The metabolic syndrome is a well-established risk factor for CVD, in which the complex pathogenic mechanism involves obesity, hypertension, atherogenic dyslipidaemia and insulin resistance(Reference Tamariz, Hassan and Palacio1). Fasting and postprandial hypertriacylglycerolaemia as well as hyperglycaemia have been suggested to induce endothelial dysfunction via oxidative stress and the production of atherogenic remnant lipoproteins, which in turn are a cause of atherosclerosis(Reference Ceriello, Taboga and Tonutti2). The presence of carotid atherosclerosis was strongly associated with the magnitude of left ventricular mass (LVM)(Reference Roman, Pickering and Schwartz3), which is independently associated with the incidence of cardiovascular and all-cause mortality(Reference Levy, Garrison and Savage4). There is considerable clinical evidence indicating that a chronic imbalance of cardiac fuel uptake and utilisation is related to an elevated risk of CVD. In the case of cardiac hypertrophy, the decline in fatty acid (FA) utilisation seemed to be compensated by a concomitant increase in glucose oxidation(Reference Stanley, Recchia and Lopaschuk5). The opposite was found in cardiomyopathy that develops in the context of insulin resistance and diabetes with an increased cardiac dependence on FA(Reference Opie6).
FA are the preferred source of energy for the working heart, whereby FA bound to albumin are the primary source followed by FA supplied from the lipoprotein lipase-triggered hydrolysis of TAG-rich lipoproteins(Reference van der Vusse, van Bilsen and Glatz7). Once the FA have crossed the endothelial cell barrier and entered the interstitial space, long-chain FA are taken up to the cardiac myocytes by a protein-mediated transport system(Reference Gimeno, Ortegon and Patel8). There are two different isoforms of the FA transport protein (FATP) family expressed in the heart; FATP1 and FATP6. FATP6 is involved in the translocation of long-chain FA across the plasma membrane and is thought to function as the predominant FATP in the heart(Reference Gimeno, Ortegon and Patel8). FATP6 is exclusively located on the sarcolemma in areas neighbouring small blood vessels where it is co-localised with CD36(Reference Gimeno, Ortegon and Patel8).
Alterations in the tissue content of fat transporters may give rise to heart diseases such as cardiac hypertrophy and diabetic heart. In spontaneously hypertensive rats lacking CD36 expression, the defective FA uptake was linked to insulin resistance and myocardial hypertrophy(Reference Hajri, Ibrahimi and Coburn9), whereby in a transgenic mouse model, the heart-specific overexpression of FATP1 caused a pathophysiological condition consistent with functional cardiomyopathy(Reference Chiu, Kovacs and Blanton10).
Because altered concentrations of cardiac fat transporters seem to be associated with a disturbed uptake of FA to the heart, we investigated the association of a potentially functional genetic variability in the 5′-untranslated region (UTR) of the heart-type FATP6 gene with variables of the fasting and postprandial lipid and glucose metabolism. Heart function was determined by echocardiography in selected participants in order to examine left ventricular heart mass among different genotypes.
Experimental methods
Study population
The Metabolic Intervention Cohort Kiel is a prospective population-based cohort study on natural incidence and risk factors of the metabolic syndrome. A total of 755 men aged 45–65 years were recruited via the registration-register of the town of Kiel, Germany. Exclusion criteria were known diabetes, liver diseases, intestinal absorption disorders, renal diseases, intestinal surgery within the last 3 months, thyroid disorders and hormone therapy. Of these recruits, 714 volunteers underwent a clinical examination including a measurement of pulse, blood pressure, weight, height, waist circumference and hip circumference. At inclusion in the study, all participants were guided by a physician to complete a standardised questionnaire concerning individual and family history, including lifestyle data, such as physical activity, dietary habits (frequency of fish, meat, vegetable, fruit consumption per week, intake of low-fat and fibre-rich products), smoking and alcohol consumption. Participants were instructed not to change their eating habits, not to use any dietary supplements of vitamins, minerals or special oil preparations and not to follow a special diet at least 3 d before and during the study period. Furthermore, the participants were instructed to restrain from abnormally high physical activity and excessive alcohol consumption (the day before the study). Assessment of the metabolic syndrome was based on the criteria of the International Diabetes Federation(Reference Alberti, Zimmet and Shaw11). The detailed design and methodology of the study have been described previously(Reference Rubin, Helwig and Nothnagel12). Volunteers underwent an oral metabolic tolerance test (OMTT) and an oral glucose tolerance test (OGTT) at different days with a minimum of 3 d in-between. The study was performed according to the Declaration of Helsinki. All participants gave written informed consent to participate in the study, which was approved by the Ethics Committee of the Medical Faculty, Christian-Albrechts University of Kiel.
Oral glucose tolerance test
A 75 g OGTT was performed, following a 12 h overnight fast and after dietary advice was given to ensure a carbohydrate intake of >150 g/d over the previous 3 d. Blood samples for glucose and insulin were taken before and 30, 60, 120, 180 and 240 min after the glucose load.
Oral metabolic tolerance test
An OMTT provided by Nutrichem diät+pharma GmbH, Roth, Germany was performed as described elsewhere(Reference Rubin, Helwig and Nothnagel12). In brief, after a 12 h fasting period and withdrawal of fasting blood samples, subjects drank 500 ml of the OMTT containing the following ingredients: 30 g protein (11·9 % energy), 75 g carbohydrate (29·6 % energy; 93 % sucrose, 7 % lactose), 58 g fat (51·6 % energy; 65 % saturated, 35 % unsaturated FA), 10 g alcohol (6·9 % energy), 600 mg cholesterol and 9 mg retiral (31·5 μmol) in the form of retinylpalmitate. The total energy content was 4255 kJ. Before and 0·5, 1, 2, 3, 4 and 5 h after ingestion, blood samples were drawn for the analysis of insulin, glucose, TAG and NEFA, and 6, 7, 8 and 9 h after ingestion for the analysis of TAG and NEFA.
Laboratory analyses
After the meals, blood samples were placed on ice and centrifuged, and plasma and serum were deep-frozen for later analysis. Serum was used for insulin, TAG and NEFA determination, and fluoride plasma for glucose determination. Leucocytes and Hb were assessed in EDTA blood. Other parameters were assessed in lithium-heparin plasma.
Lipid, glucose and insulin measurements have been described previously(Reference Rubin, Helwig and Nothnagel12). Serum NEFA were analysed enzymatically (Wako, Richmond, VA, USA). Homeostatic model assessment-insulin resistance (HOMA-IR) was calculated as follows: glucose (mmol/l) × insulin (μU/l)/22·5(Reference Matthews, Hosker and Rudenski13). Postprandial insulin sensitivity was assessed by the oral glucose insulin sensitivity test according to Mari et al. (Reference Mari, Pacini and Murphy14); this index correlated strongly with insulin sensitivity assessed by glucose clamping, the gold standard method. N-terminal pro-brain natriuretic peptide (NTproBNP) serum concentrations were measured in 580 participants by the Roche Diagnostics Elecsys NTproBNP electrochemiluminescence immunoassay (Roche Diagnostics, Indianapolis, IN, USA).
DNA extraction and genotyping
Genomic DNA was purified using the NucleoSpin Blood Isolation kit (Macherey–Nagel, Düren, Germany). The variation in the 5′-UTR of the FATP6 gene was successfully typed by TaqMan analysis in 695 participants (rs2526246). Sequences of PCR primers and TaqMan were as follows (ABI, Foster City, CA, USA): PCR forward primer: 5′-GCTGAGATCAGAGCTGTCTTCT-3′; PCR reverse primer: 5′-CCCTAGAACTGTTA GCCATGACA-3′; Taqman probe allele 1: 5′-CCCAGTAGCCCCC-3′; Taqman probe allele 2: 5′-CCCAGTTGCCCCC-3′. Taqman analysis was performed as described elsewhere(Reference Hampe, Shaw and Saiz15). The genotyping success rate was 97·5 %.
Echocardiography
Of the participants from the Metabolic Intervention Cohort Kiel, fifty-nine underwent an M-mode and Doppler echocardiographic examination. Of these, five participants were excluded from the study because of unsatisfactory echocardiographic tracings (n 3), diagnosed tachycardia (n 1) and ischaemic cardiomyopathy (n 1). After exclusion, twenty-seven homozygous carriers of allele T and twenty-seven BMI-matched homozygous carriers of allele A remained for further analysis. End-diastolic measurements of interventricular septal thickness, left ventricular internal dimension, posterior wall thickness and LVM calculations were obtained according to the Penn convention(Reference Devereux and Reichek16). LVM was indexed (LVMI) for body surface area.
Statistical analysis
The results are presented as means with their standard errors. The study population was tested for the distribution of genotypes according to the Hardy–Weinberg equilibrium (χ2 test).
Participants with missing data (genotyping, biochemical and anthropometric) were excluded from further statistical analysis, and therefore the analyses were performed in 685 subjects. Between-group comparisons were analysed in a crude model using the Mann–Whitney U test since the probability plot of the standardised residuals was not normally distributed. Associations between log-transformed quantitative traits and FATP6 genotypes were tested using ANOVA, with adjustment for waist circumference, age, smoking (yes/no), diabetes (yes/no), apoE genotype, use of lipid-lowering and anti-hypertensive drugs. The probability plot of the standardised residuals in this adjusted model was close to normal.
In a logistic regression analysis with the metabolic syndrome as the response variable, OR and corresponding 95 % CI were estimated.
The area under the curve was calculated by the trapezoidal method; the incremental area under the curve with the baseline subtracted. Statistical analyses were performed using SPSS (SPSS for windows, Release 9.0.1; LEAD Technologies Inc., Chicago, IL, USA). A two-sided P < 0·05 was considered statistically significant.
Results
The FATP6 gene (AA614135) is located on the human chromosome 5q23. The investigated SNP represents a haplotype block of four SNP in the 5′-UTR of FATP6 being in complete linkage disequilibrium(Reference Barrett, Fry and Maller17). According to in silico analysis, the T>A change is predicted to result in an allele-specific binding of PPAR(Reference Wingender, Chen and Hehl18). The genotype frequency of the FATP6 5′-UTR polymorphisms was as follows: TT, 0·40 (n 278); TA, 0·45 (n 305); AA, 0·15 (n 102). The minor allele frequency was 0·37. No deviations from Hardy–Weinberg were detected. TT- and TA-allele carriers were combined for statistical analysis since no differences between TT-homozygotes and heterozygotes were found (data not shown) and the T-allele was accordingly considered to be the dominant allele. According to the International Diabetes Federation criteria(Reference Alberti, Zimmet and Shaw11), the present study population was on average overweight, abdominally obese and marginally hypertensive (Table 1). Furthermore, participants were insulin resistant based on the criteria of Monzillo & Hamdy(Reference Monzillo and Hamdy19), defining disturbed sensitivity by HOMA-IR to be above 2·6. Dietary habits as assessed by a questionnaire did not differ between genotypes (data not shown).
Table 1 Anthropometric, fasting and postprandial metabolic variables in the entire study group and according to the fatty acid transport protein 6 rs2526246 polymorphism
(Mean values with their standard errors)

HOMA-IR, homeostatic model assessment-insulin resistance; OMTT, oral metabolic tolerance test; AUC, area under the curve; AUCi, incremental area under the curve; OGTT, oral glucose tolerance test; OGIS, oral glucose insulin sensitivity.
* P values for mean differences between genotypes (Mann–Whitney U test).
† Remained significant after adjusting for waist circumference, age, smoking (yes/no), diabetes (yes/no), apoE genotype, use of lipid-lowering and anti-hypertensive drugs (ANOVA).
Association of fatty acid transport protein 6 variation with traits of the metabolic syndrome
FATP6 genotypic groups did not significantly differ in BMI and waist circumference (Table 1). AA homozygotes showed significantly lower systolic (P = 0·003) and diastolic (P = 0·002) blood pressure compared with combined heterozygous and homozygous carriers of the wild-type allele (TT/TA) (Table 1). This holds true after adjustment (P = 0·017 and P = 0·024, respectively). Moreover, they had lower fasting TAG concentrations (P = 0·008). The differences remained significant (P = 0·021) after controlling for potential confounders. According to HOMA-IR, homozygous carriers for allele A presented with higher, but non-significant, insulin sensitivity. Carriers of the different genotypes, however, did not significantly differ in cholesterol, HDL-cholesterol, LDL-cholesterol, fasting NEFA, glucose and insulin.
AA homozygotes showed lower TAG concentrations after ingestion of a mixed meal compared with T-allele carriers (Fig. 1(a); Table 1). The individual peak concentrations of postprandial TAG following an OMTT were lower in AA homozygotes compared with T-allele carriers (2·73 (sem 0·11) v. 3·07 (sem 0·07) mmol/l; P = 0·021). Differences remained significant after controlling for confounders (P = 0·033). NEFA were lower in the late postprandial phase after the OMTT in the crude model as well as after controlling for confounders (Fig. 1(b)). There was no significant difference between genotypes in postprandial glucose concentrations following either an OMTT or an OGTT (Table 1; Fig. 1(c)). Insulin concentrations in response to the OMTT varied among FATP6 genotypes, with consistently lower concentrations in AA carriers. After controlling for confounders, the difference did not remain significant (Fig. 1(d)). Genotypic groups did not differ in insulin resistance, estimated either by the OMTT-based product of postprandial insulin × glucose concentrations or by the OGTT-based index of insulin sensitivity (oral glucose insulin sensitivity) (Table 1).

Fig. 1 Fasting and postprandial variables according to the fatty acid transport protein 6 polymorphism (rs2526246) after an oral metabolic tolerance test. (a) TAG, (b) NEFA, (c) glucose and (d) insulin concentrations in T-allele carriers (TT/TA) (○) compared with AA homozygotes (●). Values are means, with their standard errors represented by vertical bars. * Mean values were significantly different between genotypes at that time (P < 0·05) after controlling for waist circumference, age, smoking (yes/no), diabetes (yes/no), apoE genotype, use of lipid-lowering and anti-hypertensive drugs.
In compliance with these findings, carriers of the rare allele also showed a reduced risk of the metabolic syndrome (OR 0·54, 95 % CI 0·34, 0·85, P = 0·008; Table 2). This remained significant after adjusting for confounders.
Table 2 Association of fatty acid transport protein 6 polymorphism (rs2526246) with the metabolic syndrome
(Odd ratios and 95 % confidence intervals)

* Adjusted for waist circumference, age, smoking (yes/no), diabetes (yes/no), apoE genotype, use of lipid-lowering and anti-hypertensive drugs.
Association of fatty acid transport protein 6 variation with left ventricular hypertrophy
NT-proBNP, which has been proposed as an early marker of heart failure(Reference Morillas, Castillo and Quiles20), were not significantly different between genotypes (AA (n 92): 46·7 (sem 6·7); T-allele carriers (n 488): 61·3 (sem 5·9) pg/ml; P = 0·512). Homozygous dominant and heterozygous genotypes contributed the same to the phenotype as observed in the entire cohort. Before having finalised the entire evaluation, we started the investigation of the association with cardiac parameters by sonography. In order to assure a maximal effect size of the polymorphism, we recruited only BMI-matched TT and AA homozygotes for the present investigation.
There were no significant differences in anthropometric and fasting variables between TT and AA homozygotes included for echocardiographic examination (Table 3). Similarly, no differences were observed for the postprandial variables except for the TAG area under the curve, which were lower in AA homozygotes by trend (AA: 15·1 (sem 0·9) mmol/l × h; TT: 17·9 (sem 1·2) mmol/l × h; P = 0·08). Differences between genotypic groups in subjects undergoing the echocardiographic examination were similar to those in the entire cohort, but at a lower level (Table 3).
Table 3 Anthropometric and fasting metabolic variables according to the fatty acid transport protein 6 polymorphism (rs2526246) in participants undergoing echocardiographic examination
(Mean values with their standard errors)
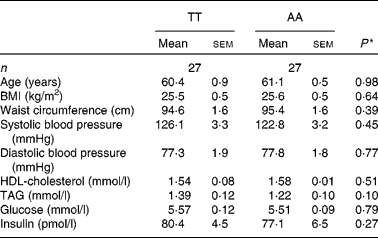
* P values for mean differences between genotypes (Mann–Whitney U test).
AA homozygotes had a significantly lower LVMI than TT homozygotes (108·4 (sem 7·1) v. 129·0 (sem 4·4) g/m2; P = 0·038) (Fig. 2).
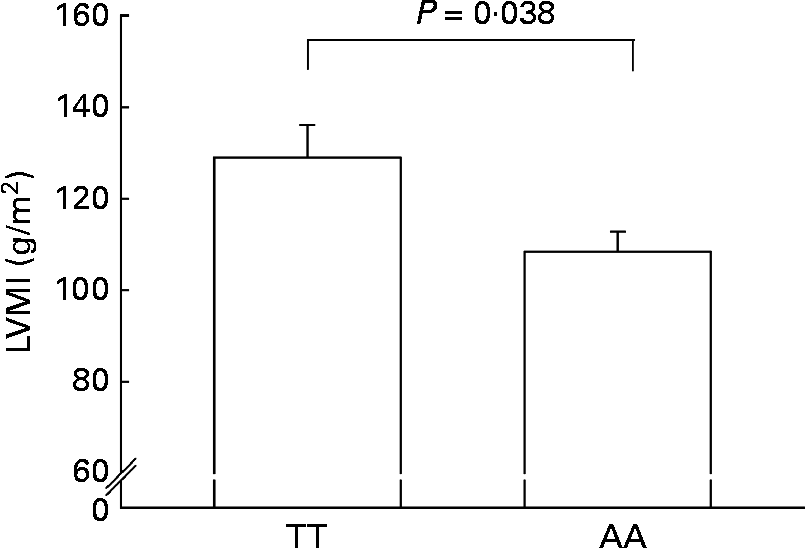
Fig. 2 Left ventricular mass index (LVMI) in AA homozygotes (n 27) and TT homozygotes (n 27) of the fatty acid transport protein 6 polymorphism (rs2526246). Participants were matched for BMI. Values are means, with their standard errors represented by vertical bars.
Discussion
So far, little is known about the impact of genetic variability in the FATP6 gene. This is the first study that addresses an association of a SNP presenting a haplotype in the 5′-UTR (–7T>A) of the FATP6 gene with traits of the metabolic syndrome and heart failure. This SNP was suggested to differ in the binding of PPAR transcription factors that are known to be critical regulators of gene expression in the heart(Reference Gilde and Van Bilsen21).
There is evidence that underlines the crucial role of FATP6 in mediating long-chain FA uptake to the heart(Reference Gimeno, Ortegon and Patel8). The present study supports this assumption because carriers of the rare allele had lower lipid and insulin concentrations (Table 1; Fig. 1). Differences in NEFA concentrations were seen only in the late postprandial phase (Fig. 1(b)), indicating that a more efficient uptake of FA released from TAG-rich lipoproteins leaves less NEFA in the plasma(Reference Peterson, Bihain and Bengtsson-Olivecrona22). Interestingly, the deficiency of CD36, which co-localises with FATP6 on the sarcolemma, resulted in elevated NEFA concentrations, which in turn impaired the lipoprotein lipase-mediated TAG clearance(Reference Goudriaan, den Boer and Rensen23). The heart is, indeed, able to maintain normal concentrations of plasma TAG due to a moderate activity of cardiac lipoprotein lipase, also in the absence of skeletal muscle and adipose lipoprotein lipase(Reference Augustus, Yagyu and Haemmerle24). Accordingly, an accelerated clearance of TAG-rich lipoproteins and a shortened residence time in the circulation induced by a potential gain-of-function of FATP6 in AA homozygotes might explain the lower postprandial TAG concentrations in that genotype. This might occur in cooperation with CD36(Reference Gimeno, Ortegon and Patel8). Higher TAG-rich lipoprotein clearance in turn is associated with lower fasting TAG concentrations(Reference Vedala, Wang and Neese25), explaining the lower fasting TAG concentrations in AA homozygotes compared with T-allele carriers, and thus the lacking difference was obtained when TAG incremental area under the curve were calculated (Table 1).
Elevated NEFA, TAG-rich lipoproteins and their corresponding remnant particles are described to be a potential cause for the development of insulin resistance(Reference Steinberg, Tarshoby and Monestel26, Reference Pedrini, Kranebitter and Niederwanger27). Lower fasting and postprandial TAG concentrations in AA homozygotes may account for the lower postprandial insulin and glucose response following an OMTT (Fig. 1(c) and (d)). NEFA differed at a later stage after the OMTT than the insulin differences, and therefore they do not seem to have a direct effect in this respect.
There was no difference in BMI and waist circumference between the two genotypic groups. Whereas partitioning of FA to the adipose tissue, for example, by PPAR agonists results in higher insulin sensitivity but also an increase in body fat mass(Reference Yamauchi, Kamon and Waki28); FATP6 SNP may enhance insulin sensitivity without increasing BMI and abdominal fat (Table 1).
Postprandial hypertriacylglycerolaemia may result in an accumulation of atherogenic particles, which then impairs endothelial function(Reference Ceriello, Taboga and Tonutti2). In addition, elevated NEFA concentrations were shown to reduce NO production, which presumably results as well in endothelial dysfunction(Reference Steinberg, Tarshoby and Monestel26). According to that and since endothelial dysfunction is closely related to hypertension, the improved blood pressure indices in AA homozygotes (Table 1) might be explained by lower NEFA concentrations in the late postprandial phase (Fig. 1(b)) and lower fasting and postprandial TAG concentrations (Fig. 1(a)).
Both dyslipidaemia as well as hypertension lead to cardiac restructuring and damage, which is evidenced by left ventricular hypertrophy(Reference Sundstrom, Lind and Vessby29). Accordingly, lower TAG concentrations and blood pressure values in AA homozygotes made us to speculate about the impact of the investigated FATP6 polymorphism on the LVM, as measured using M-mode echocardiography. Indeed, AA homozygotes were associated with a significantly lower LVMI (Fig. 2). In cardiac hypertrophy, there is an altered cardiac fuel uptake and utilisation with a shift from FA towards glucose oxidation, which was suggested to be a compensatory mechanism to support oxygen-sparing glycolytic pathways for ATP production(Reference Stanley, Recchia and Lopaschuk5), but on the long run, the reliance on glucose may only produce a relatively energy-deficient state that results in decreased contractile performance(Reference Davila-Roman, Vedala and Herrero30). Decreased cardiac FA oxidation and lipid incorporation in the infracted rat heart was accompanied by the down-regulation of FA transporters and an impaired cardiac function(Reference Heather, Cole and Lygate31). Similarly, FA utilisation was decreased and glucose utilisation increased in human idiopathic dilated cardiomyopathy(Reference Davila-Roman, Vedala and Herrero30). However, it is still an open question whether this is part of the pathological process mediating heart failure or a matter of adaptation(Reference Davila-Roman, Vedala and Herrero30).
Some limitations should be mentioned. The results of the present study should be viewed as explorative and the present observations are restricted to men in this special age group with a high prevalence of overweight; therefore, replications in other populations should be undertaken. Moreover, the genotypes in the echocardiographic cohort showed significant differences in LVMI and borderline significant differences for postprandial TAG, while differences in other traits of the metabolic syndrome were not as strong as observed in the entire cohort. This might be explained by the rather small echocardiographic study group and the concomitant limited statistical power. On the other hand, this might underline the impact of the FATP6 genotype on the LVM.
In the present study, we examined the impact of the − 7T>A polymorphism in the 5′ UTR of the FATP6 gene on some traits of the metabolic syndrome. AA homozygotes were associated with lower systolic and diastolic blood pressure, lower fasting and postprandial TAG, as well as lower insulin concentrations. In compliance with these findings, carriers of the rare allele also had a reduced risk of the metabolic syndrome (Table 2). We propose a SNP-associated gain-of-function of FATP6, which might increase the FA uptake and utilisation leading to a better energy supply in cardiomyocytes and preventing hypertrophy by reducing either blood pressure or direct myocardial effects. The Framingham Heart Study evidenced that an increase in LVM predicts a higher incidence of CVD(Reference Levy, Garrison and Savage4). Therefore, if the present findings can be confirmed in further studies, FATP6 may be an interesting pharmacological target. The investigated differences may be based on an altered translational activity, which is mediated by genotypic variations. Further studies about the underlying mechanism remain to be conducted.
Acknowledgements
This study was supported by a grant of the German Federal Ministry of Education and Research ‘Dietary fat and metabolism, gene variability, regulation and function’ (AZ 0312823 A/B). Nutrichem supported the studies of the BMBF-supported cluster and was partner in this cluster. A. A., U. H., D. R. and J. S. were responsible for the conception and design of the study; A. A., U. H., D. R., M. L., T. R., N. E. E. M., U. R. F., S. S., N. F. and J. S. were responsible for conducting the experiments and data collection; A. A. was responsible for the statistical analysis; A. A., U. H., D. R., M. P. and J. S. were responsible for the data interpretation; A. A. was responsible for drafting the manuscript; J. S. was responsible for raising the funds and supervision. All authors contributed to the final version of the manuscript and did a critical revision, and evaluated and approved the final version of the manuscript. The authors declare that there are no conflicts of interest.







