Epidemiological studies that linked low birth weight with later diseaseReference Hales and Barker1 have led to the theory of developmental programmingReference Gluckman and Hanson2. The early environment, encountered during a sensitive period, is believed to influence the development and hence the function of the organism permanentlyReference Harding3. Humans that are born small for gestational age represent an example of developmental programming. These individuals are thought to be adapted to a poor environment, and when confronted with a rich, western, environment they have an increased risk of insulin resistance, hypertension, obesity and CVD (collectively called the metabolic syndrome) in adult lifeReference Hales and Barker1, Reference Gluckman and Hanson2. Other examples of developmental programming are various animal models that manipulate the perinatal nutritional environmentReference Bertram and Hanson4–Reference Ozanne6. Studies using these models have shown that, depending on the exact nature and timing of the manipulation, programming can act in different directions. For instance, different effects on energy balance have been reported after perinatal malnutritionReference McMillen, Adam and Muhlhausler7.
In different rat models, adult food intakeReference Bassett and Craig8–Reference Zambrano, Bautista, Deas, Martinez-Samayoa, Gonzalez-Zamorano, Ledesma, Morales, Larrea and Nathanielsz11 and body fatReference Desai, Gayle, Babu and Ross9, Reference Zambrano, Bautista, Deas, Martinez-Samayoa, Gonzalez-Zamorano, Ledesma, Morales, Larrea and Nathanielsz11–Reference Vickers, Breier, Cutfield, Hofman and Gluckman14 were either increased, decreased or unchanged. In man, programming of energy balance has also been reported. Although obesity rates have been reported to be lower after perinatal malnutritionReference Ravelli, Stein and Susser15, there are now several studies that associate low birth weight with a more central distribution of fatReference Fall, Osmond, Barker, Clark, Hales, Stirling and Meade16, Reference Valdez, Athens, Thompson, Bradshaw and Stern17 and a lower lean body massReference Gale, Martyn, Kellingray, Eastell and Cooper18.
An important part of the regulation of energy balance takes place in the hypothalamus. Whereas in man, a substantial part of the development of the hypothalamus and the brain is completed in utero, in rats much of this development occurs postnatallyReference Dobbing and Sands19–Reference Koutcherov, Mai, Ashwell and Paxinos22. Therefore, we have used early postnatal food restriction (FR) in rats to study developmental programming of energy balance. We have previously shown that raising rats in large litters reduced body weight into adulthoodReference Engelbregt, Van Weissenbruch, Lips, Van Lingen, Roos and Delemarre-van de Waal12, Reference Huizinga, Engelbregt, Rekers-Mombarg, Vaessen, Delemarre-van de Waal and Fodor23 and decreased the fat percentage in adult malesReference Engelbregt, Van Weissenbruch, Lips, Van Lingen, Roos and Delemarre-van de Waal12. These animals also showed disruptions in several processes that are regulated by the hypothalamus; a delayed onset of pubertyReference Engelbregt, Houdijk, Popp-Snijders and Delemarre-van de Waal24, impaired testicular functionReference Van Weissenbruch, Engelbregt, Veening and Delemarre-van de Waal25 and changes in the growth hormone axisReference Houdijk, Engelbregt, Popp-Snijders and Delemarre-van de Waal26.
If the hypothalamus is affected in these animals, then its regulation of energy homeostasis may also be affected, which could ultimately lead to permanently altered energy balance. Changes in energy balance might contribute to the phenotype of these animals. Therefore, the aim of the present study was to elucidate whether early postnatal FR alters energy intake and resting energy expenditure (REE) in adult and middle-aged male rats.
Methods
Experimental animals
Primiparous timed-pregnant Wistar rats (Harlan, Horst, The Netherlands) arrived on day 14 or 15 of gestation and were housed individually under controlled lighting (12 h light, 12 h dark) and temperature (21·5 (sd 0·5) °C). Animals had unlimited access to tap water and standard rat chow (Ssniff R/M-H; Bio Services, Uden, The Netherlands; 12·8 kJ/g metabolisable energy, 19·0 % protein, 3·3 % fat, 36·5 % starch, 4·7 % sugar and 4·9 % crude fibre), unless mentioned otherwise. Pups were born spontaneously on day 22 or 23 of gestation. From day 20 of gestation, the presence of pups was checked daily in the morning and the first day of life was designated postnatal day 1. On day 2, male and female pups were allocated to either a control litter of ten pups or a FR litter of twenty pups using computer-generated random numbers. Male-to-female ratio was 1:1 in all fostered litters. In large FR litters, less milk has been shown to be available per pup than in control litters, resulting in undernutritionReference Fiorotto, Burrin, Perez and Reeds27. On day 25, the pups were weaned and males were housed two per cage, paired with another animal of the same experimental group. Subsets of animals were killed at different ages for another study. A subset of thirty-nine of the male animals in the experiment survived until the age of 1 year and were used in the present study (sixteen controls and twenty-three FR animals). All procedures were approved by the Animal Experimentation Ethics Committee of the Vrije Universiteit and the VU University Medical Center in Amsterdam, The Netherlands.
Body dimensions
Body weight was measured regularly throughout life. At the age of 12 months (day 380), body length was measured from the tip of the nose to the anus under pentobarbital or O2/CO2 anaesthesia before the animals were killed for further study. BMI was calculated as the ratio of body weight (g) to body length (cm) squared. In a previous study, we showed that at the age of 6 months control and FR males had a fat-free mass (FFM) of, respectively, 76 and 81 % of their body weightReference Engelbregt, Van Weissenbruch, Lips, Van Lingen, Roos and Delemarre-van de Waal12. Therefore, an estimate of FFM at 6 months was calculated as 0·76 × body weight in controls and 0·81 × body weight in FR males.
Food intake
Individual food intake was determined in sixteen adult and middle-aged control animals and twenty-three FR animals at the ages of 6 and 12 months. The animals were housed individually at least 1 d before the measurement to become accustomed to the testing cage. The food provided was weighed at the beginning and the end of a 24 h period. Individual energy intake was calculated from 24 h food intake and the energy density of the diet (12·8 kJ/g).
Indirect calorimetry
REE was determined by means of indirect calorimetry in the same sixteen control and twenty-three FR animals at the ages of 6 and 12 months. The animals were housed individually at least 1 d before the measurement to become accustomed to the testing cage. All measurements were carried out during the light, inactive, phase of the day. During the measurements, no food and water were available. A metabolic monitor (Deltatrac II MBM-200; Datex-Ohmeda, Helsinki, Finland), adapted to fit the animal cages, was used to measure resting VO2 and carbon dioxide production rate (VCO2) every minute. The lower limit for reliable measurements was 5 ml/min for both VO2 and VCO2, restricting us to the measurement of adult males; neither females nor younger animals reached this limit of reliability. Before each measurement, the metabolic monitor was calibrated with a gas mixture of 95 % O2 and 5 % CO2. Mean VO2 and VCO2 values from stable measurements with a duration of at least 20 min and a CV ≤ 5 % were used for calculations. REE was calculated using the modified Weir formulaReference Weir28: REE (kJ/24 h) = 4·184 × (5·50 × VO2 (ml/min)+1·76 × VCO2 (ml/min)), without adjustment for urinary nitrogen excretionReference Even, Mokhtarian and Pele29. To avoid possible effects of circadian rhythm on energy expenditure interfering with the group effects, control and FR animals were measured in an alternating manner. After the two energy balance measurements were completed, the animals were socially housed with the same individual as before.
Data analysis
The results were analysed using Statistical Product and Service Solutions software for Windows, version 12 (SPSS Inc., Chicago, IL, USA). All data were checked for normality and are expressed as means with their standard errors (except in Figs. 1 and 2, where standard deviations are shown for better visibility). After exclusion of animals with missing values or a CV >5 % in the indirect calorimetry, data were analysed for fourteen control and twenty-two adult FR males at 6 months and for sixteen control and twenty-one middle-aged FR males at 12 months. All outcome measures were initially analysed by means of one-way ANOVA. To confirm that the FR in the FR litters was distributed evenly over the pups within a litter, differences in variance of preweaning body weight between the groups were tested using Levene's test for homogeneity of variances. Potential confounding effects of biological and foster dams were tested in univariate ANOVA. Foster dam nested within group and the interaction between biological dam and group had no long-lasting significant effect and were omitted in further analyses. Energy utilisation is known to correlate with body size, and more specifically with FFM, and it has been recommended to adjust for FFM in an ANOVA when comparing energy utilisation between subjects with different body compositionsReference Arch, Hislop, Wang and Speakman30, Reference Toth31. In a previous study we have shown that at the age of 6 months FR males indeed have a different body composition than controlsReference Engelbregt, Van Weissenbruch, Lips, Van Lingen, Roos and Delemarre-van de Waal12, confirming the need for adjustment. At 6 months, we estimated FFM by means of the values found in this previous study. At 12 months, BMI was available as another estimate of body composition. Therefore, energy balance data were tested in a univariate ANOVA with estimated FFM (eFFM) as a covariate at 6 months and BMI at 12 months, as recommendedReference Arch, Hislop, Wang and Speakman30, Reference Toth31. If these covariates did not have a significant effect, they were omitted from the analysis.

Fig. 1 Body weight (BW) throughout the experiment and during the first month of life (inset) of male food restriction (FR) rats (■, n 23), which were food restricted during lactation, and male control rats (□, n 16). Values are means with their standard deviations depicted by vertical bars (where the error bars are not visible, they are within the symbol). Mean values of the FR group were significantly different from those of the control group: ***P < 0·001 from day 4 until 380.
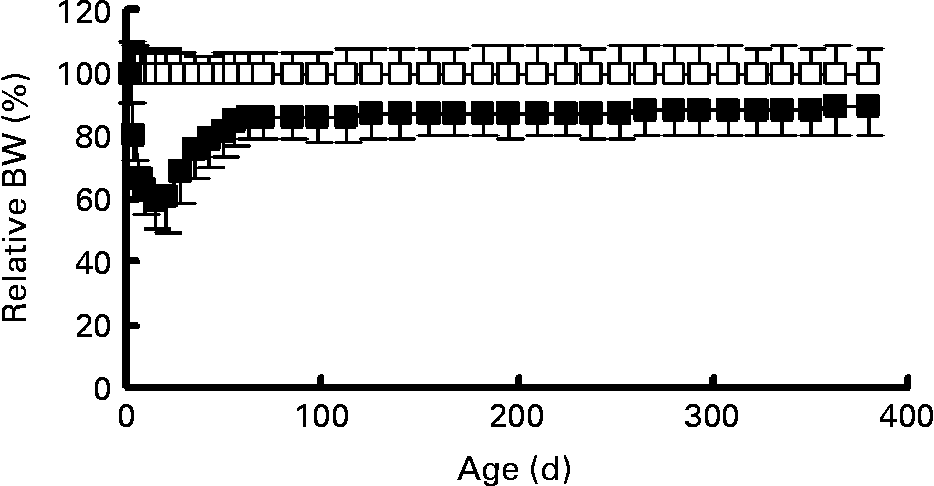
Fig. 2 Body weight (BW) expressed as a percentage of control body weight for food restriction animals (■, n 23) and control animals (□, n 16). Values are means with their standard deviations depicted by vertical bars.
Results
The thirty-nine animals used in the present study were born from twenty-one of the thirty-three dams in the complete experiment and on day 2 were fostered to six different dams for each group. The original litter size of the foster dams nurturing FR pups (12·3 (sem 1·0) pups) was not different from that of the foster dams that nurtured control pups (11·7 (sem 0·8) pups, P>0·600). Nor did the original litter size of FR pups (12·0 (sem 0·5) pups) differ from that of control pups (12·2 (sem 0·5) pups, P>0·800). Of the thirty-nine pups in this study, 85 % were cross-fostered, whereas 15 % (three control and three FR animals) remained with the same dam after the random redistribution on day 2.
Early postnatal FR resulted in a persistent reduction in body weight, body length and BMI. Mean body weights of control and FR rats are shown in Fig. 1. Body weight on day 2 (before the redistribution into control and FR litters) was 7·7 (sem 0·13) g. Body weight was lower in FR rats from day 4 until day 380 (P < 0·001). Relative to control values, body weight of FR animals decreased during lactation to 60 % at weaning. After weaning, relative body weight of FR rats increased to 86 % on day 70 and then stabilised so that on day 380 FR animals weighed 89 % of control weight (Fig. 2). During the lactation period, the variance in body weight did not differ significantly between the groups (P>0·200), although on day 21 there was a trend towards larger variance in the FR group (P = 0·093). Body dimensions of FR and control rats at 6 and 12 months are shown in Table 1. At both 6 and 12 months, body weight was lower in FR males (P < 0·001). At 6 months, eFFM, which was computed as 76 % of body weight in controls and 81 % of body weight in FR rats, was lower in FR animals (P = 0·029). At 12 months, body length (P < 0·001) and BMI (P = 0·024) were lower in FR animals than in controls.
Table 1 Body dimensions of control and food restriction (FR) males at the ages of 6 and 12 months
(Mean values with their standard errors)
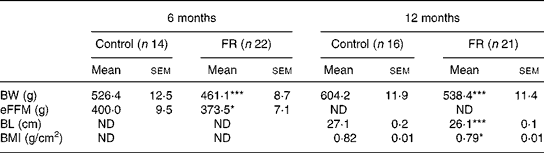
BL, body length; BW, body weight; eFFM, estimated fat-free mass; ND, no data.
Mean values were significantly different from those of the control group at the same age: *P < 0·05, ***P < 0·001.
At 6 months, we could not obtain measurements with a CV below 5 % for three animals (two controls and one FR rat), despite repeated attempts. These animals were excluded from all analyses at this time-point. At 12 months, two FR animals had to be excluded; one had to be killed prematurely, one had missing body length data at the time of killing.
Energy intake
At both 6 and 12 months, FR animals consumed a significantly smaller absolute amount of food than control animals (Table 2). Energy intake correlated with estimated body composition at both 6 months (eFFM, R 0·699, P < 0·001) and 12 months (BMI, R 0·540, P = 0·001). Energy intake was adjusted for eFFM at 6 months and for BMI at 12 months to account for differences in body composition between the groups. Adjusted energy intake at 6 months (Fig. 3 (A)) was lower in FR males (285·0 (sem 4·2) kJ/24 h) than in control males (303·4 (sem 5·3) kJ/24 h, P = 0·012). At 12 months, adjusted energy intake (Fig. 3(B)) was also lower in FR rats (284·4 (sem 5·1) kJ/24 h) than in controls (304·9 (sem 5·9) kJ/24 h, P = 0·016).
Table 2 Energy intake (EI), resting energy expenditure (REE) and EI minus REE of control and food restriction (FR) males at the ages of 6 and 12 months
(Mean values with their standard errors)

Mean values were significantly different from those of the control group at the same age: *P < 0·05, **P < 0·01.

Fig. 3 Relationship between estimated body composition and energy intake at 6 months (A) and 12 months (B) in control (□, - - -, n 14 in (A), n 16 in (B)) and food restriction rats (■, ——, n 22 in (A), n 21 in (B)). The given P values indicate a significant effect of experimental group on energy intake when the estimated fat-free mass was included as a covariate.
Resting energy expenditure
Mean values for VO2 and VCO2 were 6·5 (sem 0·1) and 6·0 (sem 0·1) ml/min at 6 months and 6·9 (sem 0·1) and 6·5 (sem 0·1) ml/min at 12 months, respectively.
At both 6 and 12 months, FR animals had a significantly lower absolute REE than control animals (Table 2). REE correlated with estimated body composition at both 6 months (eFFM, R 0·870, P < 0·001) and 12 months (BMI, R 0·680, P < 0·001). At 6 months, energy expenditure adjusted for estimated body composition (Fig. 4(A)) was not significantly different between FR males (192·0 (sem 2·2) kJ/24 h) and controls (198·1 (sem 2·8) kJ/24 h, P = 0·099), nor did adjusted energy expenditure at 12 months (Fig. 4(B)) differ significantly between FR rats (204·1 (sem 3·2) kJ/24 h) and controls (211·3 (sem 3·6) kJ/24 h, P = 0·156).

Fig. 4 Relationship between estimated body composition and resting energy expenditure (REE) at 6 months (A) and 12 months (B) in control (□, - - -, n 14 in (A), n 16 in (B)) and food restriction rats (■, ——, n 22 in (A), n 21 in (B)).
Energy intake minus resting energy expenditure
When energy balance is neutral, energy intake equals total energy expenditure, so the difference between energy intake and REE represents the amount of energy available for other functions such as locomotor activity. This parameter did not correlate with BMI or eFFM (P>0·480). Energy intake minus REE was lower in FR rats than in control rats at both 6 (P = 0·038) and 12 months (P = 0·044; Table 2).
Discussion
In the present study, early postnatal FR of male rats resulted in an acute reduction in growth, followed by incomplete catch-up growth, and permanently altered energy balance. At both 6 and 12 months, FR rats consumed and expended less energy than controls. After subtraction of REE from energy intake, FR animals had less energy available for other functions. When estimated adult body composition was taken into account, energy intake was lower after FR, whereas energy expended in rest was similar to that of controls.
Programming of energy balance
In the present study, male FR rats remained lighter than control males until the end of the experiment at the age of 12 months. This suggests that early postnatal FR can programme later size. This is in contrast to FR later in life, which has been shown to induce reversible growth restriction with complete catch-upReference Hughes32, Reference Widdowson and McCance33. BMI was also reduced in FR animals. Although BMI is not a direct measure for fat mass, it is strongly correlated with the percentage of body fat in both manReference Kopelman34 and ratsReference Novelli, Diniz, Galhardi, Ebaid, Rodrigues, Mani, Fernandes, Cicogna and Novelli Filho35. Therefore, the present results suggest that at least in rats early postnatal FR can programme a low level of adult adiposity. This may be through a reduced energy intake, but from the present data it is not possible to discern cause and effect in the relationship between BMI and food intake. Although REE was reduced in the FR animals, it seemed appropriate for the altered body composition. Therefore, programming of REE does not seem to have taken place. In the case of neutral energy balance, energy intake equals total energy expenditure. Since REE includes BMR, the thermic effect of food and energy expended for growth, the difference between total energy expenditure and REE represents activity-related energy expenditureReference Wenk, Colombani, Van Milgen, Lemme, Chwalibog and Jakobsen36. Energy intake minus REE, or activity-related energy expenditure, was reduced in FR males. Therefore, these animals may either be less active or expend less energy during their activity. In adult rats, the energy expended for growth is negligible. If during the development of these animals, energy intake was also reduced without a change in BMR, there may have been less energy available for growth. This may explain, at least in part, the permanent reduction in body weight, body length and BMI in the animals in the present study.
Early and late effects
When analysing data on late effects of early insults, it is important to separate the effects of the early insult from those of events later in lifeReference Lucas, Fewtrell and Cole37. Therefore, in the present study both unadjusted data and data adjusted for estimated adult body composition were presented. Energy intake and REE were both reduced in FR animals when early size (i.e. control or FR) was the sole independent variable. Adding estimated adult body composition as a covariate removed the effect on REE, but not that on energy intake. This suggests that later events may have been more important in determining REE than early postnatal FR, but that the FR was the most important determinant of energy intake in these animals. Here it should be noted that the adjustment for FFM as advisedReference Arch, Hislop, Wang and Speakman30, Reference Toth31 is essential for this result. When adjusted for the less recommended crude body weight instead of the metabolically active FFM, energy intake was not significantly different between the groups (data not shown). This emphasises the importance of choosing the appropriate parameter for adjustment of energy balance data. The difference between energy intake and REE, or activity-related energy expenditure, was independent of adult size and therefore the differences between the groups were most probably due to the early postnatal undernutrition in the FR group.
If the differences between the groups are to be attributed to true programming, the effects must be permanent. Therefore, the animals were tested in adulthood. Animals were retested when middle-aged at the age of 1 year to verify whether the effects were truly permanent. Since similar results were obtained at both ages studied, we are rather confident that permanent programming really occurred.
Energy balance in other models
Postnatal manipulations of litter size appear to yield consistent results. Other studies using large litters have also found a permanently reduced body weightReference Bassett and Craig8, Reference Oscai and McGarr10, Reference Engelbregt, Van Weissenbruch, Lips, Van Lingen, Roos and Delemarre-van de Waal12, Reference Faust, Johnson and Hirsch13, Reference Widdowson and McCance33 and fat massReference Engelbregt, Van Weissenbruch, Lips, Van Lingen, Roos and Delemarre-van de Waal12, Reference Faust, Johnson and Hirsch13, a lower food intake in young adulthoodReference Bassett and Craig8 and a far lower cumulative absolute food intake from weaning until over a year of ageReference Oscai and McGarr10. Studies using overfeeding in small litters have found opposite results: animals were permanently heavier than control animalsReference Bassett and Craig8, Reference Boullu-Ciocca, Dutour, Guillaume, Achard, Oliver and Grino38, Reference Plagemann, Harder, Rake, Voits, Fink, Rohde and Dorner39, had an increased fat mass or BMIReference Boullu-Ciocca, Dutour, Guillaume, Achard, Oliver and Grino38, Reference Plagemann, Harder, Rake, Voits, Fink, Rohde and Dorner39 and had a larger absolute food intake in young adulthoodReference Bassett and Craig8, Reference Boullu-Ciocca, Dutour, Guillaume, Achard, Oliver and Grino38, Reference Plagemann, Harder, Rake, Voits, Fink, Rohde and Dorner39. New in the present study is that food intake of male FR rats was not only significantly lower in absolute terms, but it was even reduced when their altered body composition was taken into account.
In comparison with the present observation of an appropriately reduced REE, a previous study using early postnatally overfed small litter male rats showed increased total energy expenditure at the age of 5 weeks, but not in older animalsReference Wiedmer, Klaus and Ortmann40.
Comparing the results of the present study with those of others that used different models of perinatal undernutrition is more complicated, however, as the direction of the changes observed appears to be highly dependent on the exact timing, type and severity of malnutritionReference Bertram and Hanson4–Reference Ozanne6. Maternal ‘caloric’ and protein restriction during gestation or lactation have produced an increased, reduced or normal body weight and fat mass in adulthoodReference Holemans, Aerts and Van Assche5, Reference McMillen, Adam and Muhlhausler7, Reference Desai, Gayle, Babu and Ross9, Reference Zambrano, Bautista, Deas, Martinez-Samayoa, Gonzalez-Zamorano, Ledesma, Morales, Larrea and Nathanielsz11, Reference Vickers, Breier, Cutfield, Hofman and Gluckman14, depending on the timing and severity of malnutrition. Moreover, 50 % FR of the dam during gestation increased adult food intake, but the same insult during lactation did notReference Desai, Gayle, Babu and Ross9, whereas a low-protein diet during lactation reduced adult food intakeReference Zambrano, Bautista, Deas, Martinez-Samayoa, Gonzalez-Zamorano, Ledesma, Morales, Larrea and Nathanielsz11. In general, a lower food intake has been found in models with incomplete catch-up growth, whereas a higher food intake was found after postnatal overnutrition or prenatal undernutrition followed by overcomplete catch-up. Studies using prenatal maternal malnutrition have found reductions in total energy expenditure with unaltered REE (suggesting reduced activity-related energy expenditure)Reference Daenzer, Ortmann, Klaus and Metges41 and an actual reduction in activity levelsReference Bellinger, Sculley and Langley-Evans42, Reference Vickers, Breier, McCarthy and Gluckman43 in adult males, a consequence also suggested by the results of the present study. In contrast, activity was not reduced in our previous study in young adult males that were prenatally growth restricted by bilateral uterine artery ligationReference Schreuder, Fodor, Van Wijk and Delemarre-van de Waal44.
Unlike the early postnatally food-restricted rats, most of the humans that are born small for gestational age or after intra-uterine growth restriction catch up during infancyReference Karlberg and Albertsson-Wikland45. However, it was shown that prepubertal children born small for gestational age that did not catch up had a food intake below the recommended energy intake for their ageReference Boonstra, Arends, Stijnen, Blum, Akkerman and Hokken-Koelega46. These data seem to be in accord with the reduced food intake in the early postnatally food-restricted rats with incomplete catch-up growth in the present study. Studies in neonates have suggested that infants that are born small for gestational age have a higher energy expenditure per kg body weight or FFM than weight-matched controlsReference Cauderay, Schutz, Micheli, Calame and Jequier47, Reference Davies, Clough, Bishop, Lucas, Cole and Cole48. Although these data on REE relate to acute instead of long-term effects, they do indicate that perinatal malnutrition can also affect energy expenditure in man.
The differences outlined above warn us to exert extreme caution when attempting to extrapolate outcomes of perinatal malnutrition, not only between rats and man, but also between different animal models. Seemingly comparable manipulations of pre- or early postnatal nutrition can yield widely differing resultsReference Bertram and Hanson4–Reference Ozanne6.
Because of the different timing of birth relative to development, early postnatal FR in rats is probably somewhat similar to undernutrition in human fetuses during the third trimester, although the potential for catch-up growth is evidently different between the two. It could be speculated that the window of plasticity for body dimensions, adiposity and food intake may close before the end of the lactation period in rats, whereas in man it may extend into the postnatal period.
Technical considerations
A concern when using large litters to reduce early postnatal food intake is the lack of control over the distribution of the available milk within litters. There may be competition between the pups over the milk supply and as a consequence the pups in a litter may be food restricted to different degreesReference Galler and Turkewitz49. The fact that the variance in body weight during the lactation period was similar between control and FR males suggested that in the present study all FR pups were food restricted roughly to the same degree.
The energy balance measurements in the present study were restricted to adult male rats. Investigating the possible effects of FR on energy expenditure in females would be interesting. Unfortunately, we were unable to investigate this, because of the limitations of the metabolic monitor.
In the present study, actual measurements of FFM were not available. The variables eFFM and BMI were chosen as estimates for FFM. By extrapolating the percentage of FFM from one population to another, we introduced an uncertainty. Especially because at 6 months the population of the present study was heavier than that used in the other studyReference Engelbregt, Van Weissenbruch, Lips, Van Lingen, Roos and Delemarre-van de Waal12, most probably because of the different diets the animals received. Therefore, energy intake and REE were also determined at another age, when BMI was available as a parameter of body composition. Although it is usually employed for its correlation with fat mass, BMI describes body weight relative to length, and hence does not discriminate between fat mass and FFM. It therefore also increases with increasing FFMReference Kyle, Schutz, Dupertuis and Pichard50. The fact that the analyses using these different covariates produced comparable results at both ages suggests that the estimates BMI and eFFM were equally suitable approximations for FFM.
Implications
In the present study, we showed that male rats that were food restricted early postnatally remained lean with a reduced food intake in adult life. This fits in with the relatively recent idea that promoting catch-up growth in low birth weight infants may not be beneficial for their long-term outcome. Several studies in man as well as in animals have suggested that fast and early catch-up, sometimes through super-nutritious food, can be detrimentalReference Vickers, Breier, Cutfield, Hofman and Gluckman14, Reference Vickers, Breier, McCarthy and Gluckman43, Reference Ong, Ahmed, Emmett, Preece and Dunger51, Reference Stettler, Stallings, Troxel, Zhao, Schinnar, Nelson, Ziegler and Strom52. On the other hand, rats with this modest phenotype may not have sufficient supplies for normal growthReference Huizinga, Engelbregt, Rekers-Mombarg, Vaessen, Delemarre-van de Waal and Fodor23, Reference Houdijk, Engelbregt, Popp-Snijders and Delemarre-van de Waal26 and possibly other matters such as reproductionReference Engelbregt, Houdijk, Popp-Snijders and Delemarre-van de Waal24, Reference Van Weissenbruch, Engelbregt, Veening and Delemarre-van de Waal25 and locomotor activity. In summary, the present study demonstrates that in rats early postnatal FR can programme energy balance in later life. The present study provides additional support for the hypothesis that early nutritional insults may have long-term metabolic consequences.
Acknowledgements
This study was not supported by financial assistance from extramural sources. The authors had no conflicts of interest regarding this manuscript. We are grateful to the VU University Medical Center Laboratory for Experimental Animal Research for technical assistance and to Professor H. N. Lafeber for helpful suggestions.








