Introduction
Information on the distribution and status of Cambodia’s wildlife is limited (Long & Swan, Reference Long and Swan2000), although information from surveys undertaken since the end of the 1990s has led to improved conservation plans for many species, including tigers and elephants (Daltry & Momberg, 2000; Long et al., Reference Long, Roth, Holden, Uck, Daltry and Momberg2000b). For primates, however, despite 10 of Cambodia’s 11 taxa being categorized as threatened on the IUCN Red List (IUCN, 2010), few studies have quantified their abundance (Long & Swan, Reference Long and Swan2000; Traeholt et al., Reference Traeholt, Bonthoeun, Rawson, Samuth, Virak and Vuthin2005). In particular, the Cardamom Mountains, west of the Mekong River, were inaccessible for c. 20 years because of the Cambodian civil war, and there have been only a few wildlife surveys in this region (Momberg & Weiler, 1999; Long & Swan, Reference Long and Swan2000).
Primates in South-East Asia are declining at a dramatic rate (Mittermeier et al., Reference Mittermeier, Ratsimbazafy, Rylands, Williamson, Oates and Mbora2007) and up-to-date information on their populations is essential for determining their national and global conservation status. Six primate species are reported to occur in the Cardamom Mountains (Table 1; Long & Swan, Reference Long and Swan2000). In Samkos Wildlife Sanctuary the pileated gibbon Hylobates pileatus, with an estimated 3,100 groups, is the only species that has been studied in detail (Traeholt et al., Reference Traeholt, Bonthoeun, Rawson, Samuth, Virak and Vuthin2005). Simulations predict a dramatic decline of the population in the Sanctuary in the next 40–50 years because of habitat loss from illegal logging (Traeholt et al., Reference Traeholt, Bonthoeun, Rawson, Samuth, Virak and Vuthin2005). In developing countries such as Cambodia infrastructure expansion and logging are major threats to forests and wildlife (Smith, Reference Smith2001). We therefore carried out the first survey of all primate species in the Sanctuary since 2000, reporting novel data on their abundance in the rainy season. Because the distribution of primate species in Cambodia is still poorly known we also provide a review of their distribution, status and threats.
Table 1 Total number of sightings and encounter rate (groups per hour and individuals per hour) of each species sighted during the census (60 hours diurnal; 25 hours nocturnal) in Samkos Wildlife Sanctuary (Fig. 1).
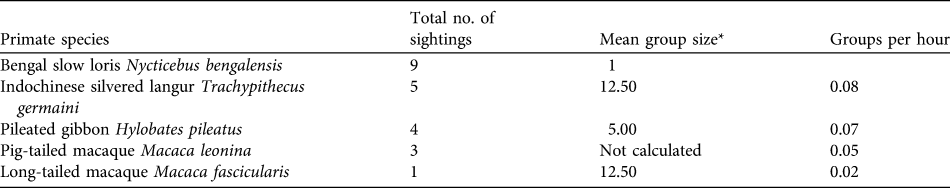
* Calculated using all sightings throughout the study period
Study area
Our study took place in the lowlands of Samkos Wildlife Sanctuary (3,338 km2) in the Cardamom Mountains (10,000 km2) in south-west Cambodia (Fig. 1). The survey area comprises lowland evergreen and dry dipterocarp forests up to an altitude of 350 m. Mean annual rainfall is 3,000–4,000 mm, with a rainy season during May–October (Rollet, Reference Rollet1972; Daltry & Momberg, 2000). The fauna of the Cardamom Mountains includes a variety of threatened and/or endemic species of birds, mammals, amphibians and plants (Momberg & Weiler, 1999; Daltry & Momberg, 2000). Some illegal activities such as timber extraction and hunting persist and local people utilize the forest legally for resin, fruit and plant collection.
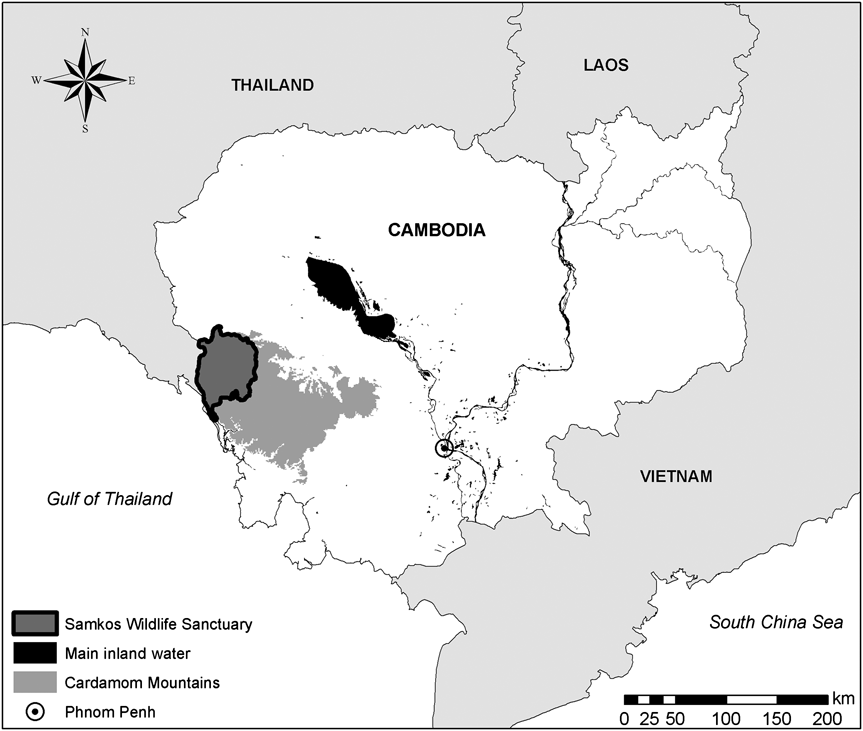
Fig. 1 Location of the Cardamom Mountains and Samkos Wildlife Sanctuary within Cambodia.
Methods
We surveyed for primates from 22 April to 31 May 2009 using line transects (Sutherland, Reference Sutherland and Sutherland2000) combined with exploration of the forest not following any transect (tracking) to increase detection and acquire descriptive data on group size. Tracking and line transects are hereafter referred to as the census.
Ten randomly selected 1-km long transects were walked twice over 10 days at 08.00–11.00 and then 2 weeks later at 14.00–17.00, and twice over 10 days at 19.00–24.00 and 24.00–04.00. Transects 1–4 were in lowland evergreen forest and transects 5–10 in dry dipterocarp forest. Eight days were spent tracking diurnal primates starting at 08.00 for 4–6 hours. Two people walked quietly at 1 km h-1, stopping frequently to scan all forest layers. We recorded date, time, transect, coordinates with a global positioning system, weather, habitat and perpendicular distance of the first animal seen from the observer or transect.
For diurnal primates we used the encounter rate biodiversity assessment technique (Sutherland, Reference Sutherland and Sutherland2000), calculating number of animals seen per survey hour. For the Bengal slow loris Nycticebus bengalensis we used the linear encounter rate per km (Nekaris et al., Reference Nekaris, Blackham and Nijman2008) and density (D) of animals per km2, calculated using D = n/2wl, where w is strip width, l is transect length and n is the number of lorises (Sutherland, Reference Sutherland and Sutherland2000).
Results
We spent 60 hours conducting diurnal surveys and 25 hours conducting nocturnal surveys. We confirmed the presence of four diurnal and one nocturnal primate species (Table 1). We sighted diurnal primates 18 times, 13 of which (72.2%) were during censuses and five opportunistically. Twelve observations (92.3%) were made during tracking and only one (7.7%) during transects (Table 1). All sightings of diurnal primates were in lowland evergreen forest. We encountered nine N. bengalensis. For the entire area surveyed the linear encounter rate was 0.45 ± SE 0.64 km-1 and density was 18.75 ± SE 26.81 km-2. More sightings (66.7%) occurred in dry dipterocarp forest, with a linear encounter rate of 0.50 ± SE 0.63 km-1 and density of 20.83 ± SE 26.35 km-2. In lowland evergreen forest (33.3% of sightings), linear encounter rate was 0.38 ± SE 0.75 km-1 with a density of 15.63 ± SE 31.25 km-2.
N. bengalensis was sighted most, followed by the Indochinese silvered langur Trachypithecus germaini, H. pileatus, pig-tailed macaque Macaca leonina and long-tailed macaque Macaca fascicularis (Table 1). We did not detect the stump-tailed macaque Macaca arctoides. We also heard H. pileatus daily between 09.00 and 10.00. Half of all diurnal species were sighted between 05.30 and 11.10 and half between 12.30 and 17.00. We observed lorises more or less equally during early and late night: 19.00–00.00 (56%) and 00.00–04.00 (44%).
Most diurnal observations (n = 17) occurred during sunny days (94.4%); no sighting occurred during rain. The mean distance from observer for diurnal primates was 19.8 m ± SD 10.1, and 5.8 m ± SD 3.6 for N. bengalensis. Mean group sizes are presented in Table 1. Group composition of H. pileatus was one adult male and female with three juveniles; the four sightings were in the same area and were probably of one group. Sightings of T. germaini and M. fascicularis comprised all age classes. Only adult N. bengalensis were seen; on three occasions individuals were observed in proximity with conspecifics.
Discussion
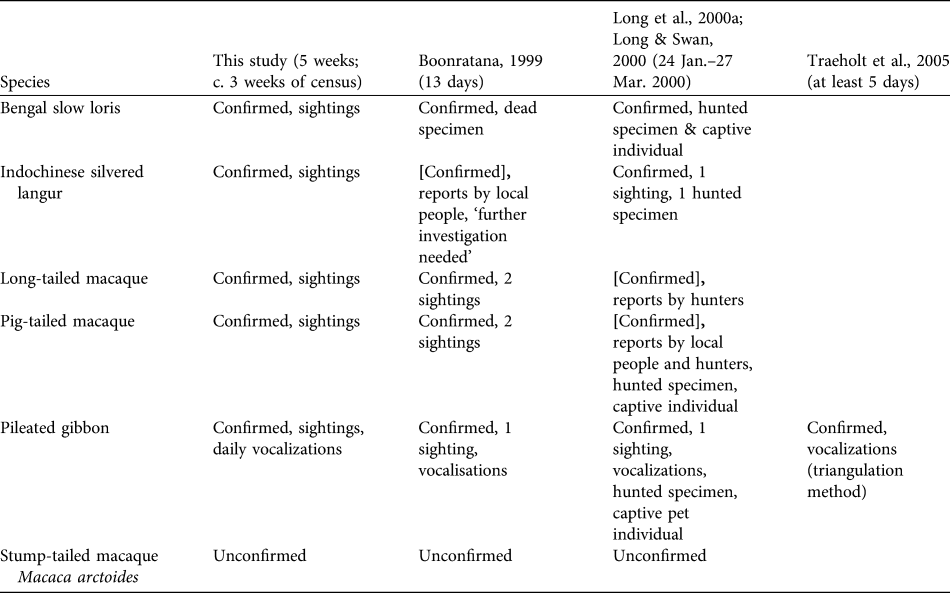
Our survey confirms the presence of four diurnal primates and the nocturnal N. bengalensis in the Cardamom Mountains. Previous surveys of these species had relied on a combination of village interviews and forest and market surveys (Table 2).
Table 2 Summary of the results of this and previous primate surveys (with surveying dates and lengths in parentheses) in the Samkos Wildlife Sanctuary (Fig. 1). [Confirmed] indicates that the presence of the species was not confirmed by sightings.
To clarify the distribution of primate species in Cambodia we compiled records for all species known from the country (Table 3). Recent changes in classification of T. germaini may increase its threat. Genetic data (Osterholz et al., Reference Osterholz, Walter and Roos2008; Roos et al., Reference Roos, Nadler and Water2008) separate T. germaini from Trachypithecus margarita, with the Mekong River a barrier between the two species (Roos et al., Reference Roos, Nadler and Water2008). Previous surveys (Boonratana, Reference Boonratana, Momberg and Weiler1999; Long & Swan, Reference Long and Swan2000; Long et al., Reference Long, Roth, Holden, Uck, Daltry and Momberg2000a) in the Cardamom Mountains identified T. germaini as Trachypithecus cristatus, consequently attributing it a much broader distribution in South-East Asia. If Roos et al.’s (2008) classification is accepted, conservation of this species in the Cardamom Mountains should be regarded as a priority.
Table 3 Confirmed records of primate species in Cambodia, with their Red List status and the areas where they have been observed, with references, west and east of the Mekong river.
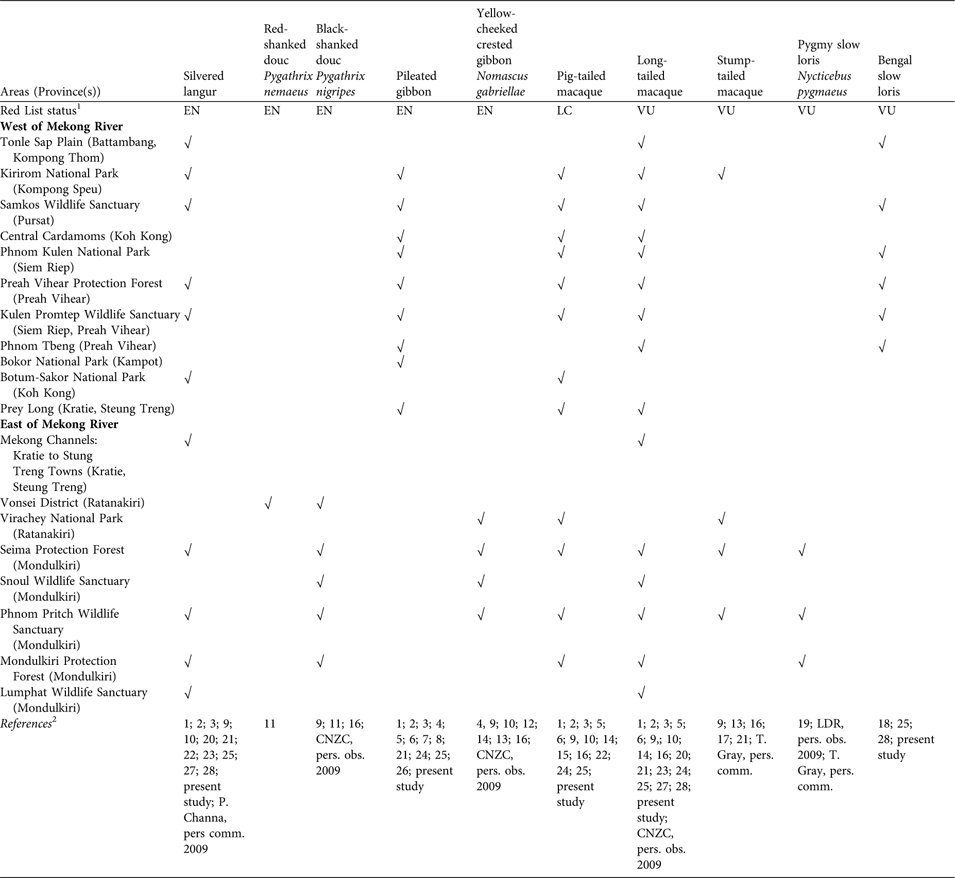
1 LC, Least Concern (i.e. not on the Red List); VU, Vulnerable; EN, Endangered (IUCN, 2010)
2 1, Boonratana (Reference Boonratana, Momberg and Weiler1999); 2, Long et al. (2000a); 3, Long & Swan (Reference Long and Swan2000); 4, Traeholt et al. (Reference Traeholt, Bonthoeun, Rawson, Samuth, Virak and Vuthin2005); 5, Daltry & Momberg (2000); 6, Emmet & Olsson (2005); 7, Neath et al. (Reference Neath, Setha, Bunnat and Stuart2001); 8, Rawson & Senior (Reference Rawson and Senior2005); 9, Pollard et al. (Reference Pollard, Clements, Hor and Ko2007); 10, Rawson (Reference Rawson2007); 11, Rawson & Roos (2008); 12, Channa & Gray (Reference Channa and Gray2009); 13, Conservation International (2007); 14, Timmins & Ratanak (2001); 15, Desai & Vuthy (1996); 16, Walston et al. (Reference Walston, Davidson and Soriyun2001); 17, Pfeffer (Reference Pfeffer1969); 18, Starr et al. (Reference Starr, Nekaris, Streicher and Leung2010b); 19, Starr et al. (Reference Starr, Nekaris, Streicher and Leung2011); 20, Campbell et al. (Reference Campbell, Poole, Giesen and Valbo-Jorgensen2006); 21, Kong Kim Sreng & Setha (2002); 22, Royan (Reference Royan2010); 23, Bezuijen et al. (Reference Bezuijen, Timmins and Seng2007); 24, ACCB data (M. Handschuh, pers. comm. 2010); 25, Wildlife Conservation Society data (H. Rainey, pers. comm. 2010); 26, Rainey et al. (2010); 27, Eames (Reference Eames2007); 28, Davidson (2006)
As H. pileatus was more easily heard than seen, using their vocalisation to census the species seems a better method to estimate density (e.g. triangulation: Traeholt et al. Reference Traeholt, Bonthoeun, Rawson, Samuth, Virak and Vuthin2005; occupancy modelling: Neilson, Reference Neilson2010). Traeholt et al. (Reference Traeholt, Bonthoeun, Rawson, Samuth, Virak and Vuthin2005) used triangulation and suggested that the Cambodian population of H. pileatus is the world’s largest and that the Cardamom Mountains are critical for the conservation of this species. Our results, with only one group sighted in an area of c. 470 ha, may be indicative that the density of the species in Samkos Wildlife Sanctuary has decreased since the study by Traeholt et al. (Reference Traeholt, Bonthoeun, Rawson, Samuth, Virak and Vuthin2005). Monitoring of this population by regular surveys is required.
Despite distribution data (Cobert & Hill, Reference Cobert and Hill1992; Rowe, Reference Rowe1996; Walston, Reference Walston and Smith2001; Francis, Reference Francis2008) suggesting the presence of M. arctoides no study has verified its presence in the Cardamom Mountains. Walston (Reference Walston and Smith2001) mistakenly cited Pfeffer (Reference Pfeffer1969) as evidence of M. arctoides west of the Mekong River but Pfeffer’s study was conducted in eastern Cambodia (Table 3). In 2000 three individuals of M. arctoides were sighted in Kirirom National Park, west of the Mekong River (Kong Kim Sreng & Setha, Reference Kong and Setha2002) and reported as having been highly reduced in numbers by hunting. Little is known about this species and its distribution in South-East Asia is not clearly defined (Fooden et al., Reference Fooden, Guoqiang, Zongren and Yingxiang1985; Choudhury, Reference Choudhury2002; Htun et al., Reference Htun, Timmins, Boonratana and Das2008). Although M. arctoides is generally found at high elevations (up to 2,700 m in India and China; Htun et al., Reference Htun, Timmins, Boonratana and Das2008), in Cambodia it was recorded at c. 250 m in Virachay National Park (Conservation International, 2007), the same altitude as our survey site. Previous surveys in the Cardamom Mountains covered a wider range of habitat including hill evergreen forest (up to 1,200 m) but did not record this species. The continued absence of M. arctoides in surveys in the Cardamom Mountains indicates either that it does not occur there or that it does so at an extremely low density (cf. Pollard et al., Reference Pollard, Clements, Hor and Ko2007).
The only previous nocturnal surveys for N. bengalensis were conducted by Daltry & Momberg (2000). Other confirmations come from dead or captive individuals (Table 2). Starr et al. (Reference Starr, Nekaris, Streicher and Leung2010b) conducted surveys for N. bengalensis throughout Cambodia but only sighted the species in Samkos Wildlife Sanctuary and Phnom Kulen National Park. The decline of this species in the wild and in traditional medicine markets, where it once was common, mean that the Sanctuary is also an important site for this species.
Previous diurnal surveys in the Cardamom Mountains were carried out during the dry season (c.f. Daltry & Momberg, 2000) when rivers dry up. Our survey, during the rainy season, was conducted next to a river that attracts some primate species, perhaps explaining why we observed some species not seen by previous researchers. Researchers often recommend to survey diurnal primates in the early morning (Sutherland, Reference Sutherland and Sutherland2000) but we observed primates active throughout the day. All of our encounters occurred during non-rainy days.
Seasonality may also affect the results of nocturnal surveys. N. bengalensis was encountered in dry dipterocarp forest characterized by grassland that this non-leaping primate uses as a substrate to reach trees. During the dry season grassland is burnt by local people for access to resin trees (Starr et al., Reference Starr, Nekaris, Streicher and Leung2011), possibly adversely affecting this species. Slow lorises also reduce their activity during the cooler period of December–February and during these months other researchers have detected relatively few (Evans et al., Reference Evans, Duckworth and Timmins2000; Starr et al., Reference Starr, Nekaris, Streicher and Leung2011). Increased activity during our study period (April–May) could explain why N. bengalensis was the most often encountered primate.
Our study provides the first encounter rates of diurnal primate species for Samkos Wildlife Sanctuary, although only an index of abundance. Given the difficult terrain, which made line transects difficult to establish and time consuming, we recommend for future research the use of occupancy surveys that use sampling of many points spread in different habitats, allowing the coverage of a large area (Royles & Nichols, Reference Royles and Nichols2003). This method also provides a detection probability for comparing different species and habitat types (MacKenzie et al., Reference MacKenzie, Nichols, Ryle, Pollock, Bailey, Hines, MacKenzie, Nichols, Ryle, Pollock, Bailey and Hines2006), something lacking in our study. Presence sampling by sign (e.g. faeces, tracks, vocalization) may be particularly suitable for the Cardamom Mountains, and not only for primates. Occupancy surveys in combination with sign counts could be useful for estimating abundance of the area’s many endemic birds (Shanahan & Possingham, Reference Shanahan and Possingham2009) and reptiles and amphibians (cf. Grismer at al., 2008). Other threatened taxa, including Endangered Asian elephant Elephas maximus, Vulnerable gaur Bos gaurus, and Endangered banteng Bos javanicus, all of which were detected during our study, leave tell-tale signs (Barnes, Reference Barnes and Kangwana1996).
Although rare and threatened species are protected by law from hunting and trade in Cambodia (Walston & Ashwell, 2005), wildlife is continually threatened by illegal activities. The end of the war was characterised by dramatic logging and increasing development of infrastructure such as roads (Momberg et al., Reference Momberg and Weiler1999), factors that still persist. During our study we heard chainsaws twice a few kilometres from our camp; illegal logging is currently increasing in the region (T. Eastoe, pers. comm.). In the Cardamom Mountains hunting and illegal trade for food, pets and medicine also accelerated after the war (Momberg et al., Reference Momberg and Weiler1999) and are still serious threats (N. Thy, pers. comm.); we saw an infant T. germaini for sale as a pet in Pramoy village. Capture of entire groups of primates such as macaques have been reported in Cambodia and could lead to local extinctions in some areas (Nijman, Reference Nijman2005; BUAV, 2008). Starr et al. (2010a) note the importance of N. bengalensis in Khmer traditional medicines. Previous visits to Samkos Wildlife Sanctuary encountered numerous hunted specimens in villages destined for medicinal use (Starr et al., Reference Starr, Nekaris, Streicher and Leung2010a).
Our study indicates the importance of Samkos Wildlife Sanctuary for primate conservation in Cambodia. We hope our findings will encourage further primate studies in the area and that the Sanctuary will become a priority for primate conservation in Cambodia.
Acknowledgements
We thank the staff of Fauna & Flora International (FFI) Cambodia Programme (T. Eastoe, O. Nelson, N. Thy, T. Wood and E. Woodfield), staff from the Ministry of Environment who authorized and facilitated the project, R. Bunthoeun, T. Clements, M. Handschuh, K.E. Hourt, A. Maxwell, B. Rawson, E. Samun, E. Pollard, H. Rainey, T. Gray, J. Eames and C. Starr for providing valuable information, anonymous reviewers for their useful comments, D. Stark for help preparing the map, and Primate Conservation Inc. and the MSc Primate Conservation at Oxford Brookes University for funding.
Biographical sketches
C.N.Z. Coudrat’s interests lie in wildlife conservation, environmental education and primatology, with a special focus on the ecology of colobines, especially douc monkeys. She is particularly interested in the Indochinese region. D. Rogers is currently involved in a biodiversity survey for the Heart of Borneo initiative. Her interests lie in the study of wildlife and its relation to habitat and conservation education both in habitat countries and worldwide. Her specific areas of interest are Hylobates, Nomascus and nocturnal mammals. K.A.I. Nekaris has studied Asian mammals in the wild and in captivity for more than 15 years. She has conducted field studies of all currently recognized taxa of slow and slender lorises and has initiated conservation awareness and capacity building projects in numerous loris range countries.






