Introduction
The behavioural and psychiatric symptoms (BPSD) in behavioural variant frontotemporal dementia (bvFTD) consist of apathy, loss of empathy, inappropriate responses, and hyperactive behaviours.Reference Mariani, Defendi, Mailland and Pomati 1 Primary progressive aphasia (PPA) patients can also manifest these behaviours over time.Reference Grossman 2 , Reference Kertesz, McMonagle, Blair, Davidson and Munoz 3 We have previously hypothesized that the pattern of evolution in BPSD for bvFTD shows initially significant disinhibition, which ultimately gives way to apathy and lack of engagement.Reference Chow, Fridhandler and Binns 4
The BPSD that frequently appear in Alzheimer’s disease (AD) are dysphoria, agitation, and anxiety;Reference Benoit, Dygai and Migneco 5 apathy is also seen in AD, at a lower frequency than in bvFTD.Reference Chow, Binns and Cummings 6
The Frontal Behavioural Inventory (FBI)Reference Kertesz, Nadkarni, Davidson and Thomas 7 assesses disinhibited behaviours and deficit behaviours (apathy). While FBI scores for the bvFTD group were not available for our previous work based on data from the University of California at San Francisco, the bvFTD group did demonstrate an initial increase in total Neuropsychiatric Inventory (NPI) scores and subsequent decline at the sixth year of illness.Reference Chow, Fridhandler and Binns 4
In the present study, we examined a new longitudinal dataset collected at a Canadian tertiary care clinic to replicate the change in BPSD trajectory with both the NPI and FBI in bvFTD, PPA and AD.
Materials and Methods
Standard protocol approvals, registrations, and patient consents
Formal and informal caregivers participated in an annual clinical telephone follow-up by summer research students at the Sam and Ida Ross Memory Clinic at Baycrest Health Sciences. Procedures for collecting the dataset were approved by the Research Ethics Board at Baycrest. Verbal informed consent to publish the anonymized data was obtained from informants during the telephone interviews.
Participant Series
Calls were made to informants for patients identified by billing or appointment records as having been evaluated, diagnosed, and treated at the Clinic in each prior year (April 1 – March 31). Of the 412 patients meeting clinical diagnostic criteria for bvFTD, PPA, or AD, 172 had informants available and willing to participate at least twice in the annual telephone follow-up. Thirty of these participants had bvFTD, 13 had PPA (including progressive non-fluent aphasia and semantic dementia) by Neary criteria,Reference Neary, Snowden and Gustafson 8 and 118 had AD (including possible AD, probable AD, and AD with cerebral vascular disease) by McKhann Criteria.Reference McKhann, Drachman, Folstein, Katzman, Price and Stadlan 9 Fifteen patients with corticobasal syndrome (CBS) and five patients with progressive supranuclear palsy were included as bvFTD or PPA, if they met the diagnostic criteria for those latter diagnoses. Corticobasal degeneration and PSP are often the underlying pathology for PPA or bvFTD, respectively.Reference Crockett DT, Koch and Parks 10 , Reference Morris 11 One of these CBS patients was autopsy-confirmed as corticobasal ganglionic degeneration. Patients with mixed dementia (AD and vascular dementia) were excluded.
Demographic information for the sample is summarized in Table 1. A majority (64%) of the patients had two sets of scores available, while the remainder had three (29%), four (6%) or five sets (1%).
Table 1 Sample Demographics
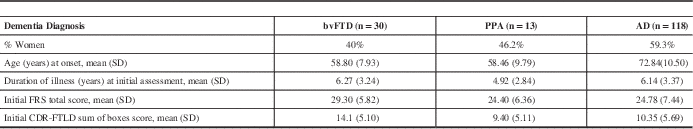
AD: Alzheimer’s disease; bvFTD: behavioural variant frontotemporal dementia; CDR-FTLD: Clinical Dementia Rating Scale modified for FTD; FRS: Functional Rating Scale; PPA: primary progressive aphasia; SD: Standard deviation.
Behavioural Assessments
Four clinical inventory assessments were administered to caregivers serially. These included: the Functional Rating Scale (FRS),Reference Crockett DT, Koch and Parks 10 the Clinical Dementia Rating Scale modified for FTD (CDR-FTLD),Reference Morris 11 , Reference Knopman, Kramer and Boeve 12 the FBI and the NPI.Reference Cummings, Mega, Gray, Rosenberg-Thompson, Carusi and Gornbein 13 We used the baseline FRS and CDR-FTLD scores to characterize the sample (see Table 1); the two latter inventories were the determinants of the rates of annual change. Specifically, the FBI assesses deficit behaviours such as apathy, aspontaneity, indifference, inflexibility, personal neglect, disorganization, inattention, loss of insight, logopenia, verbal apraxia, comprehension deficit and alien hand/apraxia; and disinhibited behaviours such as perseverations/obsessions, irritability, excessive jocularity, impulsivity, restlessness, social inappropriateness, aggression, hyperorality, hypersexuality, utilization behaviour and incontinence.Reference Kertesz, Nadkarni, Davidson and Thomas 7
The NPI assesses the frequency and severity of delusions, hallucinations, agitation/aggression, depression, anxiety, euphoria, apathy, disinhibition, irritability, aberrant motor behaviours and sleep and eating disorders.Reference Cummings, Mega, Gray, Rosenberg-Thompson, Carusi and Gornbein 13
Information on disease onset was determined by using the informant-reported year of earliest persistently abnormal clinical feature regarding language, behavioural comportment and personality, memory or executive functioning, as elicited by a physician or nurse during any clinical visit.
Some informants were not available to participate at annual intervals but were included with serial scores spanning longer than two years (27%).
Statistical analysis
We conducted statistical analyses on SPSS version 20.0 (IBM Corporation, N.Y). Linear regression analyses were used to determine the annualized rates of change (ROC) for the FBI and NPI total and sub-scores for each patient over three to five year epochs. For those with two instances, an average duration was used as the annual rate of change. In order to identify an inflection point, we re-created four binary variables from the prior studyReference Chow, Fridhandler and Binns 4 to indicate whether the first assessment took place before or after four, five, six, or seven years into illness. We used linear regression with forward entry of these four duration-related variables to ascertain the most likely predictor for annualized ROC for the behavioural inventories, with post-hoc exploration of whether the ROC before or after a cutoff duration emulated an inflection point. Post-hoc analyses included visualization on scatter plots with examinations of R2 values and independent samples t-tests.
A multivariate regression sought other predictors of the annualized ROC in the NPI and FBI total scores. Potential predictor variables in the analysis included the duration of illness at first case report form, education and the gender and relationship of informant to participant; age at onset and initial behavioural inventory scores, which had determined NPI ROC in bvFTD and AD in the previous study,Reference Chow, Fridhandler and Binns 4 were also included.
Furthermore, we used the opportunity to compare the NPI and the FBI for correlations to duration of illness for all three types of dementia. Because no inflection point was found, this aspect of post-hoc analysis entailed linear regression using the duration of illness as the dependent variable, raw total and sub-scale scores of the NPI and FBI as the independent variables (i.e., at least two data points per participant per inventory score).
Results
As expected for early-onset dementias,Reference Ratnavalli, Brayne, Dawson and Hodges 14 , Reference Rogalski EM 15 participants with bvFTD and PPA were younger than AD patients (p<0.001) (see Table 1). Although the median most recent durations of illness were seven to eight years for the three groups, the modes were much shorter for bvFTD (six years), PPA (four years), and AD (five years).
Scatter plots of apathy subscale scores against disinhibition subscale scores revealed the simultaneous occurrence of both types of behavioural disturbance (see Figure 1). Only the AD group had statistically significant bivariate correlations and these were neither inverse nor strongly positive: FBI-apathy x FBI-disinhibition r=0.39 (p<0.001) and NPI-apathy x NPI-disinhibition r=0.22 (p=0.02).

Figure 1 Apathy and disinhibition occur simultaneously across all three dementia groups. Initial apathy and disinhibition scores on the FBI are plotted. Participants with AD had r=0.39 (p<0.001) for these scores; bvFTD and PPA r values had p values >0.05. AD: Alzheimer’s disease; bvFTD: behavioural variant frontotemporal dementia; FBI: Frontal Behavioural Inventory; PPA: primary progressive aphasia.
The first level of regression analysis revealed no significant epochs for bvFTD ROC on the NPI. Annualized ROC for the FBI and FBI-apathy scores were related to observations beginning before versus after six and seven years duration of bvFTD (see Table 2), yet the respective ROC for the duration subgroups of the sample above did not show crescendo-decrescendo trajectories. Post-hoc independent samples t-tests only supported a significant difference in FBI-apathy annualized ROC observed in the first six years of illness (increasing at 6.23 points per year ± SD 5.04), as opposed to other participants observed later in their courses of illness still to increase at a much lower ROC (0.02 points per year ± 6.76, t=2.52, p=0.02).
Table 2 Linear regression for before and after various durations of bvFTD
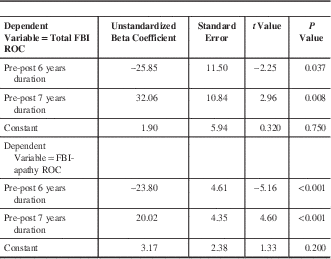
bvFTD: behavioural variant frontotemporal dementia; FBI: Frontal Behavioural Inventory; ROC: rate of change.
Other predictors revealed in the multivariate regression analyses included some initial inventory scores and education. Only the initial NPI-apathy score predicted the annualized ROC on this item (unstandardized β coefficient=−0.44, SE 0.14, p=0.005); lower initial NPI-apathy scores predicted increases in apathy and vice versa, as shown in Figure 2.

Figure 2 Scatter plot showing initial NPI-apathy subscale scores versus the annualized rate of change on the NPI-apathy subscale for bvFTD and AD. AD: Alzheimer’s disease; bvFTD: behavioural variant frontotemporal dementia; NPI: Neuropsychiatric Inventory; ROC: rate of change.
Education was a significant predictor for the ROC on the FBI-disinhibition subscale (unstandardized β coefficient=−0.54, SE 0.23, p=0.03) but not the ROC on the total FBI score. In other words, escalating disinhibition on the FBI was related to lower educational levels, and a decrease in disinhibition was appreciated among bvFTD participants with higher levels of education (see Figure 3).

Figure 3 Scatter plot showing the educational level versus the annualized ROC on the FBI-disinhibition subscale in bvFTD versus AD. AD: Alzheimer’s disease; bvFTD: behavioural variant frontotemporal dementia; FBI: Frontal Behavioural Inventory; ROC: rate of change
Linear regression for the best inventory correlates with duration of bvFTD favored neither the NPI nor the FBI above the other. As shown above, indices from either of the two inventories can be descriptive of bvFTD progression. Post-hoc Pearson’s bivariate correlations found that FBI-apathy correlated significantly with duration of illness (see Table 3).
Table 3 Statistically significant Pearson bivariate correlations with duration of illness (r, p values)

FBI: Frontal Behavioural Inventory; n/a: not applicable; NPI: Neuropsychiatric Inventory; FTD: frontotemporal dementia
There were no inflections in ROC on the FBI or NPI among participants with PPA, nor were the demographic variables predictive of ROC. Neither behavioural inventory was superior for following PPA duration of illness per linear regression, but the FBI total (r=0.512, p=0.005) and its sub-scale scores (apathy: r=0.401, p=0.034; disinhibition: r=0.494, p=0.008) and not NPI scores were significant correlates with duration of illness.
There were no NPI or FBI inflections for AD patients.
The second level of regression analysis for AD revealed education and initial scores as predictors of annualized ROC. Education predicted disinhibition as measured on the NPI (unstandardized β coefficient=−0.06, SE 0.03, p=0.03), not the FBI for AD, but the R2 value was almost neglible at 0.04. While disinhibition on the FBI was not predicted by education, the initial FBI-disinhibition score showed unstandardized β coefficient=−0.32, SE 0.07, p<0.001 for the FBI-disinhibition ROC (see Figure 4). Participants who presented with high initial FBI disinhibition scores (severe behavioural disturbance) tended to show a lessening of disinhibition over time.

Figure 4 Scatter plot showing initial FBI-disinhibition scores versus the annualized ROC on the FBI-disinhibition subscale for AD. AD: Alzheimer’s disease; FBI: Frontal Behavioural Inventory; ROC: rate of change.
Similar to bvFTD above, the initial NPI-apathy score predicted the NPI-apathy ROC, in the same inverse relationship ROC (unstandardized β coefficient=−0.34, SE 0.10, p=0.001, see Figure 2).
As with bvFTD, the NPI was not superior to the FBI in a linear regression for duration of AD. All NPI and FBI scores showed significant correlations with duration of AD by Pearson bivariate correlation (p< 0.01), except NPI-disinhibition.
Discussion
The purpose of this study was to compare the evolution of BPSD in patients with bvFTD, PPA and AD using longitudinal FBI and NPI data. We were unable to replicate our prior study’s identification of a six-year inflection point in BPSD for bvFTD.Reference Chow, Fridhandler and Binns 4 The current sample differed from the previous bvFTD sample (n=45) in two ways that may account for the disparity in results. First, the current sample (n=30) was two-thirds the size of the prior sample. It is possible that our sample was too small to power the analysis. Perhaps more relevant however, is that our study observed participants who were still closer to onset of illness than ending it, which implies that in another few years the Baycrest sample may manifest the crescendo-decrescendo in disinhibition. We may have missed any switched directions in ROC in participants whom we only captured after their sixth year of illness.
We had not expected to see educational levels correlate with disinhibition ROC in bvFTD (per FBI) and in AD (per NPI). However, higher education may have allowed patients to better compensate for brain pathology through a cognitive reserve mechanism.Reference Stern 16 Individuals with higher education in this study showed more mild ROC in disinhibition than those with lower education.Reference Woods, Aquirre, Spector and Orrell 17 Higher education is a correlate of neural plasticity.Reference Stern 18 The usual context for discussion of cognitive reserve is its protective effect against clinical signs of dementia despite the presence of plaques, neurofibrillary tangles and cerebrovascular disease.Reference Stern 18 , Reference Snowdon, Greiner, Mortimer, Riley, Greiner and Markesbery 19 There are almost no reports linking cognitive reserve to amelioration of bvFTD. Premi et al. reported that bvFTD patients with higher education harbour worse frontotemporal hypoperfusion than bvFTD patients at the same level of symptom severity but with lower education (less than five years).Reference Premi, Garibotti and Gazzina 20 Despite having worse brain physiological function, the more highly educated group had no worse disinhibition scores, raising the possibility that they were better able to compensate for severity of brain physiology.Reference Premi, Garibotti and Gazzina 20 Our results also suggest that behavioural disturbance, and not only cognition, might be influenced by reserve mechanisms.
Initial NPI-apathy scores for both bvFTD and AD were significant predictors of subsequent ROC on the same subscale. That high initial NPI-apathy scores were followed by decreases over time runs counter to our original hypothesis that apathy would predominate in later stages of bvFTD. Others have reported that patients with bvFTD present with either primarily disinhibited or apathetic behaviours,Reference Rascovsky, Hodges and Knopman 21 , Reference Le Ber and Guedj 22 but we have shown in this and in another dataset that disinhibition and apathy co-occur.Reference Chow, Binns and Cummings 23 If anything, the current samples showed a wider range of apathy scores than of disinhibition. We must revise our hypothesis to accommodate a lessening in apathy over the course of illness, as opposed to an exchange of disinhibition for apathy.
From our comparisons, the FBI was no more revealing for longitudinal observation of BPSD in bvFTD than the NPI. Arguably, the lack of Pearson correlates between NPI-disinhibition and duration of illness in AD might indicate that the FBI would be the better inventory for tracking disinhibition in AD, but our linear regression for total and subscale scores did not conclude that the FBI as a whole held more statistical significance.
Limitations of our study introduced some degrees of bias and imprecision. Initial assessments were not performed at the onset of symptoms and therefore the data were not ideally collected. The observation epochs are heterogeneous among participants within each diagnostic group. We have tried to minimize this bias by converting the data into annualized ROC but this costs us some precision. In addition, the study recruitment from a convenience sample creates a cross-sectional approach to the longitudinal follow-up. There may be both bias and imprecision inherent to this limitation.
Another bias in our current convenience sample leads us away from being able to test the hypothesis that PPA participants would show an inflection in their behavioural disturbances. By definition, there is a minimum two-year delay of behavioural disturbances after onset of aphasia in PPA.Reference Mesulam 24 This would push any observable climb and descent of disinhibition and apathy to a time frame beyond the sixth year originally observedReference Chow, Fridhandler and Binns 4 and the epochs observed for the current PPA sample.
A further limitation to the precision of our data collection is that informants may have differed for a given participant between annual assessments. As a result, it is possible that behavioural changes were not necessarily captured. However, informants changed for a minority (20%) of patients, thereby providing us with predominantly consistent reporting.
Behavioural and psychiatric symptoms of dementia upset patients, caregivers and other household members. The sample of participants available to us demonstrated general increases in apathy and disinhibition over the first six to seven years of illness. This increase occurred despite patients being treated at the Clinic, raising questions about the efficacy of treatment or the possibility of apathy having an iatrogenic cause.
We intend to continue the study protocol, in hopes of ultimately answering our question about the inflection in trajectory: revisions to the protocol at this point may be to hone the cohort to be more homogeneous in their duration of illness, i.e., to continue with only those participants for whom we can capture years four to ten of illness.
Acknowledgments
The authors thank Andrijana Dzever (University of Toronto Family Medicine), David Fam (University of Toronto Neurology), Jonathan Fridhandler (University of British Columbia Neurology), Noam Grysman (York University), Laura Shnall (Western University) and Mamie Shum (McGill University) for contributing to the data collection in this study.
The authors thank the Sam and Ida Ross Memory Clinic at Baycrest Hospital, the Women of Baycrest, and the Gordie and Colleen Howe Dementia Research Fund for financial support.
DISCLOSURES
This work (Tiffany Chow) was funded by the Sam and Ida Ross Memory Clinic, the Women of Baycrest, the Gordie and Colleen Howe Dementia Research Fund, and Déanne and Vahram Sedef. Morris Freedman receives support from the Saul A. Silverman Family Foundation as a Canada International Scientific Exchange Program and Morris Kerzner Memorial Fund. Alexandra Linds, Alana Kirstein, Uri Wolf, and Nicolaas PLG Verhoeff report no disclosures.
STATEMENT OF AUTHORSHIP
AB Linds was the primary author of this manuscript. AB Kirstein participated in data collection, literature search, analysis of the data and was responsible for drafting early versions of the manuscript. M Freedman, NPLG Verhoeff and U Wolf contributed participants and helped to revise and finalize the manuscript. TW Chow participated in the design of the study, contributed patients, supervised acquisition, analysis and interpretation of data, writing and finalization of the manuscript.









