Biological invasions have significant impacts on biodiversity, community structure, and ecosystem processes, often leading to the emergence of diseases that affect animal and human health (Dunn, Reference Dunn and Webster2009; Hatcher et al., Reference Hatcher, Dick and Dunn2012b). Changes in species distribution are often associated with human activity and its effects on the environment (Altizer et al., Reference Altizer, Ostfeld, Johnson, Kutz and Harvell2013; Bellard et al., Reference Bellard, Genovesi and Jeschke2016). Therefore, the invasion of parasites is often human-mediated, and co-introductions with their original hosts often give the parasites the chance to exploit new host communities (Prenter et al., Reference Prenter, Macneil, Dick and Dunn2004; Dunn et al., Reference Dunn, Torchin, Hatcher, Kotanen, Blumenthal, Byers, Coon, Frankel, Holt, Hufbauer, Kanarek, Schierenbeck, Wolfe and Perkins2012). During the invasion process, multiple outcomes can occur: the introduced host species may acquire parasites from their new environment and spill back to native species (Kelly et al., Reference Kelly, Paterson, Townsend, Poulin and Tompkins2009); or they can introduce novel parasites into new areas, creating opportunities for the emergence of diseases in native host species (Strauss et al., Reference Strauss, White and Boots2012).
Many aetiological agents of emerging infectious diseases (EIDs) (see Glossary in Supplementary Material S1) are considered a significant subgroup of biological invaders due to their rapid increase in incidence and geographic range (Hatcher et al., Reference Hatcher, Dick and Dunn2012b; Ogden et al., Reference Ogden, Wilson, Richardson, Hui, Davies, Kumschick, Le Roux, Measey, Saul and Pulliam2019). In the context of invasion, EIDs primarily arise when a parasite (macro or microparasite) spreads into a new geographical area and host population, or jumps into new host species without prior co-evolutionary history (Dunn and Hatcher, Reference Dunn and Hatcher2015). This can then lead to a disease outbreak or the establishment of new endemism, with enormous conservation, economic, and public health implications (Jones et al., Reference Jones, Patel, Levy, Storeygard, Balk, Gittleman and Daszak2008; Cunningham et al., Reference Cunningham, Dobson and Hudson2012).
The tapeworm Echinococcus multilocularis is the causative agent of human alveolar echinococcosis (AE) and is considered an emerging pathogen in some parts of the world (Eckert et al., Reference Eckert, Conraths and Tackmann2000; Davidson et al., Reference Davidson, Romig, Jenkins, Tryland and Robertson2012). The life cycle of this parasite is completed in a two-host predator–prey system, which includes small mammals as intermediate host (mostly rodents, e.g. voles) and wild canids such as foxes (Vulpes spp.) and coyotes (Canis latrans), but also domestic dogs, as definitive hosts. Humans can get infected and develop AE by accidental ingestion of eggs (Thompson, Reference Thompson2017). Recently, the European strain of E. multilocularis was detected in Canada in wild, domestic, and human hosts, indicating a possible invasion of this strain in Western Canada, and the emergence of a new endemism (Jenkins et al., Reference Jenkins, Peregrine, Hill, Somers, Gesy, Barnes, Gottstein and Polley2012; Gesy et al., Reference Gesy, Hill, Schwantje, Liccioli and Jenkins2013; Massolo et al., Reference Massolo, Klein, Kowalewska-Grochowska, Belga, MacDonald, Vaughan, Girgis, Giunchi, Bramer, Santa, Grant, Mori, Duignan, Slater, Gottstein, Müller and Houston2019; Santa et al., Reference Santa, Rezansoff, Chen, Gilleard, Musiani, Ruckstuhl and Massolo2021). Using the invasion process of this strain as a model, we analysed the invasion of parasites with complex life cycles (CLC) transmitted in predator–prey systems, integrating concepts used in EIDs and biological invasion research. Since the life cycle of this parasite requires multiple hosts, it must overcome several eco-physiological barriers to colonize new areas and new host communities. In this review, the ecological and evolutionary factors that play a significant role during the process of invasion of these parasites were analysed, considering both host–parasite and host–host interactions, as well as the effects on the native parasite gene pool, the competition between parasite strains and the possible impact on host–parasite and predator–prey interactions.
Ecological and evolutionary factors influencing parasite invasions
The invasion process and its stages
The criteria to identify an invasive species imply multiple factors, based on taxonomy, biogeography, causes (natural vs. human-mediated), intensity and extent of the impact, and dynamics of invasion. Usually, an invasive alien species is defined as one that has been transported beyond the limits of its native range and has established a population in an area where it was not known to occur previously, resulting in negative impacts in the new environment (Lockwood et al., Reference Lockwood, Hoopes and Marchetti2013). However, there is still some debate about the criteria to identify an invasive species. The discussion centres not only on the implications of a measurable impact (Ricciardi and Cohen, Reference Ricciardi and Cohen2007), but also on the potential for native species to become invasive and the role of humans in mediating the invasions (Nackley et al., Reference Nackley, West, Skowno and Bond2017). Colautti and MacIsaac (Reference Colautti and MacIsaac2004) proposed a conceptual framework based on the stages of the invasion process using neutral terminology to understand it as a biogeographical process of specific populations, rather than a taxonomic phenomenon. Likewise, Blackburn et al. (Reference Blackburn, Pyšek, Bacher, Carlton, Duncan, Jarošík, Wilson and Richardson2011) proposed a framework for biological invasions using a more holistic approach, integrating into a single model concept used in invasions mediated by humans, regardless of taxon or location, including management intervention strategies at different stages of the process. Thus, four stages/phases of the process of invasion have been proposed: (1) translocation or transport, (2) introduction, (3) establishment, and (4) invasive spread (Kolar and Lodge, Reference Kolar and Lodge2001). Therefore, an invasive species will be one that thrives and spreads widely, becoming dominant, with the potential to cause negative impacts in the colonized area.
During each stage of the invasion process, factors such as propagule pressure, biotic and abiotic conditions and community interactions are determinant for the success of invasion and may positively or negatively affect the spread of the invasive species (Colautti and MacIsaac, Reference Colautti and MacIsaac2004; Blackburn et al., Reference Blackburn, Pyšek, Bacher, Carlton, Duncan, Jarošík, Wilson and Richardson2011). In the case of emergent diseases, parasites are usually translocated with their hosts and spilled over to native host communities following a similar progression of stages of invasion (Dunn and Hatcher, Reference Dunn and Hatcher2015). However, for parasites with CLC transmitted in predator–prey systems, interactions within food webs and the presence of multiple intermediate and definitive hosts become important in determining the survival and spread of the parasite. Therefore, although the phases of biological invasion and disease emergence have many parallels, host–host and host–parasite interactions and their co-evolution history play an important role in the establishment and spread of the parasite (Hatcher et al., Reference Hatcher, Dick and Dunn2012b). The study of the mechanism driving new host–pathogen associations and of the different factors that can play a role before and during the invasion process, are essential to predict and counteract possible negative impacts of parasite invasions.
Drivers of parasite invasions and disease emergence
Climate change, habitat loss and fragmentation, changes in the use of water and land resources, and socio-economic activities can all be drivers for parasite invasions and disease emergence (Altizer et al., Reference Altizer, Ostfeld, Johnson, Kutz and Harvell2013; Hoberg and Brooks, Reference Hoberg and Brooks2015). Climate change, for example, has enabled species to expand and settle in regions where they previously could not survive (Walther et al., Reference Walther, Roques, Hulme, Sykes, Pyšek, Kühn, Zobel, Bacher, Botta-Dukát, Bugmann, Czúcz, Dauber, Hickler, Jarošík, Kenis, Klotz, Minchin, Moora, Nentwig, Ott, Panov, Reineking, Robinet, Semenchenko, Solarz, Thuiller, Vilà, Vohland and Settele2009). However, the identification of disease patterns has been challenging, particularly since climate change can limit the transmission of some pathogens, while creating opportunities for others (Altizer et al., Reference Altizer, Ostfeld, Johnson, Kutz and Harvell2013). For example, it has been suggested that high host specificity, CLC, and narrow climatic tolerance might increase the vulnerability of some parasites to climate change, particularly if there is some risk of coextinction with the host (Cizauskas et al., Reference Cizauskas, Carlson, Burgio, Clements, Dougherty, Harris and Phillips2017). However, different outcomes can be expected depending on the species-specific eco-physiological responses of both hosts and parasites to environmental changes (Stensgaard et al., Reference Stensgaard, Vounatsou, Sengupta and Utzinger2019). For example, for the trematode parasite Schistosoma mansoni and its intermediate freshwater snail host, risk-models predicted that some of north-eastern Africa might see a decline in transmission by more than 50% due to global warming (McCreesh et al., Reference McCreesh, Nikulin and Booth2015). In contrast, in China, an expansion of Schistosoma japonicum into non-endemic areas in the northern part of the country has been predicted (Zhou et al., Reference Zhou, Yang, Yang, Wang, Hong, Sun, Malone, Kristensen, Bergquist and Utzinger2008), suggesting a shift rather than an expansion in the geographic distribution of schistosomiasis due to climate change (Stensgaard et al., Reference Stensgaard, Vounatsou, Sengupta and Utzinger2019).
On the effect of climate change on parasites transmitted via direct vs. indirect life cycles, Molnár et al. (Reference Molnár, Dobson and Kutz2013) suggested that behavioural thermoregulation by the intermediate host may protect parasites against extreme temperatures, generating a ‘shelter effect’, which would favour parasites with indirect cycles under future global warming conditions. Similarly, it has been hypothesized that for parasites with CLC, the prolonged survival of larval stages in intermediate hosts could extend the generation time over multiple seasons, potentially increasing the availability of infective propagules in the environment during the invasion process (Hoberg, Reference Hoberg2010).
In addition to the effects of climate change on disease emergence, biological introductions due to transcontinental movements have brought to a global homogenization of flora and fauna, therefore influencing disease patterns globally (Young et al., Reference Young, Parker, Gilbert, Sofia Guerra and Nunn2017). Indeed, trade and transport are considered the most relevant drivers in biological invasions by microorganisms (Essl et al., Reference Essl, Lenzner, Bacher, Bailey, Capinha, Daehler, Dullinger, Genovesi, Hui, Hulme, Jeschke, Katsanevakis, Kühn, Leung, Liebhold, Liu, MacIsaac, Meyerson, Nuñez, Pauchard, Pyšek, Rabitsch, Richardson, Roy, Ruiz, Russell, Sanders, Sax, Scalera, Seebens, Springborn, Turbelin, van Kleunen, von Holle, Winter, Zenni, Mattsson and Roura-Pascual2020). For example, the change in the distribution of our model organisms (E. multilocularis) at a global level is associated with increased human travel, international trade and wildlife and/or domestic animal introductions (among other anthropogenic changes) which have allowed the spread of the parasite beyond historical endemic regions (Davidson et al., Reference Davidson, Romig, Jenkins, Tryland and Robertson2012). Similarly, for the parasite Echinococcus granulosus s.s., the causative agent of cystic echinococcosis, phylogenetic and phylogeographic analyses have demonstrated that the current widespread distribution and diversity of genotypes G1 (Kinkar et al., Reference Kinkar, Laurimäe, Acosta-Jamett, Andresiuk, Balkaya, Casulli, Gasser, van der Giessen, González, Haag, Zait, Irshadullah, Jabbar, Jenkins, Kia, Manfredi, Mirhendi, M'Rad, Rostami-Nejad, Oudni-M'rad, Pierangeli, Ponce-Gordo, Rehbein, Sharbatkhori, Simsek, Soriano, Sprong, Šnábel, Umhang, Varcasia and Saarma2018a) and G3 (Kinkar et al., Reference Kinkar, Laurimäe, Balkaya, Casulli, Zait, Irshadullah, Sharbatkhori, Mirhendi, Rostami-Nejad, Ponce-Gordo, Rehbein, Kia, Simsek, Šnábel, Umhang, Varcasia and Saarma2018b) have been shaped by intensive livestock trade, which has facilitated the dispersal of the parasite over vast geographic areas.
Finding a new host: introduction and spillover
The introduction of alien parasites into new areas can produce new host(s)–parasite associations. Still, the effectiveness of spillover to new hosts will depend on the ability of the parasite to use new resources. Mostly, parasites are considered resource specialists with restricted host ranges, which has led to the idea that when parasites become specialized, it is at the expense of their ability to perform in alternative hosts (Agosta et al., Reference Agosta, Janz and Brooks2010). However, host-switching seems to be commonly influencing the diversification of host–parasite associations (Araujo et al., Reference Araujo, Braga, Brooks, Agosta, Hoberg, von Hartenthal and Boeger2015). The underlying mechanism of this process is termed ‘ecological fitting’ (Janzen, Reference Janzen1985), in which parasites can colonize new host species (with no co-evolutionary history) and create new associations, thanks to their phenotypic plasticity, and to the conservation of genetic information (phylogenetic conservatism) related to traits associated with host exploitation (i.e. resource use) (Agosta and Klemens, Reference Agosta and Klemens2008). Consequently, these characteristics can facilitate invasion into new habitats and give the potential parasite fitness outside its natural range. The giant liver fluke, Fascioloides magna, for example, which originally cycled between North American ungulates (e.g. Rangifer tarandus, Cervus canadensis) as definitive hosts and freshwater snails as intermediate ones, was accidentally introduced to Europe. The parasite successfully found new intermediate and definitive hosts in native European species of snails (e.g. Galba trunculata), and ungulates (e.g. Dama dama), representing an example of multiple ecological fitting (Malcicka et al., Reference Malcicka, Agosta and Harvey2015). Likewise, the trematode Dicrocoelium dendriticum, native to Europe, was introduced to North America, encountering new hosts to complete its three-host life cycle involving terrestrial snails and ants as first and second intermediate hosts, and ruminants as definitive hosts (van Paridon et al., Reference van Paridon, Colwell, Goater and Gilleard2017). However, some degree of eco-physiological equivalence in both biotic and abiotic conditions between the native and invaded ranges (including similar hosts) was necessary for successful colonization (Malcicka et al., Reference Malcicka, Agosta and Harvey2015). Thus, the required conditions for an invasion by a parasite trophically transmitted might be even more restrictive if multiple host-switching events are necessary to complete its life cycle. However, co-introductions with the original host might help to overcome this barrier. In this way, the original host can act as a reservoir of the parasite for native hosts in a source−sink system, allowing more time for new host–parasite adaptations (Sokurenko et al., Reference Sokurenko, Gomulkiewicz and Dykhuizen2006).
Typically, a new host should be an inferior option compared to the original host if the parasite is specifically adapted (Kaltz and Shykoff, Reference Kaltz and Shykoff1998). However, host-switching can happen rapidly, and there are multiple examples of parasites having a higher fitness on allopatric than sympatric hosts. For example, the swim bladder nematode (Anguillicola crassus), a parasite of the Japanese eel (Anguilla japonica), was introduced into the United Kingdom, where successfully infected native European eels (Anguilla anguilla), causing infections with higher worm intensities and pathogenic effects than in Japanese eels (Kirk, Reference Kirk2003). A possible explanation for this phenomenon is that when alien parasites are introduced to a new area, naïve hosts, which lack co-evolved resistance or tolerance, can suffer more significant pathogenic effects than co-evolved hosts (Allison, Reference Allison, Anderson and May1982). In a review of host–parasite co-invasions, in 85% of the cases, the virulence of the introduced parasite was higher in the new host than in the introduced host (Lymbery et al., Reference Lymbery, Morine, Kanani, Beatty and Morgan2014). However, high virulence is not always directly correlated with high parasite population fitness (i.e. capacity to survive and persist) since this might be enhanced either by increased or decreased virulence, depending on the characteristics of transmission (Cressler et al., Reference Cressler, McLeod, Rozins, Van Den Hoogen and Day2016). While in early stages of invasion, higher virulence may be advantageous to the parasite, also increases selection pressure on the parasite towards a level that does not compromise long-term transmissibility (Anderson and May, Reference Anderson and May1982). Thus, the outcome of host–parasite interactions in the invasion process may not always be predictable, as different levels of virulence might be expected in an unusual host (Ebert, Reference Ebert1995). For example, differences in the co-evolutionary outcomes (resistance vs. tolerance) between the bivalve host Mytilus edulis and the invasive parasitic copepod Mytilicola intestinalis were observed in two different fronts of invasions in the North Sea, which was possibly related to local environmental differences (Feis et al., Reference Feis, Goedknegt, Thieltges, Buschbaum and Wegner2016). Therefore, spatial and temporal heterogeneity of the environment can shape the outcome of new host–parasite interactions and the process of invasion, affecting not only parasite infectivity and virulence, but also host immune responses.
Biodiversity-parasite transmission relationship and invasion dynamics
Ecosystem traits along with host community diversity and composition, are important factors influencing successful invasions and parasite transmission in new environments. However, in the last years, there has been an intense debate on whether high-diversity systems reduce or increase the risk of transmission of infectious diseases, if there is a context-dependent relationship, or if there is no direct correlation (Rohr et al., Reference Rohr, Civitello, Halliday, Hudson, Lafferty, Wood and Mordecai2020). The dilution effect hypothesis proposes that diverse ecological communities can limit pathogen spread, and so reduce disease risk (Johnson and Thieltges, Reference Johnson and Thieltges2010; Ostfeld and Keesing, Reference Ostfeld and Keesing2012). Therefore, biodiversity loss could increase the transmission of parasites by indirectly or directly regulating populations of competent hosts, or changing the behaviour of the host, parasite, or vector (Keesing et al., Reference Keesing, Belden, Daszak, Dobson, Harvell, Holt, Hudson, Jolles, Jones, Mitchell, Myers, Bogich and Ostfeld2010). However, the strength of the diversity−disease relationship varies depending on the parasite, and not all parasites are affected by changes in biodiversity (Rohr et al., Reference Rohr, Civitello, Halliday, Hudson, Lafferty, Wood and Mordecai2020). In particular, CLC parasites with intermediate hosts or vectors and free-living stages would likely exhibit strong responses to changes in community diversity and composition, since multiple biological mechanisms influence the transmission process (Johnson and Thieltges, Reference Johnson and Thieltges2010; Rohr et al., Reference Rohr, Civitello, Halliday, Hudson, Lafferty, Wood and Mordecai2020). Moreover, the response to these changes (increasing or decreasing disease risk) might depend on the host species composition rather than diversity per se. Therefore, the capacity to amplify or dilute parasite burden would differ depending on each species and their susceptibility, abundance and transmission potential (Levi et al., Reference Levi, Keesing, Holt, Barfield and Ostfeld2016), as well as the mode of transmission of the parasite (frequency- or density-dependent) (Young et al., Reference Young, Parker, Gilbert, Sofia Guerra and Nunn2017). For example, in the context of invasions, the Pacific oyster (Crassostrea gigas), introduced in the European Wadden Sea, can be infected by the native trematode Renicola roscovita; still, it is not consumed by native birds (definitive hosts). Thus, the Pacific oyster acts as a dead-end intermediate host, reducing the transmission of the parasite (Krakau et al., Reference Krakau, Thieltges and Reise2006). However, in the case of co-introductions, when new species adds more competent individuals to an unsaturated community, amplification of parasite transmission is expected since more competent host are available (Rohr et al., Reference Rohr, Civitello, Halliday, Hudson, Lafferty, Wood and Mordecai2020). In the case of E. multilocularis, changes in community composition of the intermediate host have been associated with a higher prevalence of the parasite, when there is an increase in the relative abundance of competent hosts as a result of anthropogenic landscape disturbances (Giraudoux et al., Reference Giraudoux, Craig, Delattre, Bao, Bartholomot, Harraga, Quere, Raoul, Wang, Shi and Vuitton2003; Liccioli et al., Reference Liccioli, Giraudoux, Deplazes and Massolo2015b). Since many parasites are able to infect phylogenetically close host species, thus, the phylogenetic and ecological structure of the receptive community needs to be considered to understand the diversity−parasite transmission relationship (Parker et al., Reference Parker, Saunders, Bontrager, Weitz, Hendricks, Magarey, Suiter and Gilbert2015; Young et al., Reference Young, Parker, Gilbert, Sofia Guerra and Nunn2017).
Effects of the parasite on host ecological interactions during the invasion process
Parasites can have a strong impact on host ecological interactions at all trophic levels and can play a significant role in co-invasions with their host (Prenter et al., Reference Prenter, Macneil, Dick and Dunn2004; Hatcher et al., Reference Hatcher, Dick and Dunn2006; Dunn et al., Reference Dunn, Torchin, Hatcher, Kotanen, Blumenthal, Byers, Coon, Frankel, Holt, Hufbauer, Kanarek, Schierenbeck, Wolfe and Perkins2012). Such interactions include competition, predation, intraguild predation or ecological processes in which host species only interact via the indirect effects of the parasite (i.e. apparent competition). Through these interactions, the parasite can directly or indirectly affect host survival (population density-mediated effect) or host behaviour and life history (trait-mediated effect) (Hatcher et al., Reference Hatcher, Dick and Dunn2006). During the invasion process, parasite-mediated competition can result from the differential effects of the parasite on the fitness of competing host species (introduced vs. native), altering their ability to compete and ultimately affecting the outcome of invasion (Hudson and Greenman, Reference Hudson and Greenman1998). Examples of disease-mediated invasion can be found across multiple taxa, including all kinds of parasites (macro- and microparasites, parasitoids and soil pathogens) and hosts (vertebrates, invertebrates and plants) (reviewed in Strauss et al., Reference Strauss, White and Boots2012). A classic example of disease-mediated invasion is the competitive exclusion of the populations of native red squirrel (Sciurus vulgaris) in the UK, mediated through the transmission of a Parapoxvirus that was introduced with the arrival of the North American grey squirrel (Sciurus carolinensis) (Tompkins et al., Reference Tompkins, Sainsbury, Nettleton, Buxton and Gurnell2002). Likewise, the meningeal worm (Parelaphostrongylus tenuis) transmitted through snails, facilitated the range expansion of North American white-tailed deer (Odocoileus virginianus), leading to dramatic decreases of moose (Alces alces) and caribou (R. tarandus) populations in the north-eastern United States in the mid-1900s (Anderson, Reference Anderson1972).
Parasites transmitted through predator–prey systems can also alter host interactions. For example, parasites can manipulate the behaviour of the intermediate host (prey), increasing its susceptibility to predation and thereby the probability of the parasite reaching the definitive host (Moore, Reference Moore2002; Poulin and Maure, Reference Poulin and Maure2015). Although some changes in host behaviour can be non-adaptive by-products of infection, host manipulation by parasites is a common adaptive strategy of parasites (Poulin and Maure, Reference Poulin and Maure2015). In the case of E. multilocularis, there is no direct evidence of increased vulnerability to predation in infected intermediate hosts. However, mathematical models have shown that increased susceptibility to predation enhances parasite persistence even at extremely low predator densities, which could be related to the observed prevalence rebounding after anthelmintic treatment in foxes as part of Echinococcosis control programmes (Vervaeke et al., Reference Vervaeke, Davis, Leirs and Verhagen2006). During the invasion process, host manipulation could increase parasite transmission, depending on the ability of the parasite to manipulate different intermediate hosts. For example, the invasive American brine shrimp, Artemia franciscana, and the native shrimp, A. parthenogenetica from the Mediterranean region, share a cestode parasite that causes reverse phototaxis and colour change in the native, but not in the invasive brine shrimp, leading to higher predation of the native shrimp by bird definitive hosts (Georgiev et al., Reference Georgiev, Sánchez, Vasileva, Nikolov and Green2007). Thus, the differential effect of the parasite in the new and original host has the potential to alter the characteristics of food webs, to influence types of interactions in ecological networks, affecting community structure and ecosystem stability in the invaded location (Hatcher et al., Reference Hatcher, Dick and Dunn2012a; Jephcott et al., Reference Jephcott, Sime-Ngando, Gleason and Macarthur2016).
Idiosyncratic characteristics of the invasion process: founder events and propagule pressure
During the process of invasion, the likelihood of successful establishment will strongly depend on the propagule pressure, which refers to the number of introduction events and the number of infective stages released. Both observational and experimental analyses found that propagule pressure explains significant variation in the outcome of biological invasions across different taxa and locations (Lockwood et al., Reference Lockwood, Cassey and Blackburn2005). Therefore, the repeated release of a large number of individuals in multiple locations helps to overcome problems of small population size, facilitating long-term establishment. For example, during founder events, introduced small populations can suffer genetic bottlenecks and increased inbreeding levels that reduce adaptive potential compared with larger populations resulting from multiple introductions. Hence, multiple introductions help increase the genetic variability and improve the ability of introduced populations to adapt to the novel selection pressures (Lockwood et al., Reference Lockwood, Cassey and Blackburn2005; Roman and Darling, Reference Roman and Darling2007). However, multiple introductions and high genetic variation do not seem to be indispensable for a successful invasion, and even genetically impoverished populations have the potential to evolve rapidly. In a review addressing the link between genetic diversity and invasion success, Dlugosch et al. (Reference Dlugosch, Anderson, Braasch, Cang and Gillette2015) found that genetic variation rarely shapes the invasion process. They proposed that the effect of genotypes on phenotype expression and the kind of genetic variation that is introduced, rather than the quantity, might play a more definitive role. Moreover, low variability in single or few markers is usually not an adequate measure of the species capacity to adapt to new environmental conditions.
The introduction, establishment, and invasive spread of species, despite potentially costly genetic bottlenecks, represent a genetic paradox. However, factors such as reproductive traits of most parasites (self-fertilization or high reproductive output), may prevent or increase tolerance to genetic depletion and inbreeding depression during the invasion process (Frankham, Reference Frankham2004). For a parasite like E. multilocularis, reproductive traits could be an advantage in the first stages of invasion. They are hermaphrodite, capable of self- and cross-fertilization, and have a phase of asexual multiplication that produces a large number of protoscoleces, developing thousands of sexually mature adult worms. However, although genetic admixture and reproductive traits have been proposed to explain this genetic paradox, the mechanisms behind successful invasions, despite substantial genetic depletion, inbreeding depression, and drift loads, have not been completed elucidated (Schrieber and Lachmuth, Reference Schrieber and Lachmuth2017).
Within-host parasite interactions and their effects on the local parasite population
The infection of individual hosts by multiple parasite strains or species is common in nature, generating competitive or cooperative interactions between parasites. These parasite interactions represent a primary evolutionary force shaping parasite survival, growth, reproduction, and transmission (Mideo, Reference Mideo2009), thus influencing the colonization of new host species
Intra- and inter-specific parasite competition would depend on resource availability (exploitation competition), immune response (apparent competition), or direct interference (Read and Taylor, Reference Read and Taylor2001; Lello et al., Reference Lello, Boag, Fenton, Stevenson and Hudson2004). For example, the abundance of within-host resources can be a limiting factor, leading to selection for divergence in resource use or adaptation to increase the ability to exploit the shared resource (Mideo, Reference Mideo2009). Therefore, multiple infections can promote the evolution of high virulence due to faster depletion of host resources (Alizon et al., Reference Alizon, de Roode and Michalakis2013). Although this has been predicted in mathematical models and observed in lab experiments (reviewed in Cressler et al., Reference Cressler, McLeod, Rozins, Van Den Hoogen and Day2016), higher virulence is not always found in the wild, and the genetic relatedness of coinfecting parasites may play a more important role in shaping the evolution of virulence (Read and Taylor, Reference Read and Taylor2001; Alizon et al., Reference Alizon, de Roode and Michalakis2013). Moreover, recent evidence suggests that interactions among co-infecting parasites are key for maintaining genetic variation in parasite traits such as infectivity and virulence, thereby influencing the co-evolutionary dynamics between hosts and parasites (Seppälä and Jokela, Reference Seppälä and Jokela2016).
In the context of invasion, when previously allopatric lineages come into contact and interbreed, novel allelic combinations can be generated with positive and negative effects for the invasive and native parasite population (Shi et al., Reference Shi, Joshi, Tielbörger, Verhoeven and Macel2018). Potential benefits for the invasive parasite include heterosis, decreasing inbreeding depression, and enhancing adaptive potential (Roman and Darling, Reference Roman and Darling2007; Rius and Darling, Reference Rius and Darling2014). Additionally, the emergence of novel genotypes can also have an important role in providing opportunities for local adaptation (Verhoeven et al., Reference Verhoeven, Macel, Wolfe and Biere2011; Rius and Darling, Reference Rius and Darling2014). Yet, the introduction of novel genotypes can also cause outbreeding depression, producing a ‘dilution’ of locally adapted genotypes with a subsequent increase of maladaptive genotypes (Verhoeven et al., Reference Verhoeven, Macel, Wolfe and Biere2011). However, the co-occurrence of divergent lineages does not necessarily involve genetic admixture (Rius and Darling, Reference Rius and Darling2014). Intraspecific competition between these related strains also occurs and is likely to be more intense than between different species of parasites, due to overlapping ecological niches and possible immune cross-reactions (Read and Taylor, Reference Read and Taylor2001; Alizon et al., Reference Alizon, de Roode and Michalakis2013). The effect of this competition was tested by experimentally infecting laboratory mice with the protozoan parasite, Trypanosoma brucei (causal agent of human African sleeping sickness), using strains with different levels of virulence. The experiment showed a strong mutual competitive suppression of co-infecting strains in early stages of infection, resulting in decreasing effects of infection in the host (Balmer et al., Reference Balmer, Stearns, Schotzau and Brun2009). On the other hand, high relatedness between co-infecting genotypes has also been proposed as a factor that can promote cooperation between parasites, resulting in an indirect increase in their fitness (Leggett et al., Reference Leggett, Brown and Reece2014). For E. multilocularis, mixed infections of different Echinococcus and Taenia species in individual definitive hosts have been commonly reported (Knapp et al., Reference Knapp, Bart, Giraudoux, Glowatzki, Breyer, Raoul, Deplazes, Duscher, Martinek, Dubinsky, Guislain, Cliquet, Romig, Malczewski, Gottstein and Piarroux2009; Liccioli et al., Reference Liccioli, Catalano, Kutz, Lejeune, Verocai, Duignan, Fuentealba, Hart, Ruckstuhl and Massolo2012; Umhang et al., Reference Umhang, Karamon, Hormaz, Knapp, Cencek and Boué2017; Massolo et al., Reference Massolo, Valli, Wassermann, Cavallero, D'Amelio, Meriggi, Torretta, Serafini, Casulli, Zambon, Boni, Ori, Romig and Macchioni2018; Santa et al., Reference Santa, Pastran, Klein, Duignan, Ruckstuhl, Romig and Massolo2018). Similarly, mixed infections of E. multilocularis genetic variants are relatively common (Umhang et al., Reference Umhang, Karamon, Hormaz, Knapp, Cencek and Boué2017; Santa et al., Reference Santa, Rezansoff, Chen, Gilleard, Musiani, Ruckstuhl and Massolo2021), but the heterozygosity observed in some studies was low, suggesting a rare occurrence of outcrossing (Nakao et al., Reference Nakao, Sako and Ito2003; Knapp et al., Reference Knapp, Guislain, Bart, Raoul, Gottstein, Giraudoux and Piarroux2008). Thus, self-fertilization could be common during the first stages of invasion when a small number of propagule founders is present (Lymbery, Reference Lymbery, Thompson, Deplazes and Lymbery2017). Investigating the differences in virulence and infectivity and its effect on the intraspecific competition between multiple E. multilocularis strains would help to understand their differential invasiveness potential.
After having analysed the ecological and evolutionary factors that can play a significant role during the invasion process of CLC parasites, it is clear that the influence of multiple factors on within-host interactions – including host-immune response, parasite life-traits, population density effects, phenotypic plasticity, and genotype-environmental interactions – can all change the outcome of different invasion events by the same parasite. Thus, the analysis of current processes of invasions of parasites transmitted in cycles involving multiple hosts would help understand common pathways and potential effects of similar parasite invasions.
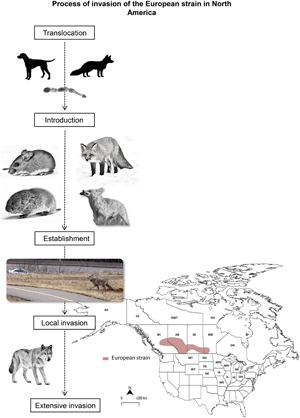
The invasion of the European strain of Echinococcus multilocularis into North America
Translocation and introduction of the European strain
There is increasing evidence that E. multilocularis is expanding both its geographic and host range, associated with changes in the distribution of its definitive hosts due to anthropogenic effects (Eckert et al., Reference Eckert, Conraths and Tackmann2000; Giraudoux et al., Reference Giraudoux, Craig, Delattre, Bao, Bartholomot, Harraga, Quere, Raoul, Wang, Shi and Vuitton2003; Davidson et al., Reference Davidson, Romig, Jenkins, Tryland and Robertson2012). In North America, E. multilocularis was first reported in the Northern Tundra Zone (NTZ) of Alaska and the Canadian Arctic. Only after the 1960s, the parasite was reported in the Northern Central region (NCR), which include 13 contiguous states of the USA and the southern area of three Canadian provinces (Alberta, Saskatchewan and Manitoba) (Eckert et al., Reference Eckert, Conraths and Tackmann2000). However, recent studies have provided evidence of the parasite presence in previously non-endemic regions in Canada such as British Columbia (BC) (Gesy et al., Reference Gesy, Hill, Schwantje, Liccioli and Jenkins2013), Southern Ontario (Kotwa et al., Reference Kotwa, Isaksson, Jardine, Campbell, Berke, Pearl, Mercer, Osterman-Lind and Peregrine2019), and the taiga region between NTZ and NCR (Schurer et al., Reference Schurer, Pawlik, Huber, Elkin, Cluff, Pongracz, Gesy, Wagner, Dixon, Merks, Bal and Jenkins2016).
Despite a significant increase in epidemiological research in the last years, information about the genetic diversity of E. multilocularis in North America is still largely unknown. Nakao et al. (Reference Nakao, Xiao, Okamoto, Yanagida, Sako and Ito2009) assessed the intraspecific genetic diversity of the parasite describing four genetic strains worldwide (Asian, European, North American and Mongolian), which are believed to were isolated during repeated glacial events during the Pleistocene. In Canada and the United States, two haplotypes of the North American strain (N1, N2) were described, each one associated with the NTZ and NCR areas. However, recent studies have revealed the circulation of European-type haplotypes in wild canids in British Columbia, Saskatchewan, and Alberta (Gesy et al., Reference Gesy, Hill, Schwantje, Liccioli and Jenkins2013; Gesy and Jenkins, Reference Gesy and Jenkins2015; Massolo et al., Reference Massolo, Klein, Kowalewska-Grochowska, Belga, MacDonald, Vaughan, Girgis, Giunchi, Bramer, Santa, Grant, Mori, Duignan, Slater, Gottstein, Müller and Houston2019) (Fig. 1), and multiple aberrant cases of AE in dogs in previously non-endemic areas of British Columbia (Jenkins et al., Reference Jenkins, Peregrine, Hill, Somers, Gesy, Barnes, Gottstein and Polley2012) and Ontario (Skelding et al., Reference Skelding, Brooks, Stalker, Mercer, de Villa, Gottstein and Peregrine2014; Oscos-Snowball et al., Reference Oscos-Snowball, Tan, Peregrine, Foster, Bronsoiler, Gottstein, Jenkins, Gesy and Bienzle2015). Moreover, there has been an unprecedented outbreak of human AE cases, with 17 locally acquired cases described since 2013 in the province of Alberta (Massolo et al., Reference Massolo, Klein, Kowalewska-Grochowska, Belga, MacDonald, Vaughan, Girgis, Giunchi, Bramer, Santa, Grant, Mori, Duignan, Slater, Gottstein, Müller and Houston2019; Houston et al., Reference Houston, Belga, Buttenschoen, Cooper, Girgis, Gottstein, Low, Massolo, MacDonald, Müller, Preiksaitis, Sarlieve, Vaughan and Kowalewska-Grochowska2021) and the first confirmed human case in Saskatchewan (Schurer et al., Reference Schurer, Tsybina, Gesy, Kolapo, Skinner, Hill and Jenkins2020), after only two locally acquired cases ever reported (in 1923 and 1977) for continental North America (Massolo et al., Reference Massolo, Liccioli, Budke and Klein2014; Klein and Massolo, Reference Klein and Massolo2015). Furthermore, molecular characterization (when possible) confirmed the European strain as the causative agent, yet none of these patients had travelled outside Canada, suggesting autochthonous transmission of this strain (Massolo et al., Reference Massolo, Klein, Kowalewska-Grochowska, Belga, MacDonald, Vaughan, Girgis, Giunchi, Bramer, Santa, Grant, Mori, Duignan, Slater, Gottstein, Müller and Houston2019).

Fig. 1. Distribution of European-type haplotypes of E. multilocularis in North America. This map shows the historical area of distribution of E. multilocularis (Em) corresponding to the Northern Tundra Zone (NTZ) and Northern Central Region (NCR), the new endemic regions, and the locations in which European-type haplotypes have been identified (based on cox1, cob and nad2 genes). Abbreviations: Alaska (AK), Yukon territory (YT), Northwest Territories (NT), Nunavut (NU), British Columbia (BC), Alberta (AB), Saskatchewan (SK), Manitoba (MB), Montana (MT), Wyoming (WY), North Dakota (ND), South Dakota (SD), Nebraska (NE), Minnesota (MN), Iowa (IA), Wisconsin (WI), Illinois (IL), Michigan (MI), Indiana (IN) and Ohio (OH). References: (Eckert et al., Reference Eckert, Gemmell, Meslin and Pawlowski2001; Nakao et al., Reference Nakao, Xiao, Okamoto, Yanagida, Sako and Ito2009; Jenkins et al., Reference Jenkins, Peregrine, Hill, Somers, Gesy, Barnes, Gottstein and Polley2012; Gesy et al., Reference Gesy, Hill, Schwantje, Liccioli and Jenkins2013; Schurer et al., Reference Schurer, Gesy, Elkin and Jenkins2013, Reference Schurer, Pawlik, Huber, Elkin, Cluff, Pongracz, Gesy, Wagner, Dixon, Merks, Bal and Jenkins2016; Gesy and Jenkins, Reference Gesy and Jenkins2015; Santa et al., Reference Santa, Rezansoff, Chen, Gilleard, Musiani, Ruckstuhl and Massolo2021).
Genetic characterization and phylogenetic analysis of the European-type haplotypes in Western Canada have shown a close relationship with original European clades (Gesy et al., Reference Gesy, Hill, Schwantje, Liccioli and Jenkins2013; Gesy and Jenkins, Reference Gesy and Jenkins2015; Massolo et al., Reference Massolo, Klein, Kowalewska-Grochowska, Belga, MacDonald, Vaughan, Girgis, Giunchi, Bramer, Santa, Grant, Mori, Duignan, Slater, Gottstein, Müller and Houston2019), which supports the hypothesis of a relatively recent introduction, facilitated through translocation of domestic dogs (Jenkins et al., Reference Jenkins, Peregrine, Hill, Somers, Gesy, Barnes, Gottstein and Polley2012), via intermediate hosts translocated with international shipping (Davidson et al., Reference Davidson, Romig, Jenkins, Tryland and Robertson2012), and/or introduced red foxes (Vulpes vulpes) imported for sport hunting from France during the last century (Kamler and Ballard, Reference Kamler and Ballard2002). Another possible indicator of a recent invasion is the increasing reports of unusual clinical manifestations of AE in dogs, with severe and often lethal infections (Jenkins et al., Reference Jenkins, Peregrine, Hill, Somers, Gesy, Barnes, Gottstein and Polley2012; Peregrine, Reference Peregrine2015). This could be related to a general increase in the exposure to the parasite (Deplazes and Eckert, Reference Deplazes and Eckert2001), for example, in urban areas (Liccioli et al., Reference Liccioli, Giraudoux, Deplazes and Massolo2015b), and the presence of introduced parasite strains with high pathogenic potential, such as the European strain (Nakao et al., Reference Nakao, Xiao, Okamoto, Yanagida, Sako and Ito2009). In Canada, there are no strict screening requirements for E. multilocularis for pets imported into the country, and there are no regulations for the relocation of dogs within and between provinces (Canadian Food Inspection Agency, 2018). Between 2013 and 2014 alone, 6200 dogs were imported into Canada from 29 countries, including from Europe (Anderson et al., Reference Anderson, Douma, Kostiuk, Filejski, Rusk, Weese, Bourque, Lee-Fuller and Rajzman2016; Julien et al., Reference Julien, Sargeant, Filejski and Harper2021), which poses a high risk of introduction of the parasite. Indeed, a risk assessment of importation of dogs from endemic countries infected with E. multilocularis into the UK found that, without the mandatory treatment with praziquantel, the probability of at least one infected dog returning to the UK was approximately 98% (Torgerson and Craig, Reference Torgerson and Craig2009). On the other hand, an introduction via intermediate hosts is less likely due to the generally low prevalence of the parasite in these hosts (Romig et al., Reference Romig, Deplazes, Jenkins, Giraudoux, Massolo, Craig, Wassermann, Takahashi, de la Rue, Thompson, Deplazes and Lymbery2017). In the UK, for example, E. multilocularis was found in a captive beaver imported from Germany (Barlow et al., Reference Barlow, Gottstein and Mueller2011); however, despite the potential risk of introduction, there have been no known domestically acquired cases in the UK.
Another possible mechanism of introduction to consider could be through the dispersal movements of arctic foxes (Vulpes lagopus), as evidenced by the satellite tracking of natal dispersal by a young female between continents, from Svalbard Archipelago (Norway) to Ellesmere Island, Nunavut (Canada), in 76 days (Fuglei and Tarroux, Reference Fuglei and Tarroux2019). However, only the Asian and North American strains have been reported in the northern territories in North America, whereas the European strain seems to be restricted to the NCR (Fig. 1) (Nakao et al., Reference Nakao, Xiao, Okamoto, Yanagida, Sako and Ito2009; Gesy et al., Reference Gesy, Hill, Schwantje, Liccioli and Jenkins2013; Gesy and Jenkins, Reference Gesy and Jenkins2015; Massolo et al., Reference Massolo, Klein, Kowalewska-Grochowska, Belga, MacDonald, Vaughan, Girgis, Giunchi, Bramer, Santa, Grant, Mori, Duignan, Slater, Gottstein, Müller and Houston2019; Santa et al., Reference Santa, Rezansoff, Chen, Gilleard, Musiani, Ruckstuhl and Massolo2021). Therefore, introduction with red foxes and/or dogs, seems to be the more plausible source of invasion, with multiple introduction events having occurred during the last century, as evidenced by the limited distribution of some European-type haplotypes in each western province (Santa et al., Reference Santa, Rezansoff, Chen, Gilleard, Musiani, Ruckstuhl and Massolo2021).
Establishment into local predator–prey systems
In the core endemic area of Europe red foxes appear to be responsible for most of the environmental contamination with E. multilocularis eggs (Eckert and Deplazes, Reference Eckert and Deplazes1999), being the primary host of the parasite, which at the same time implies a long co-evolutionary history. Likewise, it was assumed that the red fox was the primary host in the NCR in North America. However, the coyote (C. latrans) is likely another important definitive host in that area, due to its larger home range, dispersal distances and increasing presence in urban environments (Catalano et al., Reference Catalano, Lejeune, Liccioli, Verocai, Gesy, Jenkins, Kutz, Fuentealba, Duignan and Massolo2012; Gesy et al., Reference Gesy, Hill, Schwantje, Liccioli and Jenkins2013; Liccioli et al., Reference Liccioli, Giraudoux, Deplazes and Massolo2015b; Deplazes et al., Reference Deplazes, Rinaldi, Alvarez Rojas, Torgerson, Harandi, Romig, Antolova, Schurer, Lahmar, Cringoli, Magambo, Thompson, Jenkins, Thompson, Deplazes and Lymbery2017). Moreover, interspecific competition between coyotes and red foxes has decreased red fox populations densities, since coyotes seem to displace them and could even prey upon them (Gosselink et al., Reference Gosselink, Van Deelen, Warner and Joselyn2003; Liccioli et al., Reference Liccioli, Bialowas, Ruckstuhl and Massolo2015a), which may generate in turn, changes in definitive host communities and shift in the role of the different hosts in the transmission of the parasite (Liccioli et al., Reference Liccioli, Kutz, Ruckstuhl and Massolo2014).
After its introduction in North America, the European strain may have benefited from the most abundant definitive host available, the coyote. The abundance and distribution of this host species across North America have increased due, in part, to the agricultural expansion after European colonization, which created an ideal habitat for them (Gompper, Reference Gompper2002). Thus, coyotes, being naïve hosts, lacking co-evolved resistance or tolerance to the European strain, may have played a significant role in the environmental contamination with eggs by this strain. Although the dietary preferences of coyotes vary across their range, small mammals are a staple for coyotes in urban environments, and up to 80% of these prey species are competent hosts for Em (Liccioli et al., Reference Liccioli, Bialowas, Ruckstuhl and Massolo2015a). Moreover, the prevalence of Em in coyotes was reported being up to 83.8% in a hyperendemic area in the city of Calgary (Liccioli et al., Reference Liccioli, Kutz, Ruckstuhl and Massolo2014), and, on average, 24% in urban and rural environments in Alberta (Catalano et al., Reference Catalano, Lejeune, Liccioli, Verocai, Gesy, Jenkins, Kutz, Fuentealba, Duignan and Massolo2012), which supports the key role of coyotes in the transmission of the parasite.
In the endemic area of Em in Central Europe, intermediate hosts are mainly voles, Microtus arvalis, and Arvicola spp. (Romig et al., Reference Romig, Deplazes, Jenkins, Giraudoux, Massolo, Craig, Wassermann, Takahashi, de la Rue, Thompson, Deplazes and Lymbery2017). In the NCR in North America, the European strain found competent intermediate hosts in meadow voles (Microtus pennsylvanicus) and deer mice (Peromyscus maniculatus), but also newly described hosts such as the southern red-backed vole (Myodes gapperi) (Liccioli et al., Reference Liccioli, Duignan, Lejeune, Deunk, Majid and Massolo2013). The establishment of the European strain may have also affected the population dynamics of intermediate host communities through apparent competition or host manipulation, potentially altering the predator–prey relationships and the E. multilocularis transmission. Differences in metacestode development and susceptibility to infection in intermediate hosts have been observed between parasite isolates from different geographical regions (Rausch and Richards, Reference Rausch and Richards1971; Bartel et al., Reference Bartel, Seesee and Worley1992). In experimental infections, meadow voles were all susceptible to isolates from North America (St. Lawrence Island) and Europe (Germany) and developed fertile metacestodes (Obayashi et al., Reference Obayashi, Rausch and Fay1971). On the other hand, Rausch and Richards (Reference Rausch and Richards1971) observed that metacestodes in deer mice contained fewer protoscoleces than meadow voles. Moreover, coyotes were found to select against deer mice, in favour of voles, despite a relatively higher abundance of deer mice in urban areas (Liccioli et al., Reference Liccioli, Bialowas, Ruckstuhl and Massolo2015a). Therefore, even if the average prevalence of Em in deer mice is similar to meadow voles (Liccioli et al., Reference Liccioli, Bialowas, Ruckstuhl and Massolo2015a), susceptibility to different parasite strains and predator–prey relationships could influence the transmission of the parasite. However, the specific pathogenicity and zoonotic potential of the European strain have not been empirically tested or compared to other strains, and overall, the individual role of different players in this multi-host transmission system has not yet been elucidated.
The spread of the European strain and competition with native strains
Genetic studies analysing mitochondrial and nuclear DNA of E. multilocularis, have found a distant genetic relationship between E. multilocularis isolates from Europe and North America, when comparing isolates from distinct geographical regions (Bowles et al., Reference Bowles, Blair and McManus1992; Nakao et al., Reference Nakao, Xiao, Okamoto, Yanagida, Sako and Ito2009; Knapp et al., Reference Knapp, Bart, Maillard, Gottstein and Piarroux2010). Only until 2009, a haplotype closely related to a European strain from west-central Europe (E4), was detected in a dog in British Columbia, Canada (Jenkins et al., Reference Jenkins, Peregrine, Hill, Somers, Gesy, Barnes, Gottstein and Polley2012), leading to increased efforts in identifying the extent of the invasion of the European strain into North America. A subsequent survey in coyotes and foxes within the same area in British Columbia confirmed that the European-type haplotype (BC1) was the only one detected in this new endemic region in definitive hosts (Gesy et al., Reference Gesy, Hill, Schwantje, Liccioli and Jenkins2013). A different European-type haplotype (SK1) was found in coyotes from the peripheral area of Saskatoon, Saskatchewan, while, in the southwest of the province, six haplotypes similar to the North American (N2) strain were found in deer mice (Gesy and Jenkins, Reference Gesy and Jenkins2015). The situation in Alberta was even more surprising since all of the identified haplotypes were closely related to the European strain (except from one sample) (Gesy et al., Reference Gesy, Schurer, Massolo, Liccioli, Elkin, Alisauskas and Jenkins2014; Massolo et al., Reference Massolo, Klein, Kowalewska-Grochowska, Belga, MacDonald, Vaughan, Girgis, Giunchi, Bramer, Santa, Grant, Mori, Duignan, Slater, Gottstein, Müller and Houston2019; Santa et al., Reference Santa, Rezansoff, Chen, Gilleard, Musiani, Ruckstuhl and Massolo2021), despite it being a historically endemic region for the NA strain. These results suggest that the European strain is widespread within Western Canada, forming complex mosaics with the North American strain, and potentially out-competing it. After introduction and establishment, the spread of the European strain could have been boosted by the presence of highly vagile, abundant and susceptible hosts like coyotes. Additionally, wolves (Canis lupus) were recently confirmed as regular definitive host of E. multilocularis (Schurer et al., Reference Schurer, Gesy, Elkin and Jenkins2013, Reference Schurer, Pawlik, Huber, Elkin, Cluff, Pongracz, Gesy, Wagner, Dixon, Merks, Bal and Jenkins2016; Gesy et al., Reference Gesy, Schurer, Massolo, Liccioli, Elkin, Alisauskas and Jenkins2014), potentially contributing to a broader spread of the strain into northern areas, due to their large home range (>62 000 km2 in the artic) and dispersal distances (50–800 km) (Walton et al., Reference Walton, Cluff, Paquet and Ramsay2001) (Fig. 2).

Fig. 2. Suggested process of invasion of the European strain of Echinococcus multilocularis in North America. The European strain successfully overcame geographic, ecological, and evolutionary barriers, allowing its widespread in Western Canada. Although multiple driving factors for the invasion have been identified during its translocation, introduction, establishment and spread stages, there are still unknown factors to explain the extent of the invasion of this strain. IH: Intermediate host, DH: Definitive host. Em: Echinococcus multilocularis.
In the NCR, the European strain was found in AE cases of dogs with no travel history (Kotwa et al., Reference Kotwa, Isaksson, Jardine, Campbell, Berke, Pearl, Mercer, Osterman-Lind and Peregrine2019). Moreover, in the city of Calgary, a study found one out of 218 dog faecal samples to be positive to Em after molecular confirmation (Massolo et al., Reference Massolo, Liccioli, Budke and Klein2014), whereas in a survey across the Canadian provinces, the parasite was not detected in 1608 faecal samples from shelter dogs (Villeneuve et al., Reference Villeneuve, Polley, Jenkins, Schurer, Gilleard, Kutz, Conboy, Benoit, Seewald and Gagné2015). Since limited information is available about the true prevalence of intestinal infection, the definitive role of domestic dogs in the transmission of E. multilocularis, and the genetic variants circulating in dog populations in North America still need to be determined (Massolo et al., Reference Massolo, Liccioli, Budke and Klein2014; Toews et al., Reference Toews, Musiani, Checkley, Visscher and Massolo2021). Certainly, dogs are highly susceptible to Em infection and the parasite has a high biotic potential in this host (Kapel et al., Reference Kapel, Torgerson, Thompson and Deplazes2006). For example, in highly endemic areas of central Asia, free-roaming dogs have shown a prevalence of up to 26% (Ziadinov et al., Reference Ziadinov, Mathis, Trachsel, Rysmukhambetova, Abdyjaparov, Kuttubaev, Deplazes and Torgerson2008). Therefore, domestic dogs might play a pivotal role in the transmission of the European strain in North America, potentially acting as the main source of infection in humans as suggested by recent findings (Massolo et al., Reference Massolo, Klein, Kowalewska-Grochowska, Belga, MacDonald, Vaughan, Girgis, Giunchi, Bramer, Santa, Grant, Mori, Duignan, Slater, Gottstein, Müller and Houston2019) due to their large population in urban areas (e.g. >125 000 licensed dogs in 2013 in Calgary, AB) and increased exposure to E. multilocularis linked to the presence of coyotes in urban areas.
The missing link: an ecological framework for CLC parasites transmitted in predator–prey systems
Parasitic diseases caused by parasites with CLC come from a wide range of taxonomic groups, yet they share many features in their life histories. From an evolutionary perspective, incorporating an intermediate host can maximize parasite fitness by increasing the number of surviving progeny in the next generation (Chubb et al., Reference Chubb, Ball and Parker2010). In predator–prey systems, intermediate hosts are species that occupy key positions in food webs, thus facilitating transmission of the parasite (Poulin and Maure, Reference Poulin and Maure2015). Infecting predators that prey upon multiple individuals in a relatively short time is also an efficient way for parasites to meet a sexual partner increasing the opportunities for cross-fertilization (Brown et al., Reference Brown, Renaud, Guégan and Thomas2001). Moreover, reaching long-lived and highly vagile definitive hosts such as large carnivores promotes higher growth (with consequently high fecundity), longevity and broad spatial dispersion (Ewald, Reference Ewald1995; Parker et al., Reference Parker, Chubb, Ball and Roberts2003). Therefore, for a parasite transmitted in prey–predator systems, all these characteristics can give an advantage for the successful colonization of new environments and hosts, helping to increase spatial distribution across heterogeneous environments and over extended time frames (Hoberg, Reference Hoberg2010). Nonetheless, the complexity of the transmission process may also prevent substantial changes in the parasite distribution and host range (Cleaveland et al., Reference Cleaveland, Laurenson and Taylor2001). If suitable hosts for all parasite life cycle stages are not present, or the parasite cycle becomes truncated in dead-end hosts, the parasite will not become established (Torchin et al., Reference Torchin, Lafferty, Dobson, McKenzie and Kuris2003). This problem may be overcome in the case of co-introductions of parasites and their hosts. For example, the local establishment of E. multilocularis into the Svalbard Archipelago in the Norwegian Arctic was enabled by the co-introduction of the sibling vole (Microtus rossiaemeridionalis), since no suitable intermediate host was previously present (Henttonen et al., Reference Henttonen, Fuglei, Gower, Haukisalmi, Ims, Niemimaa and Yoccoz2001).
Many alien parasites are co-introduced with an alien host species, and the successful establishment of parasites with CLC is not unusual. A review by Lymbery et al. (Reference Lymbery, Morine, Kanani, Beatty and Morgan2014) found that, of 98 studies on co-introductions of different taxa, 36% involved parasites with an indirect life cycle. However, when considering the impact of the invasive parasite species, only one species (Plasmodium relicta) out of the eight parasites included in the IUCN list of worst invasive alien species has an indirect cycle (Hatcher et al., Reference Hatcher, Dick and Dunn2012b). Hence, most harmful emergent diseases, impacting human and animal health, are transmitted through direct cycles (Jones et al., Reference Jones, Patel, Levy, Storeygard, Balk, Gittleman and Daszak2008; Tompkins et al., Reference Tompkins, Carver, Jones, Krkošek and Skerratt2015). However, this could instead reflect more significant research and surveillance efforts towards emerging diseases affecting humans and domestic animals, which calls for more efforts to understand the actual extent of invasions for parasites with CLC involving wildlife. Under current climate change, CLC parasites transmitted in predator–prey systems may have an advantage under extreme temperatures and greatest ability to disperse, becoming an important source of future emergence diseases.
In the previous sections of this review, we identified ecological and evolutionary factors that could influence the invasion process of parasites with CLC. Based on the conceptual invasion frameworks developed by Colautti and MacIsaac (Reference Colautti and MacIsaac2004) and Blackburn et al. (Reference Blackburn, Pyšek, Bacher, Carlton, Duncan, Jarošík, Wilson and Richardson2011), and the process of invasion of the European strain of Em in North America, we propose a framework (Fig. 3) to summarize the mechanisms previously identified, integrating concepts used in EIDs and biological invasion research to understand the invasion process of parasites transmitted in predator–prey systems from an eco-evolutionary perspective. We used the model of ‘stages’ and ‘filters’ that the potential invader must overcome, and included key processes occurring at each stage of invasion, the role of the interactions between the host−parasite−environment and the differences in the temporal-space scale. In the first stage of invasion, the translocation of the parasite can be mediated by indirect factors such as anthropogenic disturbances that generate in turn changes in the distribution of the hosts; or can be directly human-mediated through the transport of propagules, or the movement of the original hosts along with the parasite, associated with local/global trade and transport (Hatcher et al., Reference Hatcher, Dick and Dunn2012b; Altizer et al., Reference Altizer, Ostfeld, Johnson, Kutz and Harvell2013). During this stage, the number of propagules translocated and geographical barriers that historically prevented natural dispersal are critical, facilitating or not the release of the parasite beyond the limits of its native range. In the second stage of invasion, the introduction and spillover to native hosts would depend on specific traits of the parasite (e.g. reproduction strategy, phenotypic plasticity), the characteristics of the local hosts (e.g. susceptibility, biotic potential), the number and frequency of infective stages introduced, if the parasite is co-introduced with its original hosts, and environmental matching, that would help to overcome biotic and abiotic barriers to survive and form new host–parasite associations (Frankham, Reference Frankham2004; Lockwood et al., Reference Lockwood, Cassey and Blackburn2005; Dunn and Hatcher, Reference Dunn and Hatcher2015). At the same time, these factors can positively or negatively affect interactions between the parasite, and the intermediate and definitive hosts (native/alien) within the predator–prey system. These interactions occur at an individual level, at local or regional geographic scale (depending on the number of founder events), with possible differential impacts on the native/alien host, which could facilitate or not the transmission of the parasite (Prenter et al., Reference Prenter, Macneil, Dick and Dunn2004; Hatcher et al., Reference Hatcher, Dick and Dunn2006; Lymbery et al., Reference Lymbery, Morine, Kanani, Beatty and Morgan2014). Therefore, during the introduction and establishment stages, the parasite must overcome survival and reproductive barriers to rapidly adapt to the new environment. In the third stage of invasion, the persistence of the parasite in the recipient area would depend on the pre-adaptation characteristics of the parasite via the eco-evolutionary experience in previous environments, allowing individuals to survive as an infective propagule, find and infect new intermediate and definitive hosts, and to reproduce, completing their life cycle with the establishment of a self-sustaining population in the long-term (Agosta and Klemens, Reference Agosta and Klemens2008; Ogden et al., Reference Ogden, Wilson, Richardson, Hui, Davies, Kumschick, Le Roux, Measey, Saul and Pulliam2019). Additionally, the characteristics of the predator–prey community, such as diversity/richness and hosts population size and density, would influence the transmission of the parasite, amplifying or diluting parasite burden (Keesing et al., Reference Keesing, Belden, Daszak, Dobson, Harvell, Holt, Hudson, Jolles, Jones, Mitchell, Myers, Bogich and Ostfeld2010; Young et al., Reference Young, Parker, Gilbert, Sofia Guerra and Nunn2017). Moreover, if the parasite is introduced with its original hosts, they could act as a reservoir for native hosts, allowing more time for new host–parasite adaptations (Sokurenko et al., Reference Sokurenko, Gomulkiewicz and Dykhuizen2006). At the same time, ecological interactions between different species of definitive and intermediate hosts within the predator–prey community might be influenced by the parasite, via direct or indirect effects on host behaviour and survival, thus, potentially promoting competition and predation and enhancing the transmission of the parasite (Hudson and Greenman, Reference Hudson and Greenman1998; Hatcher et al., Reference Hatcher, Dick and Dunn2006; Dunn et al., Reference Dunn, Torchin, Hatcher, Kotanen, Blumenthal, Byers, Coon, Frankel, Holt, Hufbauer, Kanarek, Schierenbeck, Wolfe and Perkins2012). At this stage, the parasite population is small and localized and numerically rare. However, in the fourth stage of invasion, the population can become either localized and dominant, or widespread but rare.
The local or regional dispersal, and dominance of the parasite over other species/strains, would depend on the within-host intra- and interspecific interactions and the ability to exploit the shared resource, as well as the dispersal patterns and distribution of the host populations (Mideo, Reference Mideo2009; Alizon et al., Reference Alizon, de Roode and Michalakis2013). Furthermore, the spatial and temporal heterogeneity of the founder events and the environmental conditions can also shape the outcome of new host–parasite interactions and the process of invasion, limiting or promoting an extensive spread of the parasite. In the last stage of the invasion, the parasite population can become widespread and dominant after overcoming geographical and dispersal barriers to find new suitable host communities at multiple sites. This process would depend on the stability, complexity and structure of the ecological network in which the parasite is transmitted, and the potential effect of the parasite on the interactions within the food web, that will allow the spread to multiple predator–prey communities over extensive areas away from the point of introduction (Hatcher et al., Reference Hatcher, Dick and Dunn2012b; Jephcott et al., Reference Jephcott, Sime-Ngando, Gleason and Macarthur2016). Thus, incorporating network analysis concepts is pivotal to understand the ecology and heterogeneity of parasite transmission and its potential invasive spread, especially for parasites that depend to some extent on the behaviour of the host to enable transmission. The application of social networks to study the epidemiology of wildlife parasites has been growing in the last years, especially for EIDs (Godfrey, Reference Godfrey2013). However, a broader scope and incorporation of parasites transmitted in complex host–parasite systems is still needed.

Fig. 3. Framework for the invasion process of parasites trophically transmitted in predator–prey systems. During each stage, biological processes are occurring at different time, space and ecological scales. These processes depend upon intrinsic characteristics of the predator–prey system and the interactions between biotic and abiotic components. This framework shows the main factors that are determinant during the invasion process and may affect the host–parasite interactions within the predator–prey community and the transition to subsequent stages of invasion. The + and − symbols indicate positive and negative effects. Solid and broken lines represent direct and indirect effects, respectively. P: Parasite, DH: Definitive host, IH: Intermediate host. Lower case letters ‘a’ and ‘n’ stand for alien and native species.
Concluding remarks
Biological invasions are multifactorial, complex phenomena that involve largely idiosyncratic ecological characteristics. Despite the vast body of knowledge about underlying processes influencing invasion dynamics, there is an incomplete understanding of the interaction between macro and micro-evolutionary processes and what drives the potential for invasion. Additionally, the research on parasitism in the context of biological invasions has advanced very slowly, compared to the study of biological invasions by animals or plants (Ogden et al., Reference Ogden, Wilson, Richardson, Hui, Davies, Kumschick, Le Roux, Measey, Saul and Pulliam2019). The capability of identifying the introduction of non-indigenous parasites is limited by the absence of comprehensive taxonomic inventories of parasites, including molecular characterization. Therefore, molecular-based surveillance is key to exploring the distribution and diversity of exotic and native species, and to be able to understand the evolutionary and biogeographic history of invasion processes.
Although parasites with CLC may have to overcome more ecological and physiological barriers to invade new environments successfully, there are examples of colonization in heterogeneous environments, including parasites with high impact on animal and human health (Henttonen et al., Reference Henttonen, Fuglei, Gower, Haukisalmi, Ims, Niemimaa and Yoccoz2001; Malcicka et al., Reference Malcicka, Agosta and Harvey2015; van Paridon et al., Reference van Paridon, Colwell, Goater and Gilleard2017). The analysis of the invasion process of the European strain of E. multilocularis in North America is particularly important to our understanding of the specific factors influencing the invasion process of those parasites that are trophically transmitted in predator–prey systems. Understanding the historical origins and complex components of these new host–parasite mosaics is essential in formulating predictions about future invasions, and elucidate the effects of climate change and ecological disturbance on the potential for invasion. Moreover, since most parasites are introduced with their original hosts, there is a need for integration between the study of biological invasions and disease ecology, which would allow the design of comprehensive predictive frameworks assessing the risk of invasion and lead to possible management strategies. This mandates for multidisciplinary studies on mechanisms, effects, and the control of parasite invasions, focusing not only on host–parasite interactions, but also on the broader impacts of these invasions at the ecosystem level.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0031182021001426
Author contributions
AM and MAS conceived the review. MAS searched the literature and drafted the manuscript. MM, AM, and KER revised and edited the manuscript. All authors edited and reviewed the final manuscript.
Financial support
This work was supported by Alberta Conservation Association (A.M., grant number 10010809); Natural Sciences and Engineering Research Council of Canada-NSERC (A.M, grant number 10013904), (K.E.R., grant number RGPIN-2018-03913); Mitacs (A.M., grant number 10018836), and Elanco Canada (A.M., grant number 10017067).
Conflict of interest
The authors declare there are no conflicts of interest.
Ethical standards
There is no ethical issue involved in this review article.