Vasovagal syncope is a common subtype of neural-mediated syncope. It is prevalent in children and adolescents and may critically affect the quality of life of patients. In recent years, the management of children with vasovagal syncope has attracted more attention. Reference Wang, Wang and He1–Reference Shen, Sheldon and Benditt4 The prognosis of most children with vasovagal syncope was good; however, many children continued with recurrent syncope, accompanied by sinus arrest lasting for more than 3 seconds or severe hypotension and bradycardia. Currently, it is believed that vasovagal syncope with syncopal recurrence and sinus arrest lasting for more than 3 seconds is malignant. Reference Pentousis, Cooper and Cobbe5 Malignant vasovagal syncope seriously affects the physical and mental health of children. Therefore, more attention should be paid to the diagnosis and treatment of malignant vasovagal syncope, as its occurrence in children is unpredictable. Any prolonged cardiac arrest may be associated with the risk of sudden death. Pacemaker implantation theoretically offers powerful protection. However, in clinical practice, many children with malignant vasovagal syncope have good quality of daily life, so the rate of acceptance of implanting a pacemaker is low, and this treatment is still controversial for malignant vasovagal syncope children with cardiac arrest. Reference Sanatani, Chau and Fournier6,Reference Maggi, Solari and Brignole7
The conventional treatment of vasovagal syncope includes oral rehydration saline, β-receptor blockers, and orthostatic training. Reference Tao, Xu, Liao, Li, Jin and Du8,Reference Tao, Chen, Li, Tang, Du and Jin9 However, the above-mentioned methods cannot prevent cardiac arrest in children with malignant vasovagal syncope effectively and so it is important to explore effective treatment for this type of syncope.
It is well known that in children with cardiac arrest-associated malignant vasovagal syncope, the prominent characteristic is vagus-mediated cardiac inhibition. Reference Maggi, Solari and Brignole7 Therefore, it is crucial to find treatment to decrease the vagal nerve tension. The cardiac autonomic nervous system is a complex network consisting of convergence of multiple autonomic ganglion plexuses, distributed in the fat pad of the heart, playing an important role in regulating the autonomic nervous function of the sinoatrial and the atrioventricular nodes. Reference Armour10,Reference Chiou, Eble and Zipes11 Previous studies on adults with malignant vasovagal syncope revealed that vagal nerve ablation may reduce the frequency of syncope in patients; Reference Lu, Wei, Upadhyay and Tung12–Reference Tu, Wu and Hu13 however, the efficacy and safety of ganglionated plexus ablation in children are not clear. Previous studies have reported that decelerated capacity showed a superior diagnostic value in patients with vasovagal syncope, Reference Tu, Wu and Hu13 as it reflected cardiac parasympathetic tension, and we assume that it can also be seen as an indicator for cardiac neuroablation. Our study aimed to explore the efficacy of catheter ablation in ganglionated plexus in children with malignant vasovagal syncope.
Materials and methods
In this study, children diagnosed with malignant vasovagal syncope were enrolled in Beijing Children’s Hospital affiliated with Capital Medical University. All underwent catheter ablation treatment of ganglionated plexus between August 2017 and June 2022. A detailed medical history, physical examination, and other detailed medical tests to exclude the possibility of cardiogenic syncope, neurological diseases, and metabolic diseases were obtained for each patient. Malignant vasovagal syncope was diagnosed with the head-up tilt test. 14 The diagnostic criteria of malignant vasovagal syncope were as follows: 14 (1) syncope occurring in older children; (2) persistent standing, mental tension, fear, and muggy environment, which were the most common precipitating factors; (3) fulfilling the positive criteria of head-up tilt test; and (4) other diseases that could cause syncope were excluded. This study was approved by the Ethics Committee of Beijing Children’s Hospital affiliated with Capital Medical University, and written informed consent was obtained from a parent/guardian of each enrolled child.
Head-up tilt test 14
Drugs which could influence autonomic nervous system activity were stopped for at least 5 half-lives, and children were kept fasting for 4 hours before the head-up tilt test, which was conducted in a quiet and dim environment. Electrocardiography, heart rate, and blood pressure were monitored continuously by tilt bed system (Standard Medical Technology, Beijing, China). First, children were positioned flat on the bed for 10 minutes, after which the bed was tilted to 60°for 45 minutes or until a positive response occurred.
The positive diagnostic criteria in head-up tilt test were the following: ① decrease of blood pressure; ② drop of heart rate; ③ sinus arrest; or ④ second-degree or greater atrioventricular block and asystole persisting for > 3 seconds. On the basis of the diagnosis of vasovagal syncope, if cardiac arrest lasted for ≥ 3 seconds in association with syncope, diagnosis of malignant vasovagal syncope could be made. Sinus node dysfunction should be excluded. Clinically, it is feasible to further evaluate the function of the sinus node by electrocardiogram exercise stress test.
Pre-ablation preparation
All children were asked to discontinue drugs for at least 5 half-lives. All the procedures were performed under local anesthesia. The changes of electrocardiogram, blood oxygen saturation and blood pressure were continuously monitored during the procedure. The filtering range of electrocardiogram is 30–500 Hz and the speed is 100 mm/s. Electrodes were place in the coronary sinus and the right ventricle from the subclavian vein and femoral vein access. Modified atrial septal puncture was performed under X-ray fluoroscopy. Reference Yao, Ding and Chen15 The 3-dimensional geometry of the left atrium was conducted under the guidance of the En site-Navx Velocity 5.0 system (Abbott) or Carto3 Version 6.0 system (Johnson & Johnson). Target mapping and ablation were performed in the left atrium using a cold saline irrigated-tip ablation catheter. High frequency stimulation mapping was performed on the endocardial surface of the left atrium to search for positive response points and radio-frequency ablation. During the operation, ventricular pacing was automatically output by a temporary pacemaker to avoid ventricular electrode connection with an EP4 simulator or high frequency stimulation by ventricular electrode output accidentally caused by the operator. Power and temperature were set with upper limits of 40 W and 60°C, respectively. The end point of the ablation procedure was taken as lack of vagal response induced with repeat high-frequency stimulation.
Catheter ablation of ganglionated plexus in the left atrium guided by high-frequency stimulation
Each ganglionated plexus was ablated by trial discharge methods. First, radiofrequency energy was released in the anatomical location area of each ganglionated plexus for 10 seconds. If the vagal response was triggered, including sinus arrest, atrioventricular block, ventricular escape, and the average R-R interval was extended by 50 per cent, continuous discharge was repeated in this area for at least 30 seconds, until the vagus response disappeared. On the contrary, if there was no vagal reaction within 10 seconds, the discharge was stopped and the ablation of adjacent parts attempted again under the guidance of three-dimensional mapping system. The end point of autonomic ganglion plexus ablation under anatomic guidance was that no vagal reaction was caused by five consecutive attempts of ablation of any ganglionated plexus anatomical location area, for instance, when ablation catheter was placed on the anterior wall of the right pulmonary vein in vasovagal syncope cardio-inhibitory children.
Protocol of follow-up after catheter ablation
After the operation, the patients stopped all drugs and were re-examined in the outpatient or inpatient department at 1 month, 6 months, and 1 year, until 5 years after the operation to record the recurrence of post-operative syncope in detail. Six months after the therapy, follow-up was conducted by a professional investigator by telephone or outpatient visits. As indicated before, symptom score was calculated before and at the end of the follow-up depending on the occurrence and frequency of syncope: 0 point indicated no syncopal event; 1 point = 1 event per month; 2 points = 2–4 events per month; 3 points = 2–7 events per week; and 4 points = > one per day. When the symptom score decreased by at least 1 point, the treatment was considered to be effective. Reference Zheng, Sun and Qiao19 Head-up tilt test and 24-hour electrocardiogram were performed during the follow-up.
Calculation of deceleration capacity
The calculation of deceleration capacity was derived from the results of 24-hour electrocardiographic monitoring, and the recordings were digitised at 128 Hz and automatically processed with specific software based on the phase-rectified signal averaging algorithm. Reference Tao, Chen, Li, Tang, Du and Jin9,Reference Zheng, Sun and Qiao19 First, the heartbeat intervals longer than the preceding interval are called anchors. R-R interval prolongations 5% were excluded to avoid errors caused by artefacts. Segments of the same size around the anchors were selected and aligned at the anchors and signals X within the aligned segments were averaged. Deceleration capacity is quantified using the following equation: deceleration capacity = (1/4)[X0 + X1−(X −1)−(X −2)]. X0 and X1 are the averages of the anchor points and the following R-R intervals, while X −1 and X −2 are the averages of the 2 R-R intervals preceding the anchor points. Deceleration capacity measures were calculated for the entire 24 hours from 8:00 to 23:00 (daytime deceleration capacity) and from 23:00 to 8:00 (night-time deceleration capacity).
Previous studies proposed that deceleration capacity reflected the cardiac parasympathetic tension. Reference Tu, Wu and Hu13
Statistical analysis
Data analysis was performed using SPSS version 22.0 (IBM, Armonk, New York). Data were expressed as mean ± SD, and the Shapiro–Wilk test was applied to evaluate the normality of the distribution of continuous data before statistical analysis. Non-normal distribution variables are expressed in quartile by median (Q1, Q3). The paired t test was applied to compare the symptom scores and deceleration capacity values between pre-ablation and post-ablation.
Results
Clinical outcomes
The mean age of enrolled children was 12 (range 8–15) years, with 13 girls and 7 boys. After follow-up for 2.5 (range 0.6–5) years, the syncope symptom scores were decreased significantly compared with before treatment [3 (range 2–4) versus 5 (range 3–8) scores, P < 0.01]. Eighty-five per cent (17/20) children no longer experienced syncope, whilst 80 per cent (16/20) children showed negative head-up tilt test after treatment. No adverse effects such as cardiac arrhythmia (atrial fibrillation, atrial tachycardia, and inappropriate sinus tachycardia) occurred in children (Table 1 and Table 2). Deceleration capacity values were significantly decreased in 1 month, 6 months, and 1 year after ablation compared with pre-ablation (Table 3, Fig. 1).

Figure 1. DC values were significantly decreased at 1 month, 3 months, 6 months, and 1 year after ablation compared with pre-ablation.
Table 1. Information of vasovagal syncope cardioinhibitory prior to cardio-neuroablation
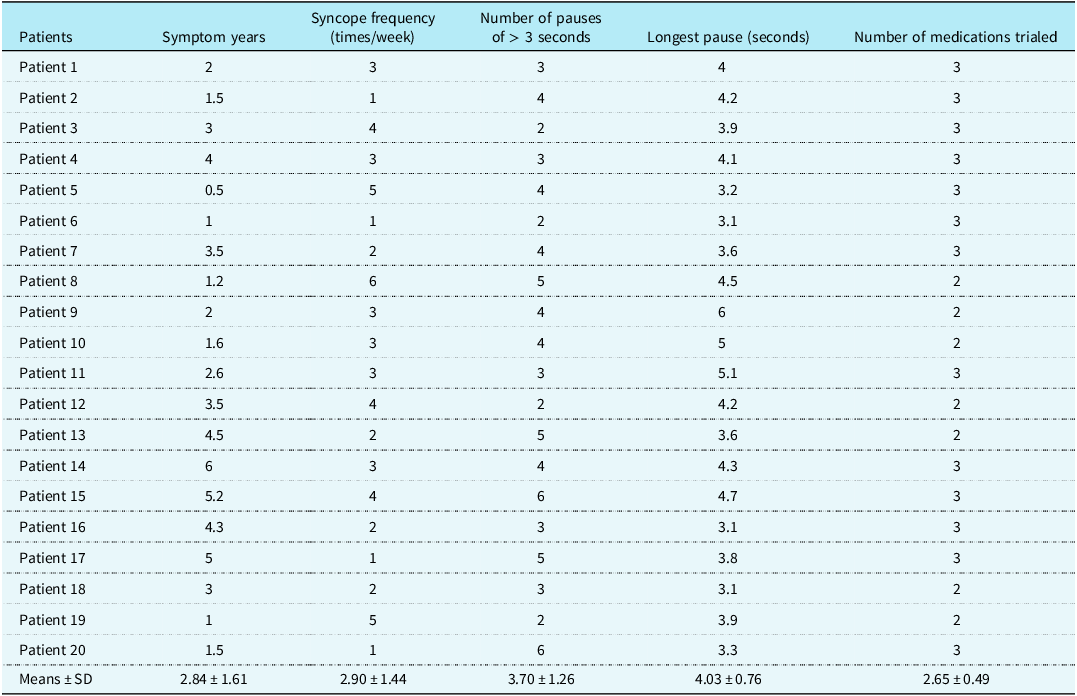
Table 2. Comparison of parameters in vasovagal syncope children between pre-procedure and post-procedure
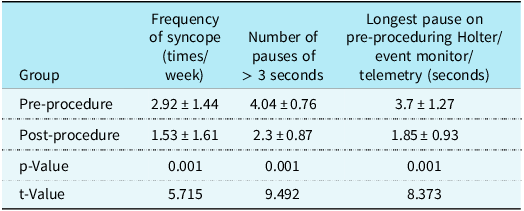
Table 3. Comparison of DC values between pre-ablation and post-ablation
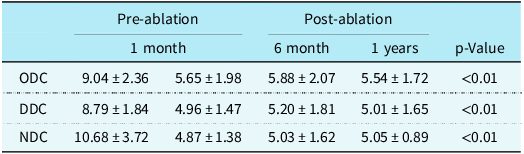
ODC = overall deceleration capacity; DDC = daytime deceleration capacity; NDC = nighttime deceleration capacity.
P < 0.01 refering to comparison with DC values of pre-abltation.
Catheter ablation of ganglionated plexus in left atrium guided by high-frequency stimulation
Twenty children with malignant vasovagal syncope underwent ablation of ganglionated plexus. A total of 46 ganglionated plexus sites induced vagal response were ablated, including 24 (52%) left inferior ganglionated plexus, 9 (19.6%) left inferior pulmonary vein ganglionated plexus, 6 (13%) left superior ganglionated plexus, and 7 (15.4%) left superior pulmonary vein ganglionated plexus. The average operation time was 42.5 ± 4.9 minutes, and the average ablation time was 400 ± 121.6 seconds. The locations of ganglionated plexus were left superior ganglionated plexus, superolateral area around the root of the left superior pulmonary vein, left inferior ganglionated plexus, infero-posterior area around the root of the left inferior pulmonary vein, right anterior ganglionated plexus, supero-anterior area around the root of the right superior pulmonary vein, and right inferior ganglionated plexus, infero-posterior area around the root of the right inferior pulmonary vein (Fig. 2).

Figure 2. The locations of ganglionated plexus were left superior ganglionated plexus, superolateral area around the root of the left superior pulmonary vein, left inferior ganglionated plexus, inferoposterior area around the root of the left inferior pulmonary vein, right anterior ganglionated plexus, supero-anterior area around the root of the right superior pulmonary vein, and right inferior ganglionated plexus, infero-posterior area around the root of the right inferior pulmonary vein.
The change of electrocardiogram in head-up tilt test between pre-ablation and post-ablation, during head-up tilt test pre-ablation, a ventricular pause lasting for at least 3 seconds (secondary to atrio-ventricular block followed by a sinus pause of 2 seconds) was observed. In contrast, after ablation, atrio-ventricular block and sinus pauses were no longer noted (Fig. 3).

Figure 3. During head-up tilt test pre-ablation, a ventricular pause lasting for at least 3 seconds (secondary to atrio-ventricular block followed by a sinus pause of 2 seconds) was observed. In contrast, after ablation, atrio-ventricular block and sinus pauses were no longer noted.
Discussion
Oral rehydration saline, β-receptor blockers, and orthostatic training are the conventional treatments for vasovagal syncope in children. However, these conventional treatments cannot prevent cardiac arrest effectively in these children. It is therefore essential to explore effective treatment for them. Our study found that catheter ablation of ganglionated plexus was effective and safe in these children and could be used as a treatment option. Furthermore, long-term efficacy was well maintained, which was confirmed by head-up tilt test and 24-hour Holter monitoring records.
The occurrence of vasovagal syncope is closely related to abnormal B-J reflex, which would lead to increased vagal and decreased sympathetic excitability. Reference Ali, Pachon Maetos and Kichloo17 Therefore, treatment measures that can reduce vagal nerve excitability are relatively effective and fundamental measures. Pacemaker implantation is a powerful protective method in theory. Reference Paech, Wagner and Mensch18 However, pacemaker implantation is also associated with complications, such as local bleeding, infection, and risk of arrhythmia. In addition, additional factors need to be taken into account, such as the service life of pacemakers, the children’s growth and development, and the potential psychological burden after surgery. These factors need to be carefully considered when making treatment decisions. Pacemaker implantation is still controversial in children with malignant vasovagal syncope accompanied by cardiac arrest. Reference Maggi, Solari and Brignole7 Therefore, it is imperative to explore a novel and effective treatment.
In recent years, it has been found that the cardiac vagal ganglion was located in the epicardium. Reference Zheng, Sun and Qiao19,Reference Xu, Zhao and Duan20 In 2005, Pachon et al. Reference Paech, Wagner and Mensch18 suggested that radiofrequency catheter ablation of the epicardial ganglions from the surface of the endocardium would be a new treatment strategy for vasovagal syncope. In 2011, Pachon et al. reported that catheter ablation was effective in 43 adult patients with vasovagal syncope cardio-inhibitory and only 3 patients had recurrent syncope during the 2-month follow-up period. Reference Pachon, Pachon and Cunha Pachon21 Zhao et al. treated a 57-year-old female vasovagal syncope patient with ablation of the posterior wall of the coronary sinus orifice and the pulmonary vein orifice under the guidance of high-frequency stimulation. Reference Liang, Jiayou, Zonggui and Dening22 No recurrence of syncope was found in the follow-up 12 months after the operation. However, little is known about the study of catheter ablation for children with malignant vasovagal syncope.
A total of 20 children diagnosed with malignant vaosovagal syncope were enrolled in our study, treated with catheter ablation, and had good results. After follow-up for 2.5 (range 0.6–5) years, the syncope symptom scores were decreased significantly compared with before treatment [3 (2–4) versus 5 (3–8) scores, P < 0.01]. Eighty-five per cent (17/20) children no longer experienced syncope, whilst 80% (16/20) children showed negative head-up tilt test after treatment. No adverse effects such as cardiac arrhythmia occurred. Deceleration capacity values were significantly decreased at 1 month, 6 months, and 1 year after ablation compared with pre-ablation. The cardiac vagal ganglion located in the epicardium could be responsible for the results. It is known that the cardiac nerve consists of parasympathetic nerve, sympathetic nerve, and sensory nerve systems. The post-ganglionic neurons of the parasympathetic nerve are close to the heart and located in the epicardium or peri-epicardium (epicardium fat pad), whilst sympathetic nerve and sensory nerve of the post-ganglionic neurons of the nerve are located in the paravertebral ganglion chain or central nervous system. Reference Tao, Xu, Liao, Li, Jin and Du8,Reference Tao, Chen, Li, Tang, Du and Jin9 In summary, the vagus nerve is the only post-ganglionic nerve innervating the heart located in the epicardium. Endocardial catheter ablation can cause damage to post-ganglionic neurons of the vagus nerve, thus causing endocardial degeneration, which provides a basis for catheter ablation of epicardial vagal ganglion from the surface of the endocardium. However, the sympathetic and sensory nerves are only affected by nerve endings, which is reversible. Consequently, catheter ablation is a safe and effective method for treating malignant vasovagal syncope in children.
Our study has some limitations. The population sample was small, so larger sample and multi-centre studies are needed to confirm the safety and efficacy of catheter ablation. Furthermore, the end point of catheter ablation is not clear. Although vagal reflex disappearance could be taken as the end point of ablation, there is still a lack of evidence. Therefore, currently, multi-centre randomised controlled trials are needed to further improve and establish the role of this new technology.
Data availability statement
The data involved in the study was included in the manuscript.
Author contribution
Hongxia Li and Wei Shao are contributed equally to the study.
HL had primary responsibility for the protocol development, patient enrolment, data collection, and preliminary data analysis and wrote the draft. WS analysed the data together and revised important content. XY assisted with the study design, data collection, data analysis, and draft editing. LG gave important advice on study design, supervised the data collection, and reviewed the manuscript for important intellectual content. LG and YY supervised the design and execution of the study, checked the data analysis, contributed to the writing of the manuscript, and had a final approval of the manuscript submitted. All authors read and approved the final manuscript and agreed to be accountable for all aspects of the work.
Financial support
None.
Competing interests
None.
Ethical standard
The studies involving human participants were reviewed and approved by the Ethics Committee of Beijing Children’s Hospital affiliated with Capital Medical University, National Center for Children’s Health. Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin.









