A general hypothesis regarding most theropod dinosaurs is that they were active predators or scavengers (Paul Reference Paul1988). Fossil evidence of this behavior is well known in the fossil record of Laurasia, but not in Gondwana (Fastovsky et al. Reference Fastovsky, Huang, Hsu, Martin-McNaughton, Sheehan and Weishampel2004). The scarce information on the diet of South American theropods is due to the lack of discoveries of worn teeth, or even of bones with tooth marks, only a few of which have yet been found. In Brazil, theropod teeth from the Marília Formation of the Sítio Paleontológico de Peirópolis comprise one of the largest and most diverse fossil tooth collections in South America.
Little is known about the feeding biomechanics of theropod dinosaurs, especially the role of teeth on bite force and the estimated pressure produced by them (bite force/tooth contact area). As observed in crocodiles by Erickson et al. (Reference Erickson, Gignac, Steppan, Lappin, Vliet, Brueggen, Inouye, Kledzik and Webb2012a), these biomechanical aspects might be related to variations in rostral and dental morphologies, body size, feeding ecology or other evolutionary variations. The current knowledge about tooth pressure (the forces inflicted in the act of biting the prey and on tooth-on-tooth contact) of dinosaurs is still scarce, and previous research on this subject has concentrated on ornithischian dinosaurs, mammals, sharks and other fish (e.g., Lee et al. Reference Lee, Constantino, Lucas and Lawn2011; Mallon & Anderson Reference Mallon and Anderson2014; Erickson et al. Reference Erickson, Gignac, Lappin, Vliet, Brueggen and Webb2014; Rowe et al. Reference Rowe, Erickson, Sawyer and Krick2014).
Some studies described striated surfaces on dinosaur teeth and suggested they are directly related to mastication (Weishampel Reference Weishampel1984; Fiorillo Reference Fiorillo, Kielan-Jaworowska, Heintz and Nakrem1991, Reference Fiorillo, Sun and Wang1995, Reference Fiorillo, Currie and Padian1997, Reference Fiorillo1998; Weishampel & Norman Reference Weishampel and Norman1989; Upchurch & Barrett Reference Upchurch, Barrett and Sues2000; Barrett Reference Barrett and Carpenter2001; Rybczynski & Vickaryous Reference Rybczynski, Vickaryous and Carpenter2001; Sankey et al. Reference Sankey, Brinkman, Guenther and Currie2002; D'Amore Reference D'Amore2009; Erickson et al. Reference Erickson, Krick, Hamilton, Bourne, Norell, Lilleodden and Sawyer2012b, Reference Erickson, Sidebottom, Kay, Turner, Ip, Norell, Sawyer and Krick2015; Sereno Reference Sereno2012; Mallon & Anderson Reference Mallon and Anderson2014). Most of these studies analysed herbivorous ornithischians and sauropods, by comparing and contrasting the feeding habits of theropods, varanids, crocodyliforms and mammals. By observing the feeding mechanism of mammals, Weishampel (Reference Weishampel1984) noticed that herbivorous hadrosaurid dinosaurs show similar parallel striations on the enamel of opposing tooth facets, which suggests that this was probably related to the direction of tooth occlusion during mastication.
Farlow & Brinkman (Reference Farlow, Brinkman, Rosenberg and Wolberg1994) and Schubert & Ungar (Reference Schubert and Ungar2005) pointed out that tyrannosaurids have almost the same wear patterns as found in other theropod teeth, which suggests occlusion of opposing teeth. This partially corroborates Williamson & Brusatte (Reference Williamson and Brusatte2014), although they also indicated that the parallel striations on the lateral teeth of tyrannosaurids were produced during predation by contact between the tyrannosaurid teeth and the bones of the prey.
Predation marks on bones, produced by theropods, are another source of information on their feeding behaviour (D'Amore Reference D'Amore2009). Carnivorous dinosaurs had a varied feeding behaviour, and their tooth marks have been reported on other dinosaur and pterosaur bones (Currie & Jacobsen Reference Currie and Jacobsen1995; Erickson et al. Reference Erickson, Van Kirk, Su, M. E. Caler and Carter1996; Erickson & Olson Reference Erickson and Olson1996; D'Amore Reference D'Amore2009). Schubert & Ungar (Reference Schubert and Ungar2005) observed that the wear surfaces of tyrannosaurid teeth have specific shapes and orientations that can be classified as spalled surfaces caused by antemortem enamel flaking, and attritional facets produced by tooth-to-tooth contact during feeding.
According to Jacobsen (Reference Jacobsen1998), patterns of bone marks produced by some crocodilian and mammalian carnivores are comparable to the ones left by theropod dinosaurs. Studies of tooth marks on dinosaur bones from North America have been used to identify the feeding behaviour of theropods at both a family and a generic level (Holz et al. Reference Holtz, Brinkman and Chandler1998; Jacobsen & Bromley Reference Jacobsen and Bromley2009).
The shape of the denticles, and the distance between them in the jaws, provides clues for the identification of tooth marks. In Laurasian theropods (dromaeosaurids and tyrannosaurids), parallel and serration marks have been identified (Chandler Reference Chandler1990; Abler Reference Abler1992; Currie & Jacobsen Reference Currie and Jacobsen1995; Brochu Reference Brochu2003; D'Amore Reference D'Amore2009).
Research on tooth wear surfaces is potentially important to a palaeoecological study of Brazilian Late Cretaceous dinosaurs. The present study aims to analyse the tooth macrowear and microwear of theropods from Sítio Paleontológico de Peirópolis and correlate it with feeding habits.
2. Material and methods
All the specimens analysed are from the Marília Formation (Maastrichtian) of Peirópolis, Uberaba City, Minas Gerais State, Brazil (Fig. 1), a fossil site where many theropod bones have been found. The known theropod taxa include abelisaurids, carcharodontosaurids and several taxa of indeterminate theropods (Candeiro et al. Reference Candeiro, Currie and Bergqvist2012).
Fig. 1 Location map of Peirópolis site, Minas Gerais State, Brazil.
The studied specimens include teeth of Abelisauridae (CPP 020, 136, 150, 205, 242, 243, 271, 452/1), Carcharodontosauridae (CPP 124, 129a, 152, 199, 376, 447, 449) and unidentified theropods (CPP 135, 154, 161/1, 198, 371, 377, 446, 451/1, 476, 478).
The macrowear surfaces were initially analysed using a hand lens, and light microscopy, to elucidate details such as the nature of the enamel–dentine transition or the presence of secondary enamel ridges. The microwear analysis was undertaken with the support of a scanning electron microscope.
The taxonomic classification of the analysed fossil assemblage follows the identifications proposed by Candeiro et al. (Reference Candeiro, Currie and Bergqvist2012). The classification of the pattern of tooth wear follows the proposals of Molnar (Reference Molnar1998), Schubert & Ungar (Reference Schubert and Ungar2005) and Hendrickx et al. (Reference Hendrickx, Mateus and Araújo2015).
Institutional abbreviations. CPP, Centro de Pesquisas Paleontológicas Llewellyn Ivor Price, Peirópolis, Uberaba, Minas Gerais State, Brazil.
3. Results
3.1. Microwear – attritional striations
Specimens: CPP 129a, 129b (Abelisauridae); CPP 199 (Carcharodontosauridae).
The enamel on the labial and lingual surfaces of CPP 129a, 129b and 199 is characterised by shallow depressions on the apices. The wear striations are vertically oriented, deeper along the central portion of each groove, and have irregular edges. (Fig. 2). The main microwear features present on the tooth surface are narrow striations, occasionally oriented perpendicular to the deeper striations (Fig. 2A), in a similar way as in Daspletosaurus torosus (see Schubert & Ungar Reference Schubert and Ungar2005). These features suggest two types of interactions caused by dental enamel friction (Fig. 2B, C): the deeper wear surfaces were made in an occlusal direction; whereas the perpendicular striations were possibly produced by contact with prey bone (see Schubert & Ungar Reference Schubert and Ungar2005).
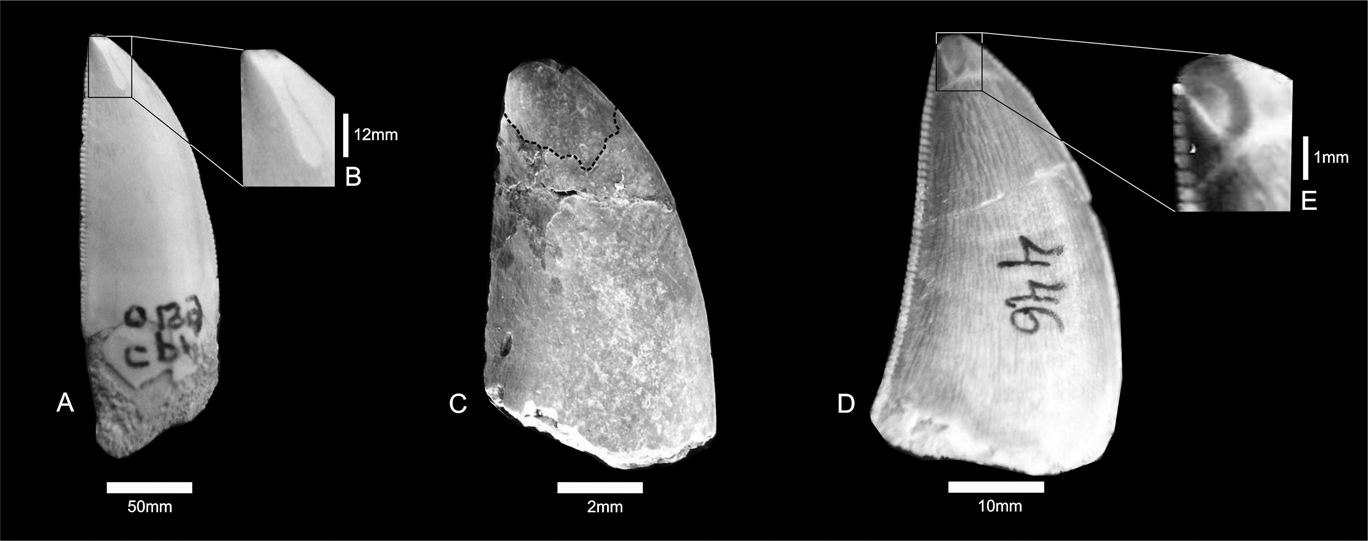
Fig. 2 (A–B) Carcharodontosauridae indet., CPP 199; close-up of tooth surface showing vertical striations. (C) Abelisauridae indet., CPP 129b. (D–E) Theropoda indet., CPP 446; close-up of apical surface showing broad wear.
3.2. Microwear – oval shape
Specimens: CPP 124, 131, 132, 135, 136, 152, 154, 161/1, 198, 199, 205, 242, 243, 271, 371, 377, 446, 447, 449, 451/1, 452/1, 463, 476, 478.
These teeth show oval apical wear, clearly exposing the enamel dentine joint (EDJ). These oval surfaces with shallow striations are present on both sides of the teeth, but they are smoother on concave surfaces (Fig. 3). The striations are predominantly oriented anteroposteriorly. The enamel surfaces are covered by numerous shallow scratches that are mostly oriented perpendicular to tooth borders. This is true for both sides of the teeth, but the scratches are more common on the labial side.
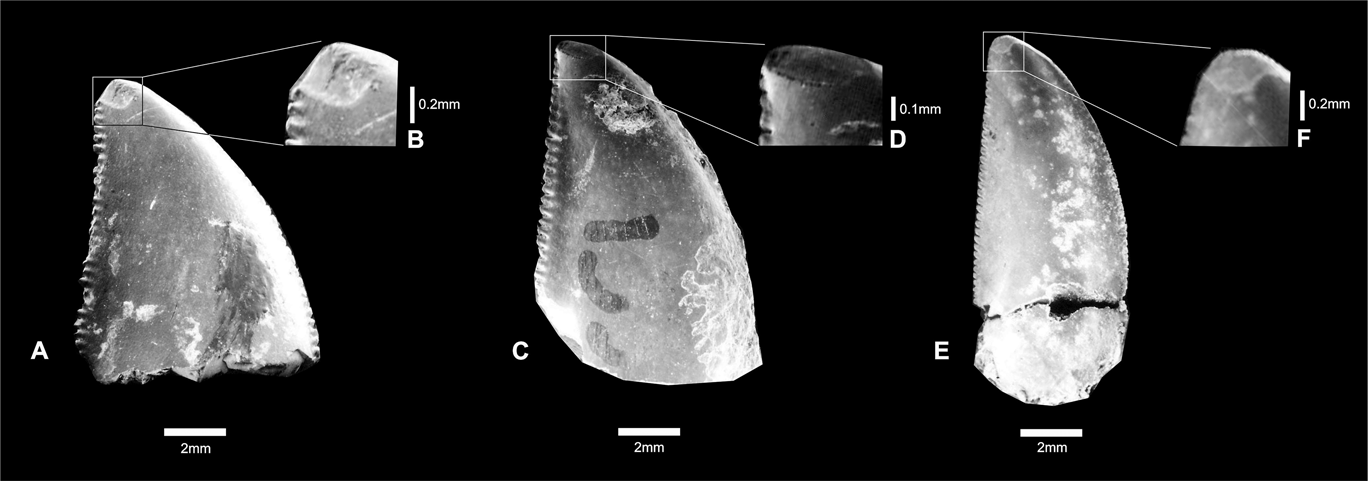
Fig. 3 (A–B) Theropoda indet., CPP 132; close-up showing oval wear surface. (C–D) Carcharodontosauridae indet., CPP 133; close-up showing oval wear surface. (E–F) Abelisauridae indet., CPP 154; close-up showing oval wear surface.
3.3. Macrowear – abrupt fracture
Specimens: CPP 020, 129a.
The macrowear patterns found in some theropod dinosaurs are very conspicuous in these specimens. The apical ends of them are abruptly fractured and it is especially evident on the labial surfaces (Fig. 4). The tooth borders are slightly abraded, but do not reach the EDJ. Attritional striations are horizontally oriented, and in some cases they extend to the posterior edge of the tip of the crown (Fig. 4B). The abrasion of the enamel of specimen CPP 129a (Fig. 4B) is very conspicuous, because both lingual and labial surfaces are thinner and irregular, exposing the dentine. It is possible that some of the wear on CPP 129a was caused by acidic liquids of the gastrointestinal tract.

Fig. 4 Macrowear abrupt fractures: (A) Abelisauridae indet., CPP 020; (B) Carcharodontosauridae indet., CPP 129a. Arrows indicated broken surface.
4. Discussion and conclusions
The tooth surface of theropods was continuously worn out throughout its use. Tooth wear is represented by vertical abrasion caused by friction, and some of these wear facets have perpendicular striations (Fig. 2A). These two types of striation were produced by different jaw movements.
On both lingual and labial faces, attritional striations are mainly observed on the apical region of the teeth. The striations get narrower towards the apex. Wear striations have been used to indicate the orientation of the force of dental occlusion, as one tooth slides over another (Abler Reference Abler1992; Schubert & Ungar Reference Schubert and Ungar2005; D'Amore Reference D'Amore2009). Attritional striations are more prominent at the tooth tips, and become more attenuated towards the roots. Candeiro (Reference Candeiro2002) asserted that the wear surfaces tend to be oval near the tips, but subsequently become elongated. If this is correct, the initial oval wear facets on theropod teeth must have been produced by tooth contact with bone surface during feeding, because attritional striations only occur during occlusion (sensu Abler Reference Abler1992; Schubert & Ungar Reference Schubert and Ungar2005; D'Amore Reference D'Amore2009).
The macrowear and microwear patterns on the theropod teeth from the Late Maastrichtian of the Marília Formation have different extents and shapes. First, the observed shapes (oval shape and abrupt fracture) could have been interpreted as a result of either tooth-to-tooth contact (Schubert & Ungar Reference Schubert and Ungar2005; D'Amore Reference D'Amore2009) or tooth-to-bone contact. In some cases, this type of occlusion may expose the dentine. Some teeth from the Marília Formation show this type of oval wear. These teeth are usually not affected on their anterior and posterior carinae, which still possess small denticles.
A conspicuous type of wear observed in specimens CPP 129a, 129b and 199 is a characteristic elongated groove that starts at the tooth apex. It suggests that penetration into hard surfaces spalled off enamel flakes from the tooth. The grooves are present on both labial and lingual faces of the enamel and expose the dentine. This evidence confirms the direction of the bite and shows greater force was used during the bone-penetrating bite.
The attritional striations, the oval wear facets and the apical grooves observed in the teeth analysed suggest that theropods from the Upper Maastrichtian Marília Formation had the same range of feeding habits as theropods from other parts of the world. Abelisaurids and carcharodontosaurids are medium-to-large sized, top predators and/or scavengers that fed especially on large prey. This behaviour probably produced the range of tooth wear observed in these dinosaurs. No published studies have yet analysed the hardness and resistance of the bones of herbivorous dinosaurs from the Marília Formation. A study of this nature would contribute to the understanding of how these abrasions are formed.
The oval shape, attritional striations and abrupt tooth fractures are tooth wear patterns comparable to those seen on the occlusal facets of other reptiles. It shows a tooth-on-tooth contact between opposing teeth that occurs repeatedly in one direction, in a scissor-like occlusion pattern (sensu Schubert & Ungar Reference Schubert and Ungar2005). The different patterns of enamel wear surfaces indicate different feeding mechanisms amongst the theropod dinosaurs of the Sítio Paleontológico de Peirópolis. The patterns of wear surfaces, such as their attritional striations and oval shapes, were probably formed by their effective cutting teeth (like a scissor with serrated blades), which was similarly observed by Schubert & Ungar (Reference Schubert and Ungar2005) on tyrannosaurid teeth. This type of occlusion is probably related to higher bite forces that produce striations on the lingual and labial surfaces, extending to the crown surfaces of the teeth – a feature similar to those observed by Molnar (Reference Molnar1998). The seemingly powerful bite force of these theropods could have produced the abrupt fractures present on the specimens described here. Thus, the apexes of some teeth were probably susceptible to breaking during the powerful friction caused by tooth occlusion. Nevertheless, such inferences regarding their diet are somewhat speculative.
Future studies regarding theropod teeth should include biomechanical tests to analyse the hardness of enamel and dentine, as well as the degree of resistance present in the bones of potential prey species from the Marília Formation of the Sítio Paleontológico de Peirópolis.
5. Acknowledgments
We are much indebted to Elsa Panciroli (University of Edinburgh) and Paulo Victor Oliveira (Universidade Federal do Rio de Janeiro), for valuable comments on the manuscript. This contribution was partially supported by the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq). RC and LB are also grateful to CNPq for the Produtividade em Pesquisa fellowship.






