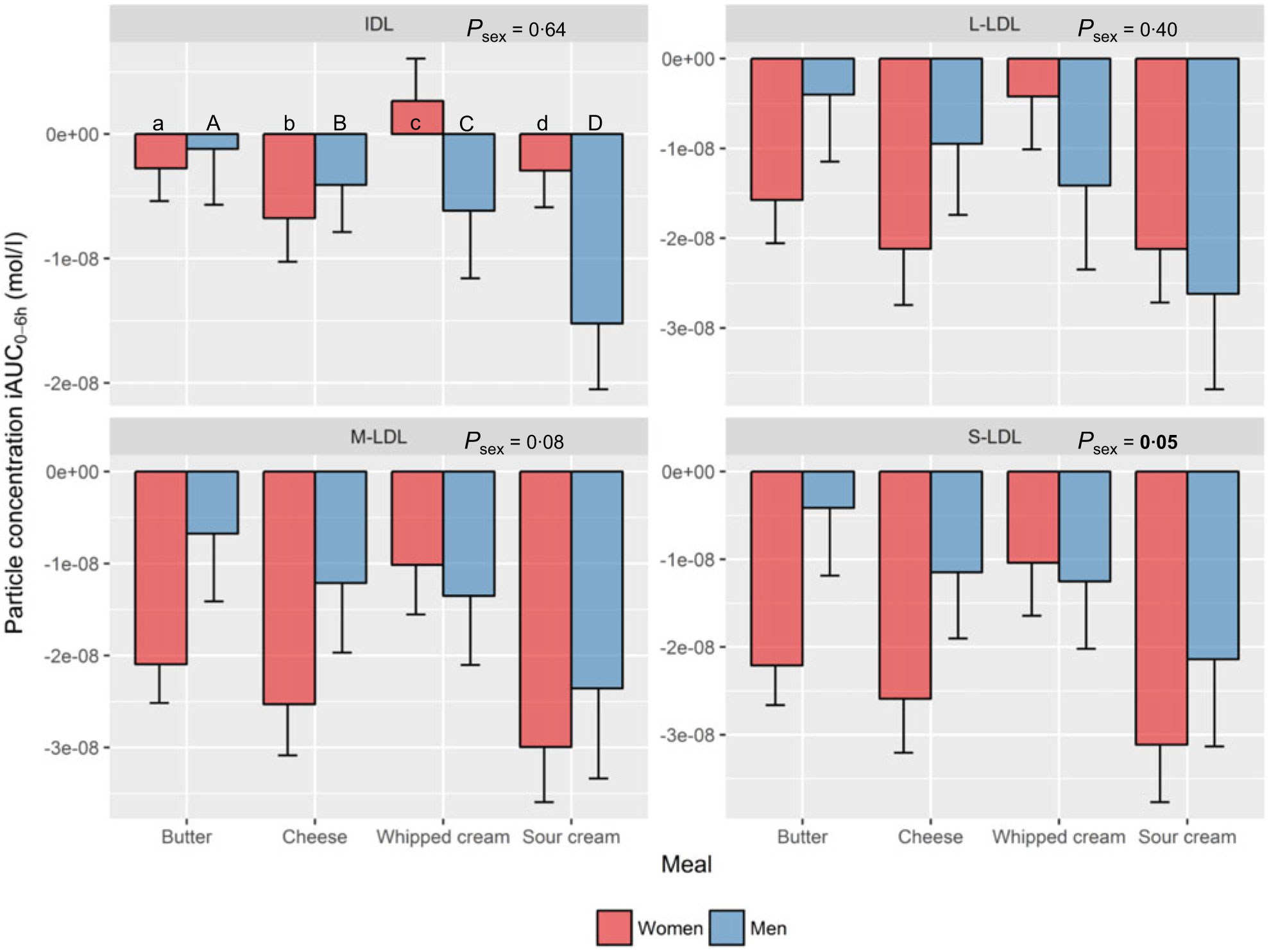
Fig. 4. Plasma 0–6 h incremental AUC (iAUC0–6h) particle concentrations of Intermediate-density lipoprotein (IDL) and LDL subclasses after intake of meal with butter, cheese, whipped cream and sour cream in healthy men and women. Values are means, with standard errors represented by vertical bars. The original linear mixed model included meal, age, BMI, sex, a sex × meal interaction and visit number as fixed effects. All subclasses presenting a P value for sex as a main effect (P sex) had no significant sex×meal interaction, and the interaction was consequently excluded from the model for those subclass analyses. (a) n 26, (A) n 10, (b) n 23, (B) n 12, (c) n 26, (C) n 12, (d) n 25, (D) n 11. (a) Response in women after intake of meal rich in fat from butter; (A) response in men after intake of meal rich in fat from butter; (b) response in women after intake of meal rich in fat from cheese; (B) response in men after intake of meal rich in fat from cheese; (c) response in women after intake of meal rich in fat from whipped cream; (C) response in men after intake of meal rich in fat from whipped cream; (d) response in women after intake of meal rich in fat from sour cream; (D) response in men after intake of meal rich in fat from sour cream. L, large; M, medium; S, small.
Meal-induced differences
VLDL subclasses. Intake of cheese induced the largest increase in XXL-VLDL-P (77 % larger v. butter: P<0·001; 76 % larger v. whipped cream: P<0·001), XL-VLDL-P (77 % larger v. butter: P=0·006; 76 % larger v. whipped cream: P=0·006) and L-VLDL-P (94 % larger v. butter: P=0·04) (online Supplementary Table 3). In men only, intake of sour cream induced a significant decrease in XS-VLDL-P (v. butter: P=0·001; v. cheese: P=0·04; v. whipped cream: P=0·006) (online Supplementary Table 3).
HDL subclasses. Intake of sour cream induced the largest increase in XL-HDL-P (69 % larger v. butter: P=0·04; 87 % larger v. cheese: P=0·03; 113 % larger v. whipped cream: P<0·001), L-HDL-P (241 % larger v. cheese: P=0·01; 110 % larger v. whipped cream: P=0·04) and M-HDL-P (v. cheese: P<0·001). In contrast, intake of cheese induced the largest decrease in S-HDL-P (101 % larger decrease v. butter: P=0·03; 103 % larger decrease v. whipped cream: P=0·01) (online Supplementary Table 4).
Intermediate-density lipoprotein and LDL subclasses. Intake of sour cream induced the largest decrease in L-LDL-P (219 % larger decrease v. whipped cream: P=0·04), M-LDL-P (153 % larger decrease v. whipped cream: P=0·006) and S-LDL-P (158 % larger decrease v. whipped cream: P = 0·02) (online Supplementary Table 5).
Discussion
The two major findings from the present study are that men and women have different postprandial lipoprotein subclass responses after intake of similar meals, and that different dairy products cause different responses within men and women, respectively. To the best of our knowledge, this is the first study to investigate sex differences in postprandial lipoprotein subclass responses to high-fat meals with different dairy products.
Regarding the postprandial iAUC0–6h particle concentrations of the HDL subclasses, sex had a significant effect on the response in XL-HDL-P and L-HLD-P with a consistently larger response seen in women compared with men. Intake of sour cream induced the largest increase in XL-, L- and M-HDL-P, which is in accordance with our previous findings from the same study where sour cream induced the largest postprandial increase in HDL-C(Reference Hansson, Holven and Øyri31). This could be due to sour cream being a homogenised dairy product, which means that sour cream consists of more and smaller fat droplets that generate increased initial lipid digestion in the gastrointestinal tract(Reference Liang, Qi and Wang38, Reference Islam, Devle and Comi39). This increase in lipolysis may potentially lead to escalated formation of nascent pre-β HDL particles in the enterocytes, which could partly explain the increase in HDL particles after intake of sour cream(Reference Camont, Chapman and Kontush40). Interestingly, even though there was no significant meal by sex interaction for any of the HDL subclasses, the figures indicate that the strong increase in XL-, L- and M-HDL-P after intake of sour cream mainly occurred in the women. Holmes et al. found that the particle concentrations of non-fasting XL-, L-HDL and M-HDL were inversely associated with myocardial infarction in Chinese adults, whereas S-HDL-P had neutral effect on myocardial infarction but was positively associated with ischaemic stroke. Furthermore, the correlations between HDL subclasses and coronary artery calcification have been studied in both women and men with or without type 1 diabetes(Reference Colhoun, Otvos and Rubens41), showing an inverse association between large HDL subclasses (corresponding to M-, L- and XL-HDL in the present study) and coronary artery calcification in both women and men without diabetes. Thus, it could be that intake of sour cream generates a more favourable postprandial HDL profile than butter, cheese and whipped cream, especially in healthy women. This provides one possible explanation for the neutral and sometimes positive epidemiological associations between intake of fermented dairy products and cardiovascular health. However, the potential health benefits of increased postprandial HDL particle concentrations remain to be investigated.
Regarding the postprandial iAUC0–6h particle concentrations of the VLDL subclasses, men showed an overall larger response in M-VLDL-P and a borderline larger response in XXL-VLDL-P and S-VLDL-P compared with women. These findings are in line with the observed borderline significant effect of sex on the serum TAG response in our previous publication(Reference Hansson, Holven and Øyri31). Based on the response patterns in Fig. 2, the lack of significant sex effects could potentially be due to the lower number of men compared with women in the study. Intake of cheese induced the largest iAUC0–6h for XXL-, XL- and L-VLDL-P, which is a deviating finding from our previous result showing a significantly larger TAG-iAUC0–6h from intake of sour cream(Reference Hansson, Holven and Øyri31). This could be explained by the different methods of measurements. Firstly, the NMR technology only measures the TAG content inside the lipoproteins, which gives a somewhat incomplete picture of the total postprandial TAG concentration, as the early postprandial phase is characterised by high lipoprotein lipase activity and, thus, a substantial amount of hydrolysed or partly hydrolysed TAG in the circulation, which are not captured with NMR. Secondly, the NMR technology has difficulties measuring very large and TAG-rich chylomicrons that can be present after a high-fat meal. It may therefore be that the sour cream generated more of these very large TAG-rich chylomicrons. This has been supported by Vors et al. who found that emulsified fat (similar to homogenised fat droplets) induced a larger increase in chylomicrons/apo-B48 and fatty acid spillover(Reference Vors, Pineau and Gabert42). The most apparent deviation in this sub-study, though, may be the observed decrease in XS-VLDL-P in men, but not in women, induced by intake of sour cream. This lipoprotein has an average diameter close to the one defining IDL and is therefore more of a remnant particle than a TAG-rich particle, with an assumed atherogenic capacity to enter the arterial wall(Reference Carmena, Duriez and Fruchart43). Non-fasting XS-VLDL-P has been linked to increased odds for myocardial infarction and ischaemic stroke in a nested case–control study(Reference Holmes, Millwood and Kartsonaki14). In addition, findings from the JUPITER (Justification for the Use of Statins in Prevention) trial showed reduced residual risk for CVD when lowering the fasting concentration of small-sized VLDL particles (with diameters corresponding to XS-VLDL measured in the present study) in statin-treated subjects(Reference Lawler, Akinkuolie and Harada15, Reference Lawler, Akinkuolie and Chu16). Interestingly, lowering the concentrations of medium and large-sized VLDL particles did not result in further risk reduction in that study. Applying these findings to our study indicates that sour cream may generate a more favourable postprandial VLDL profile than butter, cheese and whipped cream in men, potentially through a higher clearance rate of XS-VLDL particles.
Regarding the postprandial iAUC0–6h particle concentrations of IDL and the LDL subclasses, men showed an overall larger response in S-LDL-P compared with women, which is one of the lipoprotein subclasses considered to be the most atherogenic(Reference Holmes, Millwood and Kartsonaki14, Reference Diffenderfer and Schaefer44). Intake of sour cream induced significantly larger decreases in L-, M- and S-LDL-P compared with whipped cream. The LDL-cholesterol concentration is expected to temporarily decrease 5–10 % in the postprandial state due to the competition of lipoprotein lipase between intestinally derived chylomicrons and hepatic VLDL particles, where chylomicrons are the preferred lipoproteins(Reference Cohn45–Reference Bjorkegren, Packard and Hamsten47). As whipped cream induced overall smaller increases in the largest VLDL subclasses (and thus possibly less chylomicrons) compared with sour cream, this may possibly partly explain the smaller decrease in L-, M- and S-LDL-P after intake of whipped cream.
We found that men and women responded differently to the dairy meals for some lipoprotein subclasses, with women showing a pattern of less increased VLDL-P and more increased HDL-P compared with men. These findings have support in the literature(Reference Lairon, Lopez-Miranda and Williams7, Reference Wojczynski, Glasser and Oberman8, Reference Teng, Chang and Kanthimathi48, Reference Freedman, Otvos and Jeyarajah49) and one proposed mechanism is a higher lipoprotein lipase capacity in women, generating more lipolysis(Reference Lairon, Lopez-Miranda and Williams7). This could explain both the partly lower VLDL-P responses and the partly higher HDL-P responses in women, as increased lipolysis stimulates the production of HDL particles by releasing more surface components to be incorporated into new HDL(Reference Camont, Chapman and Kontush40).
Our study has several strengths including a randomised controlled cross-over design with four dairy meals containing dairy products with different matrices. The meals were equal in fat content and not adjusted for differing nutrient contents since we were interested in the effect of the fat from dairy products as whole foods. This also means that we did not adjust the amount of fat based on body mass, and differences in responses due to variations in the amount of fat ingested can therefore be ruled out. The limitations of the study are what we cannot ensure absolute standardisation of the evening meal and amount of physical activity the day before each test day, even though the participants received guidelines and reminders before each visit. The male group had higher BMI than the female group; however, the median was within normal range for both groups, and BMI was adjusted for in the analysis. The Bonferroni method was applied to the meal and meal×sex comparisons to reduce the risk of false-positive findings. Since this was an exploratory sub-study, the number of parameters was not adjusted for.
In conclusion, the present study shows that intake of meals with the same amount of fat from different dairy products induces different postprandial effects on lipoprotein subclass concentrations, with sour cream potentially being the most healthy option. We also show that women and men respond differently to high-fat dairy meals in general, but also to certain dairy products in particularly, and that women seem to respond more beneficially to high-fat dairy meals than men.
Acknowledgements
We would like to thank Navida Akhter Sheikh for help with blood sampling and all logistics, and Anne Randi Enget for help with blood sampling. We would also like to thank all the participants in the study for their time and efforts.
The present study was funded by the Research Council of Norway (IPN244633), University of Oslo, and the Throne-Holst Foundation for Nutrition Research, Oslo, Norway. TINE AS provided the dairy products included in the test meals, but was not involved in the study design, statistical analysis or interpretation of findings.
P. H., K. B. H., L. K. L. Ø., H. K. B., M. T. and S. M. U. designed research; P. H., K. B. H. and S. M. U. conducted research; P. H., L. K. L. Ø. and M. T. performed statistical analyses; G. O. G. provided essential material; P. H. , K. B. H., L. K. L. Ø., H. K. B., G. O. G., M. T. and S. M. U. wrote the paper; P. H., K. B. H. and S. M. U. had primary responsibility for the final content of the paper. All authors read and approved the final manuscript.
S. M. U. has received research grants from Mills DA and Olympic Seafood, none of which are related to the content of this manuscript. K. B. H. has received research grants and/or personal fees from Mills DA, Olympic Seafood, Kaneka, Amgen, Sanofi and Pronova, none of which are related to the content of this manuscript. TINE AS partially funded the study via the Research Council of Norway. S. M. U and K. B. H. have received funding from TINE AS, and G. O. G. is employed at TINE. She owns no stocks in the company. The other authors have no financial relationships relevant to disclose. P. H., K. B. H., L. K. L. Ø., H. K. B., M. T. and S. M. U. have no conflicts of interest.
Supplementary material
For supplementary materials referred to in this article, please visit https://doi.org/10.1017/S0007114519001429








