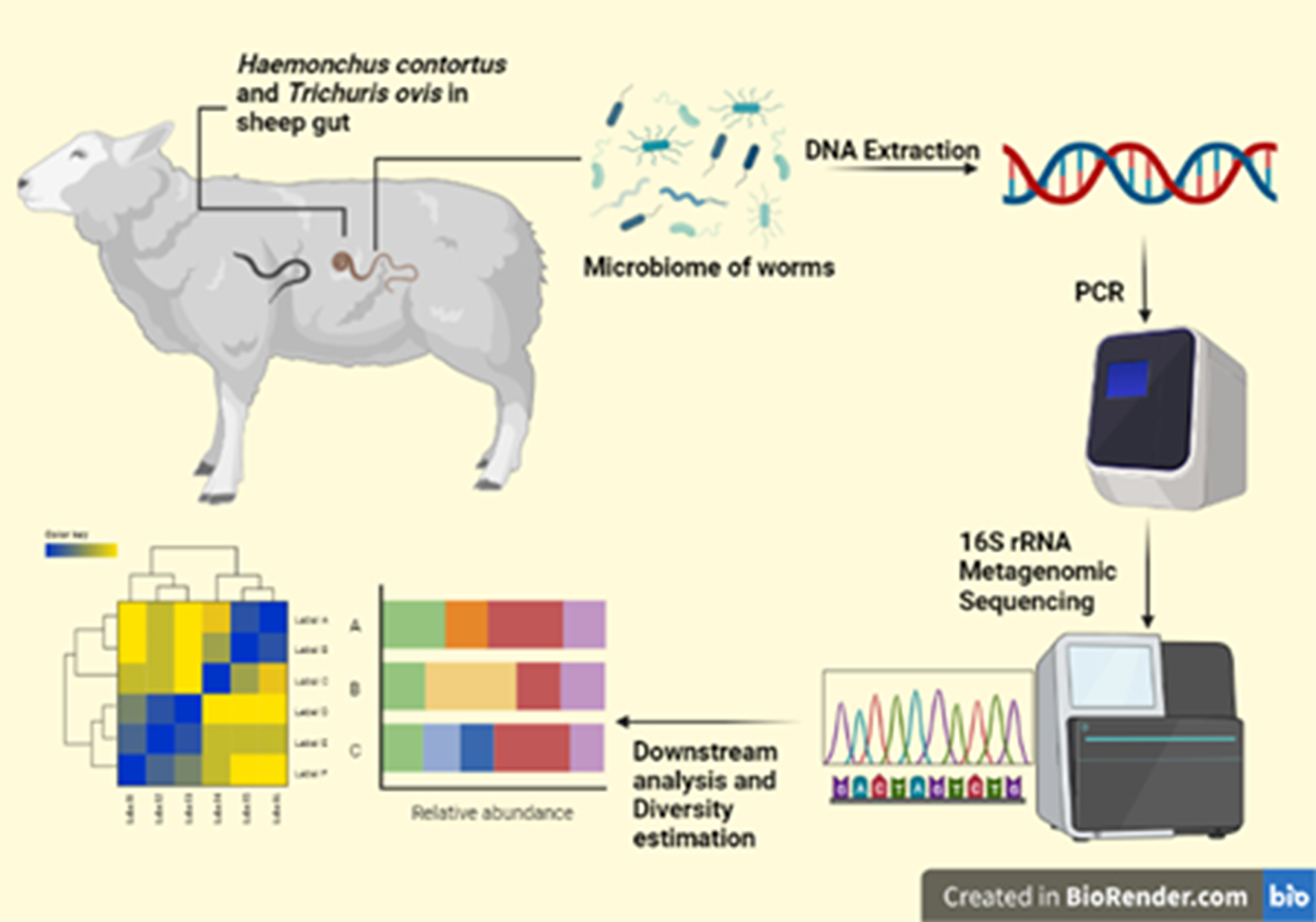
Introduction
Kashmir Merino sheep, a crossbreed of the Kashmiri, Tasmanian Merin, and Delain breeds of sheep (Ovis aries) has been extensively domesticated in the Kashmir region of India since 1960. However, like other ruminants, Kashmir Merino sheep face a substantial threat from gastrointestinal nematodes leading to a significant reduction in their productivity (Rather et al. Reference Rather, Shanaz, Alam, Hamadani, Malik, Bashir, Shah and Khan2021). Among the various nematode species, Haemonchus contortus stands out as one of the most prevalent and pathogenic parasites affecting sheep worldwide. This parasite has gained attention due to its increasing resistance to anthelmintic treatments, making its control a daunting challenge (Kaplan Reference Kaplan2004). Despite numerous attempts employing botanicals, vaccines and synthetic drugs, none have proven highly effective in managing this parasite (Ali et al. Reference Ali, Rooman, Mussarat, Norin, Ali, Adnan and Khan2021; Wang et al. Reference Wang, Li, Zhang, Yang, Ahmad, Li, Du and Hu2017). The other highly prevalent nematode in Kashmir Merino sheep is Trichuris ovis. This nematode though not much pathogenic, contributes to the histological deterioration in the caecum of sheep in heavy infections (Junquera Reference Junquera2022). Other species of the same genus, Trichuris muris, and Trichuris trichiura are highly pathogenic and lead to comorbidities in mice and humans, respectively (Hayes et al. Reference Hayes, Cliffe, Bancroft, Forman, Thompson, Booth and Grencis2017; Hayes and Grencis Reference Hayes and Grencis2021; Stephenson et al. Reference Stephenson, Holland and Cooper2000).
Both H. contortus and T. ovis have a direct life cycle involving a single host (sheep), H. contortus residing in the abomasum of sheep lays around 5000–15,000 eggs/day (Emery et al. Reference Emery, Hunt and Le Jambre2016). Eggs are deposited outside with faeces where they hatch into the L1 stage in 24 hours, which moults two times in about 5-6 days into L3 (infective stage). L3 finds its way to grass blades and then enters into sheep while grazing. Inside the abomasum, with two more moults, an L5 adult is formed to complete the life cycle (Naeem et al. Reference Naeem, Iqbal and Roohi2021). Trichuris ovis eggs are deposited with faeces where, after 20–22 days, L1 (infective stage) larva develop inside the eggs, which are then consumed by sheep while grazing. Inside the intestines, L1 hatches and moults four times to form an adult worm that inhabits the caecum to complete the life cycle (Gobind Singh and Kr. Suresh Reference Gobind Singh and Kr. Suresh1954; Hayes et al. Reference Hayes, Bancroft, Goldrick, Portsmouth, Roberts and Grencis2010).
Like all metazoan organisms, gastrointestinal nematodes contain microbiome which is thought to render important metabolic functions for the survival of these nematodes (Jenkins et al. Reference Jenkins, Brindley, Gasser and Cantacessi2019). Recent studies have revealed the association of bacteria with H. contortus which suggests the possible role of these bacteria in the survival and effective procreation of H. contortus and thus can be targeted to control the parasite indirectly (Mafuna et al. Reference Mafuna, Soma, Tsotetsi-Khambule, Hefer, Muchadeyi, Thekisoe and Pierneef2021; Sinnathamby et al. Reference Sinnathamby, Henderson, Umair, Janssen, Bland and Simpson2018). Notably, filarial nematodes have been indirectly controlled by killing their endosymbiotic bacteria Wolbachia (Landmann et al. Reference Landmann, Voronin, Sullivan and Taylor2011; Taylor et al. Reference Taylor, Bandi, Hoerauf and Lazdins2000). Study of the microbiome of H. contortus and T. ovis can help us understand the relationship between the parasite survival and pathogenicity and its associated microbiota. With the increase in anthelmintic resistance among nematodes, studies are conducted to find alternate strategies for controlling these nematodes (Papadopoulos Reference Papadopoulos2008). One such strategy could be either modulating and targeting parasite or host microbiome. With the increasing knowledge on the role of the microbiome in host–parasite relationship, many studies have diverted their focus on studying the composition and function of parasite microbiome in the adaptation, infectivity, pathogenicity and host immune modulation.
Wescott (Reference Wescott1968) found that mice microbiome renders resistance to nematode infection and pathogenesis. In contrast, host (mice, human) microbiome particularly Clostridia and Escherichia coli species have been found to induce the hatching of T. muris and T. trichiura eggs (Hayes et al. Reference Hayes, Bancroft, Goldrick, Portsmouth, Roberts and Grencis2010; Sargsian et al. Reference Sargsian, Chen, Lee, Robertson, Thur, Sproch, Devlin, Tee, Er and Copin2022). In addition to this, parasites also modulate host microbiota to favor their adaptation, for example, Teladorsagia circumcincta increases proinflammatory bacteria in the gut of sheep, while H. contortus also alters the sheep gut microbiota (Cortés et al. Reference Cortés, Wills, Su, Hewitt, Robertson, Scotti, Price, Bartley, McNeilly and Krause2020; El-Ashram et al. Reference El-Ashram, Al Nasr, Abouhajer, El-Kemary, Huang, Dinçel, Mehmood, Hu and Suo2017; Mamun et al. Reference Mamun, Sandeman, Rayment, Brook-Carter, Scholes, Kasinadhuni, Piedrafita and Greenhill2020).
Understanding the tripartite interaction between the host, parasite and microbiome in greater detail can help us develop strategies for the successful control of nematode parasites. Because H. contortus resides in the abomasum and feeds on the blood of its host, while T. ovis lives in the caecum and feeds on the intestinal contents of sheep, this study aims to reveal and compare the bacterial diversity associated with these two parasitic nematodes to find differences between their microbiome based on their predilection site and mode of feeding. The work can be used in conjunction with other pertinent studies to define the core microbiota (commonly occurring bacteria) and its function in these parasitic nematodes and reveal such bacteria that could serve as targets for the control of these worms.
Methodology
Collection and identification of parasites
A total of 30 gastrointestinal tracts of Kashmir Merino sheep were collected from the local abattoir and brought to the Parasitology Lab, Department of Zoology, University of Kashmir, where the abomasum and caecum portions were dissected open and cleaned with distilled water. Live parasites were removed from the gut carefully and washed with sterile PBS (pH 7.4) to remove any residual gut contents from the parasitic surface. Sheep guts were processed immediately after sheep death to minimize postmortem changes in microbiome diversity.
Morphological identification of H. contortus was carried out based on vulvar (in females) and spicule (in males) features (Jacquiet et al. Reference Jacquiet, Cabaret, Cheikh and Thiam1996; Kuchai et al. Reference Kuchai, Ahmad, Chishti, Tak, Javid, Ahmad and Rasool2012). T. ovis was identified by the presence of a long whip-like anterior portion, spicule characteristics in males and vulvar features in females (Cutillas et al. Reference Cutillas, German, Arias and Guevara1995). For the confirmation of species, the parasitic worms were processed for molecular identification.
Sample pooling
Out of 30 sheep guts, 23 were found infected with gastrointestinal nematodes. For further investigation, 10 sheep guts, 5 infected with H. contortus, and 5 infected with T. ovis were selected. Thus, a total of 10 samples were made, 5 each of H. contortus and T. ovis. Each sample contained 10 to 16 worms in the case of H. contortus and 4 to 6 worms in the case of T. ovis (male-to-female worm ratio was kept equal). The sample pools were made based on worm size to make equal amount of homogenate to be processed. Because H. contortus is smaller in size, more worms were homogenized as compared to T. ovis, compensating for the size-related bias in diversity results (Reese and Dunn Reference Reese and Dunn2018).
Parasite and microbiome DNA isolation
For the isolation of parasite and microbiome DNA, adult worms were washed with PBS thoroughly, then with antibiotic solution (streptomycin/erythromycin 20 mg/ml) for 3 h and finally washed with 4% sodium hypochlorite five to six times and finally again with sterile PBS to remove any surface contamination (Mafuna et al. Reference Mafuna, Soma, Tsotetsi-Khambule, Hefer, Muchadeyi, Thekisoe and Pierneef2021; Sinnathamby et al. Reference Sinnathamby, Henderson, Umair, Janssen, Bland and Simpson2018). The whole sterilized worms were then homogenized. The homogenized material was put in a 2 ml Eppendorf tube and centrifuged at 8000 rpm for 5 min, supernatant was discarded carefully and 400 μl lysis buffer (20mM Tris HCL pH 8,100 mM disodium EDTA) and 200 μl of 10% SDS were added to the pellet. The mixture was vortexed for 2 min and then 15 μl Proteinase K solution (20 mg/ml) were added to the tube. The mixture was vortexed for 5 min again and then kept for incubation at 65°C for 2 h and then lysozyme (20 mg/ml) was added to the tube and kept for incubation at 30°C for an hour and at room temperature overnight. Lysis buffer, SDS, Proteinase K and lysozyme were also added to double distilled water in separate tubes to be used as a negative control. Parasite and bacterial DNA was isolated from these samples using PureLink Microbiome DNA Purification Kit Catalog number: A29790 as per the manufacturer’s protocol. The DNA samples extracted and eluted were stored at –20 °C until further processing. This meticulous procedure aimed to ensure the purity of the DNA samples by eliminating surface contaminants allowing for accurate and reliable analysis of the parasite and microbiome DNA.
Parasite DNA amplification
Approximately 25 ng of DNA was used to amplify the ITS-2 region of H. contortus and the COX1 gene of T. ovis (Stevenson et al. Reference Stevenson, Chilton and Gasser1995; Wang et al. Reference Wang, Liu, Li, Xu, Ye, Zhou, Song, R-Q and Zhu2013). The reaction included Promega Master Mix catalog number: M7122 and 1μl (1μM concentration) of forward and reverse primers each (Table 1). The PCR conditions involved an initial denaturation step of 95°C for 5 min followed by 35 cycles of 95°C for 30 s, annealing at 55°C for 30 s and 72°C for 1 min with a final extension of 72°C for 5 min. The PCR products approximately 350 bp for ITS-2 and 618 bp for COX1 were screened by gel electrophoresis in 1.5% agarose gel run at 110 V for 40 min, and the product sizes were confirmed with a 100 bp Promega DNA marker. The PCR products were purified using AMPure beads (1.6X) to remove unused primers and later sent for sanger sequencing.
Table 1. Primer sequences used to amplify ITS2 in H. contortus and COX1 in T. ovis. F represents forward and R represents reverse

Library preparation and microbiome DNA sequencing
For the microbiome, 25 ng of DNA was used to amplify 16S rRNA (V3-V4 hypervariable region). The reaction included Gotaq Green Master Mix catalog number: M712 and 1 μM final concentration of forward and reverse primers (Table 2) (Jian et al. Reference Jian, Luukkonen, Yki-Järvinen, Salonen and Korpela2020; Klindworth et al. Reference Klindworth, Pruesse, Schweer, Peplies, Quast, Horn and Glöckner2013). The PCR conditions involved an initial denaturation step of 95°C for 5 min followed by 35 cycles of 95°C for 30 s, 55°C for 45 s and 72°C for 30 s and a final extension step at 72°C for 5 min. The PCR products in both cases were screened by gel electrophoresis in 1.5 % agarose gel run at 110 V for 40 min, and the product sizes were confirmed with a 100 bp Promega DNA marker. Unused primers were removed using AMPure beads (1.6X). Additional eight cycles of PCR were performed using Illumina barcoded adapters to prepare the sequencing libraries as per Illumina 16s Metagenomic Sequencing Library Preparation guide. The sequencing was then carried out in the Illumina MiSeq 2×300 bp paired-end chemistry to generate 0.1M reads per sample.
Table 2. Primer sequences used to amplify the V3V4 region in bacteria. F represents forward, and R represents reverse

Data processing and analysis
The data was checked for quality of bases, % bases above Q25, %GC and sequencing adapter contamination using FastQC v.0.12.0 (Table S1) (Andrews Reference Andrews2010).
Primer and adapter sequences along with low-quality bases were removed from the reads using Trimgalore v.0.6.7 wrapper with default parameters (Krueger Reference Krueger2015). QC passed forward and reverse reads were merged to make contigs using the make.contig function of mothur v.1.48.0 software (Schloss et al. Reference Schloss, Westcott, Ryabin, Hall, Hartmann, Hollister, Lesniewski, Oakley, Parks and Robinson2009). The contigs were checked for ambiguous bases. The processed contigs were screened for duplicate sequences to merge them. Gaps and overhangs at the ends of the contigs were removed and processed for chimera removal. The filtered contigs were processed and classified based on the SILVA_v138.1 database in mothur v.1.48.0 using the align.seqs and classify.seqs functions (Quast et al. Reference Quast, Pruesse, Yilmaz, Gerken, Schweer, Yarza, Peplies and Glöckner2012). The contigs were then clustered into operational taxonomic units (OTUs) in mothur software using the dist.seqs and cluster functions.
The relative abundance of OTUs was calculated in R studio 2023.03.0 Build 386 using a Mothur-produced taxonomy and OTU count table (Team Reference Team2015). Alpha diversity was estimated in mothur software using the summary.single function to get indices like Shannon, Simpson, Ace, Chao, Simpson evenness and Shannon evenness. The normality of the diversity data was evaluated using the Shapiro Wilcoxon test in R studio 2023.03.0 Build 386. The diversity data, not being normal, was analyzed for statistical significance using the Kruskal–Wallis test in R studio 2023.03.0 Build 386.
Beta diversity was calculated in mothur software with the dist.shared function using Bray–Curtis and Jaccard distance methods. Nonmetric multidimensional scaling (NMDS) was done using Bray–Curtis and Jaccard distances, and the plots were visualized with R studio 2023.03.0 Build 386. The statistical significance of beta diversity indices was calculated using permutational multivariate analysis of variance (PERMANOVA) (Anderson Reference Anderson2014). Further analysis and visualization were done in R studio 2023.03.0 Build 386 using vegan, phyloseq, tidyverse, tidyr, dplyr, phangorn and ggplot2 packages (McMurdie and Holmes Reference McMurdie and Holmes2013; Oksanen et al. Reference Oksanen, Kindt, Legendre, O’Hara, Stevens, Oksanen and Suggests2007; Schliep Reference Schliep2011; WickhamAverick et al. Reference Wickham, Averick, Bryan, Chang, McGowan, François, Grolemund, Hayes, Henry and Hester2019; Wickham et al. Reference Wickham, Chang and Wickham2016; Wickham et al. Reference Wickham, François, Henry, Müller and Wickham2019; Wickham and Wickham Reference Wickham and Wickham2017).
Results
Morphological and molecular identification of worms
Parasitic worms collected were confirmed to be H. contortus and T. ovis based on morphometric features (Table 3) as validated by molecular analysis through nBlast (Table 4).
Table 3. Morphometric measurements of H. contortus and T. ovis. Measurements are in millimeters (mm)

Table 4. GenBank accession number of H. contortus and T. ovis samples. Known sequences with >95% match and overlap were used to identify worm species in nBlast search

Microbiome associated with H. contortus and T. ovis at phylum level
A total of 28 different phyla were identified with 22 occurring in both H. contortus and T. ovis. Six phyla (Elusimicrobiota, Entotheonellaeota, Spirochaeta, RCP2-54, Dependentiae, Nitrospirota) were unique to T. ovis, and one phylum (MBNT15) was unique to H. contortus. Haemonchus contortus was dominated by phyla Proteobacteria (57%), Firmicutes (25%), Bacteroidota (15%), Actinobacteriota (3%), while T. ovis showed Proteobacteria (78%) followed by Firmicutes (8%), Bacteroidota (8%), Actinobacteriota (1%), Fusobacteriota (1%) and other phyla (4%) (Figure 1).

Figure 1. Stacked bar chart showing mean relative abundance of microbiome diversity of H. contortus and T. ovis at phylum level.
Microbiome composition of worms at genus level
The total number of OTUs found in both the nematode species was 700 (Table S2); 236 OTUs were present in both and 198 OTUs were unique to H. contortus while 266 OTUs were unique to T. ovis.
Haemonchus contortus showed Acinetobacter (25%), Massilia (10%), Prevotella (9%), Faecalibacterium (4%), Staphylococcus (4%), Streptococcus (3%), Stenotrophomonas (3%), Variovorax (3%), Phyllobacterium (3%) and other genera (Figure 2). Trichuris ovis was predominated by genus Escherichia/Shigella (69%) followed by UCG-005 (4%), Acinetobacter (3%), Prevotellaceae-UCG-003 (3%), Flavobacterium (2%), Pseudomonas (2%), Alphaproteobacteria (2%), Bacteroides (2%) and other genera (Figure 3).

Figure 2. Krona plot showing abundance of different bacterial genera reported in H. contortus.

Figure 3. Krona plot showing abundance of different bacterial genera reported in T. ovis.
Out of 236 common bacterial genera, some potentially harmful ones include Pseudomonas, Streptococcus, Staphylococcus, Sphingomonas, Klebsiella and Enterococcus.
Major unique bacterial genera
Haemonchus contortus showed 198 unique genera of which predominant ones include Spiroplasma (10.21%), Achromobacter (6%), Clostridium_sensu_stricto_1 (6%), Sphingobacterium (5.6%), Collinsella (5%), Roseburia (4.7%), Dialister (4%), Holdemanella (4%), Agathobacter (4%), Veillonella (3.3%), Catenibacterium (3.9%), Megamonas (3.3%), Haemophilus (2.7%), Butyricicoccus (2%) and Empedobacter (2%).
Trichuris ovis contained 266 unique genera predominated by Prevotellaceae_UCG-003 (36%), Campylobacter (9.3%), Bibersteinia (3.8%), Leptotrichiaceae_unclassified (3.4%), Mailhella (2.3%) and Frisingicoccus (1.6%).
Bacterial diversity statistics of worms
Even though Shannon diversity index and evenness indices were higher in the case of H. contortus. These indices are indicative of greater overall diversity and even distribution of bacteria within the H. contortus. Conversely, the mean observed number of OTUs, Simpson, Ace and Chao indices were found to be higher in T. ovis (Table 5, Figure 4). These indices suggest that T. ovis exhibited a greater richness and higher dominance of certain bacteria compared to H. contortus. In the Kruskal–Wallis test, significant difference in microbiome diversity was found in Observed, Shannon, Simpson and evenness indices while Ace and Chao richness indices did not differ significantly between the two parasite species.
Table 5. Alpha diversity measures for H. contortus and T. ovis. Statistical significance was calculated with the Kruskal–Wallis test at p < 0.05 (* represents significant difference)


Figure 4. Alpha diversity measures: Boxplot of alpha diversity indices of H. contortus and T. ovis.
Beta diversity
The NMDS analysis based on Bray–Curtis and Jaccard dissimilarity measures revealed distinct clustering patterns for the bacterial profiles of H. contortus and T. ovis (Figure 5). This clustering indicates that the microbial communities associated with these two parasitic nematode species are significantly different from each other.

Figure 5. NMDS ordination of microbiota in H. contortus and T. ovis based on Bray–Curtis distances. Each dot represents the microbiome profile of a sample. The ellipse represents the spread of the microbiome community of a group.
Additionally, the heatmap patterns (Figure 6) further underscore the dissimilarities in the bacterial profiles of H. contortus and T. ovis. Notably, H. contortus exhibited darker blue colours, suggesting greater within-species variation within its bacterial community compared to T. ovis. To statistically validate these differences, PERMANOVA was conducted on Bray–Curtis and Jaccard dissimilarity distances with 1000 permutations. The results of the PERMANOVA indicated that there were indeed significant differences in the bacterial profiles of the two parasite species, with a p-value of 0.009. This statistical analysis confirms that the observed dissimilarities in bacterial composition between H. contortus and T. ovis are not due to random chance but are significant and meaningful.

Figure 6. Heatmap of microbiome profile of H. contortus and T. ovis based on Bray–Curtis and Jaccard distances. Each small box represents the distance between two samples.
Discussion
In this study, we assessed the microbiome associated with two important and highly prevalent nematode parasites of sheep viz; H. contortus and T. ovis. Though residing in the same sheep breed, this study sought to identify variations in the bacterial profile of H. contortus and T. ovis having differences in mode of nutrition, pathogenicity, predilection site and their life cycle. The results revealed that both the nematodes have their own microbiome composition with some similarities among them.
The most abundant bacterial phyla in H. contortus are in agreement with previous assessments for the species, although previous studies reported Tenericutes in H. contortus, which we have not found (Bouchet et al. Reference Bouchet, Deng and Umair2022; El-Ashram and Suo Reference El-Ashram and Suo2017; Mafuna et al. Reference Mafuna, Soma, Tsotetsi-Khambule, Hefer, Muchadeyi, Thekisoe and Pierneef2021; Sinnathamby et al. Reference Sinnathamby, Henderson, Umair, Janssen, Bland and Simpson2018). Though comparing the microbiome of different life stages of H. contortus, these studies have not compared it with the microbiome of any other nematode species inhabiting the same host. This study fills that gap while comparing the microbiome of H. contortus with that of T. ovis.
Even though, at the phylum level, there seems to be less difference between the microbiome of two nematodes, at the genus level, the difference resolves better with H. contortus being dominated by Acinetobacter (25%), Massilia (10%), Prevotella (9%), Faecalibacterium (4%), Staphylococcus (4%), Streptococcus (3%), Stenotrophomonas (3%), Variovorax (3%) and Phyllobacterium (3%) and T. ovis being dominated by Escherichia/Shigella (69%), UCG-005 (4%), Acinetobacter (3%), Prevotellaceae-UCG-003 (3%), Flavobacterium (2%), Pseudomonas (2%), Alphaproteobacteria (2%) and Bacteroides (2%). These results contrasted with Mafuna et al. (2021) and Sinnathamby et al. ( 2018), which reported Escherichia/Shigella to be the dominant genus in the H. contortus microbiome. The discrepancy may be caused by the host and habitat because the environment can change the microbiome in the gut of sheep, which could then impact the microbiome of their parasitic nematodes (Huang et al. Reference Huang, Li and Luo2017). The distinction in the relative abundance of bacterial genera between H. contortus and T. ovis could be because of their differences in overall biology, predilection site and mode of nutrition. However, these are only assumptions at a primitive level. To look for the consistency in the microbiome structure of these parasites for management strategies, microbiome studies of these worms in different experimental conditions need to be done. In addition, T. ovis showed higher alpha diversity than H. contortus in all diversity indices except Shannon, which was lower for T. ovis than H. contortus.
The higher diversity in T. ovis might be due to its size and nature of feeding directly on the intestinal contents of the host, rich in bacteria, as food and increase in body size has been correlated with increased bacterial diversity (Reese and Dunn Reference Reese and Dunn2018). Significant differences in microbiome diversity were identified in the Observed, Shannon, Simpson and evenness indices, implying that there are statistically significant variations in OTU richness, diversity and even distribution of OTUs between the two parasitic worms. However, Ace and Chao richness indices did not display a significant difference, indicating that the estimated total number of OTUs (richness) did not significantly differ between H. contortus and T. ovis. These findings shed light on the differences in the microbiome composition and diversity between these two parasitic nematode species, highlighting both similarities and distinctions in their microbial communities.
Haemonchus contortus showed more diversity variation between samples as compared to T. ovis. The possible reason could be higher activity in H. contortus which moves within the abomasum and to the upper portion of the duodenum, exposing itself to different areas of the sheep gut leading to variations in its microbiome diversity, whereas T. ovis is always found very inactive in the caecum of sheep. It does not move actively to different sections of the hindgut, which could explain more consistency of its microbiome.
Furthermore, this study revealed some unique genera in H. contortus that were not found by previous studies (Bouchet et al. Reference Bouchet, Deng and Umair2022; El-Ashram and Suo Reference El-Ashram and Suo2017; Mafuna et al. Reference Mafuna, Soma, Tsotetsi-Khambule, Hefer, Muchadeyi, Thekisoe and Pierneef2021; Sinnathamby et al. Reference Sinnathamby, Henderson, Umair, Janssen, Bland and Simpson2018). These include Spiroplasma, Collinsella, Dialister, Holdemanella, Agathobacter, Veillonella, Catenibacterium, Megamonas, Butyricicoccus and Empedobacter which could have been incorporated from the host. Trichuris ovis showed a few unique genera including Campylobacter, Bibersteinia, Mailhella and Frisingicoccus. Some of these bacteria have a positive role and help in metabolic processes while others have been proven to be pathogenic in other hosts. Some of the potentially harmful bacteria found in both nematodes, including Pseudomonas, Streptococcus, Staphylococcus, Sphingomonas, Klebsiella and Enterococcus, suggest that these parasite species may act as vectors for such harmful bacteria and thus increase the chances of secondary infections to the host. García-Sánchez et al. (Reference García-Sánchez, Miller, Caldeira and Cutillas2022) also found many pathogenic bacteria (Aeromonas, Bartonella, Clostridium, Mycobacterium, Rickettsia, and Salmonella) associated with T. trichiura and T. suis, suggesting the role of Trichuris species being carriers of bacterial diseases. This study and relevant research can be used to determine the core microbiota of parasitic nematodes. The term ‘core bacteria’ in this context refers to the main bacteria that have been identified in all studies on the nematode microbiota. By examining the findings of several experiments, we might be able to identify frequently prevalent bacteria in these nematodes. With the aid of additional investigation into the functional role of such bacteria, we can successfully manage nematodes while concentrating on these bacteria.
In conclusion, the objective of this research was to determine the bacterial makeup of two nematode parasites, H. contortus, and T. ovis, that inhabit the same sheep breed but differ in their methods of feeding, pathogenicity, preferred locations and life cycles. The results of the study showed that both parasites have distinct microbiomes, although there are some similarities at the phylum level. The dominant phylum for both parasites was Proteobacteria, followed by Firmicutes and Bacteroidota. However, the differences between the microbiomes of the two nematodes were more noticeable at the genus level. T. ovis had a higher level of diversity, which may be due to its feeding on the intestinal contents of the host that are rich in bacteria. Studies comparing the microbiota in sheep blood and the gut are lacking, which may help to explain any potential relationships between the variation of microbiome diversity and the dietary preferences of the various nematodes. The study of the host–parasite microbiome is in its early stages. Further work needs to be done to understand these intriguing variations in the microbiome diversity of various nematode species. This study also identified new bacterial genera that were not previously reported in studies on these parasites. Furthermore, the presence of potentially harmful bacteria in both nematodes indicates their role as vectors for secondary infections in the host emphasizing the need to consider nematodes as potential contributors to bacterial diseases in hosts.
In summary, this study contributes valuable insights into the unique microbiome compositions of H. contortus and T. ovis, highlighting both commonalities and differences between these parasitic nematodes. These findings pave the way for further research into the potential implications for host health and the development of targeted management strategies.
Supplementary material
The supplementary material for this article can be found at http://doi.org/10.1017/S0022149X23000573.
Acknowledgements
We are thankful to the Department of Zoology and Centre of Research for Development, University of Kashmir for providing the necessary lab facilities. We are also obliged to the local abattoirs for providing sheep gut used in our investigation. The authors are also highly grateful to Clevergene Biocorp Pvt. Ltd. Bengaluru, India for offering NGS services.
Financial support
The authors are grateful to the Centre of Scientific and Industrial Research Human Resource Development Group, India, for providing financial assistance for this study.
Competing interest
The authors declare no potential conflicts of interest concerning the research, authorship and/or publication of this article.
Ethical standard
No live animals were used in any of the experiments for our study. Local abattoirs provided the sheep gastrointestinal tracts used in this study.