Conjugated linoleic acid (CLA), a collective term for the positional and geometric isomers of linoleic acid (LA), has been investigated extensively for its purported ability to induce weight loss and body-compositional changes such as lower fat mass, as well as elicit alterations in circulating lipids in both animals and human subjects(Reference Wang and Jones1). The main isomer occurring naturally in foods is cis-9, trans-11 (c9, t11)-CLA. Trans-10, cis-12 (t10, c12)-CLA is a quantitatively minor isomer in CLA-containing foods, but can be chemically synthesised from vegetable oils and is therefore available in equal proportions to the c9, t11 isomer in commercial dietary supplements. Physiological effects cited above have been mainly associated with the t10, c12 isomer(Reference Wahle, Heys and Rotondo2). While data emerging from animal and in vitro studies are predominantly consistent in their support for the effects of CLA, evidence from clinical trials has been less convincing, with the discrepancies attributable to variations in experimental factors(Reference Plourde, Jew and Cunnane3).
Early studies demonstrating health benefits of CLA in human subjects were conducted with mixtures containing small amounts of isomers other than the c9, t11 and t10, c12 isomers, giving rise to the possibility that some of the minor isomers may have contributed to the observed effects. This perspective has led to the suggestion that there may be value in investigating the effects of the minor, lesser-known CLA isomers(Reference Banni4). One such isomer is trans-8, cis-10 (t8, c10)-CLA, which used to be present as a minor component and considered undesirable in commercially produced dietary supplements(Reference Reany, Liu, Westcott, Yurawecz, Mossoba, Kramer, Pariza and Nelson5). However, it has been reported that c9, t11-CLA heated under specific conditions isomerises into an equimolar mixture of c9, t11 and t8, c10 isomers(Reference Destaillats, Japiot and Chouinard6). Due to the paucity of data on t8, c10-CLA, an animal study was conducted wherein Syrian golden hamsters were fed a semi-purified diet supplemented with a t8, c10+c9, t11 isomeric mixture at 2 % (w/w) of the diet, to primarily investigate its effect on plasma lipids(Reference Bissonauth, Chouinard and Marin7). Feeding animals this CLA-supplemented diet for 28 d resulted in significantly higher circulating VLDL-TAG and -cholesterol concentrations. In a subsequent in vitro experiment, 3T3-L1 preadipocytes supplemented with the t8, c10+c9, t11-CLA mixture during differentiation exhibited significantly lower TAG concentration compared with the c9, t11 pure isomer(Reference Joseph, Miller and McLeod8). However, the adiponectin content of these cells was not altered, a finding that was favourable and in contrast to cells treated with a t10, c12+c9, t11-CLA mixture, which contained markedly lower amounts of adiponectin compared with control and other fatty acid treatments.
The objective of the present study was, therefore, to further explore the biological effects of the t8, c10+c9, t11-CLA mixture, particularly in relation to body composition in male Syrian golden hamsters. Because the t10, c12 isomer has been shown to consistently induce liver hypertrophy due to steatosis in mice(Reference Poirier, Niot and Clement9, Reference Clement, Poirier and Niot10), it was important to establish that any beneficial effects we might observe with the t8, c10+c9, t11-CLA mixture in the present study occur in the absence of such negative effects.
Experimental methods
Diets
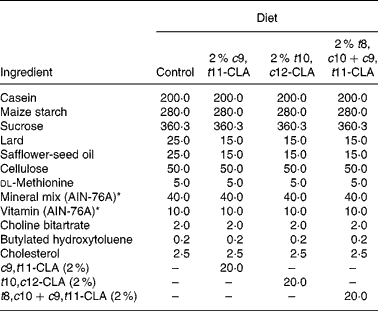
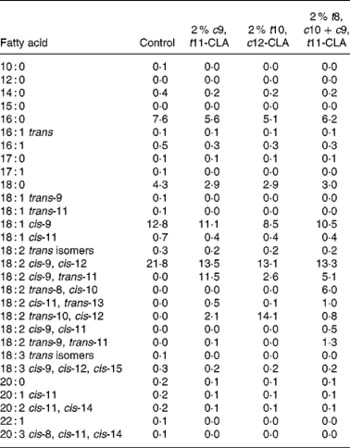
The composition of the experimental diets is presented in Table 1. The control diet used in the present study was a semi-purified, hypercholesterolaemic diet (American Institute of Nutrition (AIN)-76A; Harlan Teklad, Madison, WI, USA) containing 5 % dietary fat and 0·25 % cholesterol. This diet was supplemented at the 2 % level with three different CLA preparations at the expense of lard and safflower-seed oil. The three CLA treatments tested in the study were (1) 2 % c9, t11-CLA, (2) 2 % t10, c12-CLA and (3) 2 % t8, c10+c9, t11-CLA mixture (containing approximately 30 and 26 % of the respective isomers). The fatty acid profile of the experimental diets is presented in Table 2.
Table 1 Composition of experimental diets (g/kg)
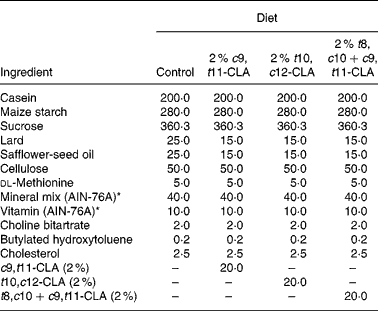
c9, t11, cis-9, trans-11; CLA, conjugated linoleic acid; t10, c12, trans-10, cis-12; t8, c10+c9, t11, trans-8, cis-10+cis-9, trans-11.
* American Institute of Nutrition (AIN)-76A (Harlan Teklad, Madison, WI, USA).
Table 2 Fatty acid composition of experimental diets (g/kg)

c9, t11, cis-9, trans-11; CLA, conjugated linoleic acid; t10, c12, trans-10, cis-12; t8, c10, trans-8, cis-10.
Test fatty acids
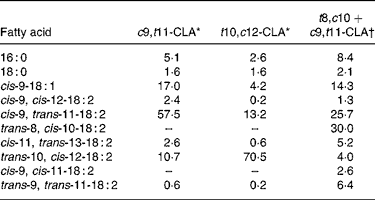
The c9, t11- and t10, c12-CLA were obtained from Lipid Nutrition (Wormerveer, The Netherlands). The t8, c10+c9, t11 isomeric mixture, which at present is not commercially available, was synthesised according to the method previously described(Reference Destaillats, Japiot and Chouinard6). Briefly, c9, t11-CLA oil was heated in the oven of a 5890A gas chromatograph (Hewlett-Packard, Palo Alto, CA, USA) at 200°C for 6 h whereupon it isomerised into an approximately 50:50 mixture of the t8, c10 and c9, t11 isomers. The fatty acid composition of the three CLA treatments is presented in Table 3.
Table 3 Fatty acid composition of conjugated linoleic acid (CLA) supplements (relative area %)
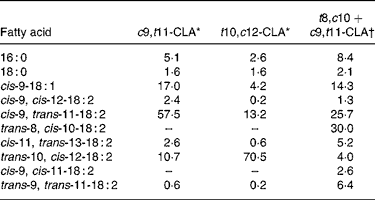
c9, t11, cis-9, trans-11; t10, c12, trans-10, cis-12; t8, c10, trans-8, cis-10.
* From certificate of analysis provided by Lipid Nutrition (Wormerveer, The Netherlands).
† Initial product contained 7·7 % of 16 : 0, 2·6 % of 18 : 0, 15·4 % of cis-9-18 : 1, 2·6 % of cis-9, cis-12-18 : 2, 55·6 % of cis-9, trans-11-18 : 2, 11·1 % of trans-10, cis-12-18 : 2 and 5·1 % of trans-9, trans-11-18 : 2.
Study protocol and animals
Sixty male Syrian golden hamsters (Charles River Laboratories, Pointe-Claire, QC, Canada) weighing 50–60 g upon arrival were individually housed in plastic cages with a continuous light–dark cycle (09.00–21.00 hours) at a constant temperature of 20 (sem 2) °C. Animals were placed on a non-purified commercial rodent diet (Nestle Purina, St Louis, MO, USA) until the start of the experiment. Upon reaching an average body weight of approximately 130 g, hamsters were randomly assigned to one of the three cholesterol-enriched CLA diets or cholesterol-enriched control diet. The diet developed in our laboratory with the addition of 0·25 % cholesterol has been previously shown to induce hypercholesterolaemia in Syrian golden hamsters(Reference Harding, Zhao and Marinangeli11–Reference Spady and Dietschy13). Animals were provided food ad libitum for 28 d, with assessment and documentation of food intake occurring every third day, and body weight occurring weekly. At week 4, animals were placed in metabolism cages for the collection of 2 d faeces. On day 25, the energy expenditure of the animals was measured by indirect calorimetry for 14 min with the use of a respiratory gas exchange system specific for rodents (MM-100; CWE, Inc., Pennsylvania, PA, USA). Energy expenditure was expressed as O2 consumption/g body weight. On day 28, animals were anaesthetised with isoflurane, and blood samples collected by cardiac puncture. Subsequent to killing by removal of the heart under anaesthesia, livers were excised, weighed and frozen in liquid N2 for storage at − 80°C. The body composition of the animals was determined by dual-energy X-ray absorptiometry using General Electric's Lunar Digital Prodigy Advance which was set up to measure small mammals(Reference Harding, Zhao and Marinangeli11). The University of Manitoba's Animal Care Committee in accordance with the Canadian Council on Animal Care Guidelines approved all procedures conducted on the animals.
Laboratory analysis
Serum measurements
Blood was collected into uncoated blood collection tubes, centrifuged at 1500 rpm for 20 min to separate blood cells from serum and stored at − 80°C until analysis. Serum concentrations of glucose, TAG, total cholesterol and HDL-cholesterol, and enzymes that reflect liver function (alanine transaminase, aspartate transaminase and γ-glutamyl transpeptidase) were measured on a VITROS® 350 Chemistry System (Ortho-Clinical Diagnostics, Raritan, NJ, USA). Non-HDL-cholesterol, which accounts for the sum of VLDL-, LDL- and intermediate-density lipoprotein (IDL)-cholesterol, was calculated by subtracting HDL-cholesterol from total cholesterol in blood.
Hepatic lipid content
Total hepatic lipids were extracted with chloroform–methanol (2:1, v/v) using the method of Folch et al. (Reference Folch, Lees and Sloane Stanley14). Further, commercially available enzymic kits were used to measure hepatic concentrations of TAG (Triglycerides/GB) and cholesterol (cholesterol oxidase–p-aminophenazone (CHOD-PAP) method) according to the manufacturer's instructions (Roche Diagnostics, Laval, QC, Canada).
Faecal fat content
Total faecal fat content was measured in lyophilised and powdered faeces as previously described(15). Briefly, faecal samples were subjected to acid hydrolysis with 4 m-HCl for 30 min (Soxtec System 1047 Hydrolysing Unit; Tecator, Inc., Höganäs, Sweden), followed by total lipid extraction with anhydrous diethyl ether (Soxtec System HT 1043 Extraction Unit; Tecator Inc.).
Statistical analysis
Data were analysed with SPSS (version 11.5 for Windows; SPSS Inc., Chicago, IL, USA). Means of the different treatment groups were compared using one-way ANOVA followed by Tukey's post hoc test to identify averages that were significantly different from one another. Results were considered to be statistically significant at P ≤ 0·05. All data are presented as mean values with their standard errors.
Results
Effect of conjugated linoleic acid supplementation on daily food intake
Hamsters fed the t10, c12-CLA-supplemented diet consumed less food compared with control animals (P = 0·024). No significant differences were observed in the average daily food intake between the control and the two other CLA diet groups, nor between all three CLA groups (Table 4).
Table 4 Food intake, oxygen consumption, body weight and body composition of hamsters following 28 d of experimental diets
(Mean values with their standard errors for fifteen animals per group)

c9, t11, cis-9, trans-11; CLA, conjugated linoleic acid; t10, c12, trans-10, cis-12; t8, c10, trans-8, cis-10.
a,b,c Mean values within a row with unlike superscript letters were significantly different (P < 0·05).
* Eleven or twelve animals per group.
Effect of conjugated linoleic acid supplementation on body weight and body composition
Results on body weight and body composition are presented in Table 4. The three different CLA treatments supplemented at 2 % of the diet did not have an impact on the body weight of the hamsters compared with that of the control. Additionally, no significant differences were found between the body weights of animals fed the three CLA diets. Hamsters in the t10, c12-CLA group exhibited 18 and 13 % higher lean body mass compared with that of the control and the c9, t11-fed animals, respectively (P < 0·001). The body fat mass of the hamsters fed the hypercholesterolaemic diet supplemented with the t10, c12 isomer was found to be lower compared with that of all the other diet groups (P < 0·0001). Notably, hamsters in the t8, c10+c9, t11-CLA mixture group had lower body fat mass compared with that of the control (P = 0·022).
Effect of conjugated linoleic acid supplementation on energy expenditure
Compared with the control diet, the CLA diets had no significant effect on energy expenditure in the hamsters. Similar results were obtained when the effects of the three different CLA diets were compared with one another. These data are presented in Table 4.
Effect of conjugated linoleic acid supplementation on blood glucose
Results on blood glucose levels following CLA supplementation (Table 5) show that there were no differences in serum glucose concentrations between the control and three CLA-enriched diets (P = 0·973). In addition there were no significant differences between the CLA diets.
Table 5 Concentrations of serum glucose, and serum, liver and faecal lipids in hamsters following 28 d of experimental diets
(Mean values with their standard errors for fifteen animals per group)

c9, t11, cis-9, trans-11; CLA, conjugated linoleic acid; t10, c12, trans-10, cis-12; t8, c10, trans-8, cis-10.
a,b,c Mean values within a row with unlike superscript letters were significantly different (P < 0·05).
* Thirteen to fifteen animals per group.
† Mean faecal fat content from faeces collected on day 26.
‡ Coefficient of digestibility = (fat intake − fat excreted)/fat intake.
Effect of conjugated linoleic acid supplementation on serum lipid profiles
Treatment effects on serum lipids presented in Table 5 show that feeding hamsters for 28 d with different CLA-supplemented diets had no significant effect on serum concentrations of TAG, total cholesterol or HDL-cholesterol. However, animals in the t10, c12-CLA group exhibited higher concentrations of non-HDL-cholesterol (sum of VLDL-, LDL- and IDL-cholesterol) compared with the c9, t11-CLA-fed animals (P = 0·018).
Effect of conjugated linoleic acid supplementation on liver weight and hepatic lipids
Data on the effect of the four different diets on liver parameters are presented in Tables 5 and 6. Hamsters fed the t10, c12-CLA-supplemented diet had livers that were larger compared with those of animals fed either the control or the two other CLA diets (P < 0·0001). Liver weights of animals in the control, c9, t11-CLA and t8, c10+c9, t11-CLA mixture groups did not significantly differ from one another. No significant effects of CLA supplementation on hepatic total TAG and cholesterol concentrations were observed in comparison with the control.
Table 6 Liver weights and serum concentrations of hepatic enzymes of hamsters following 28 d of experimental diets
(Mean values with their standard errors for fifteen animals per group)

c9, t11, cis-9, trans-11; CLA, conjugated linoleic acid; t10, c12, trans-10, cis-12; t8, c10, trans-8, cis-10; BW, body weight; ALT, alanine transaminase; AST, aspartate transaminase; GGT, γ-glutamyl transpeptidase.
a,b Mean values within a row with unlike superscript letters were significantly different (P < 0·05).
Effect of conjugated linoleic acid supplementation on hepatic enzymes
The effects of CLA supplementation on liver enzymes are presented in Table 6. Only serum alanine transaminase concentration was higher (P < 0·0001) in the t10, c12-CLA-fed animals when compared with the control and the other CLA treatments.
Effect of conjugated linoleic acid supplementation on faecal fat content
Results on the effect of CLA supplementation on faecal fat content are presented in Table 5. Faecal fat contents of hamsters in the t10, c12- and the t8, c10+c9, t11-CLA diet groups were not different compared with the control. However, the faeces of c9, t11-CLA-fed animals contained a lower amount of fat (% DM basis) compared with controls, as well as animals fed the two other CLA diets (P < 0·0001). We also observed that these animals had higher fat digestibility ((fat intake − fat excreted)/fat intake) compared with all the other groups (P < 0·001).
Discussion
The present study provides evidence that provision of a t8, c10+c9, t11-CLA mixture may aid in fat-mass reduction in hamsters when supplemented at 2 % (w/w) of a hypercholesterolaemic diet. To our knowledge, this is the first evidence for a body composition-altering activity of this isomeric mixture. Data on the physiological effects of the t8, c10 isomer are limited, since interest in the activity of CLA has been directed exclusively towards the c9, t11 and the t10, c12 isomers thus far. Evidence in support of the effects of c9, t11 and the t10, c12 isomers on body composition has emerged mainly from studies in animal models(Reference Park and Pariza16), with results in human subjects being inconsistent and not as marked as those observed in vivo. Similarly, CVD risk factors including blood lipid and lipoprotein levels have been shown to be less responsive to CLA supplementation in human subjects(Reference Desroches, Chouinard and Galibois17–Reference Venkatramanan, Joseph and Chouinard19). While these discrepancies might be a reflection of variations in experimental conditions, it has also been suggested that the numerous minor isomers that are present in commercially prepared CLA mixtures might potentially exert a metabolic influence. It was demonstrated a few years ago that the c9, t11-CLA in fats and oils isomerises into an equimolar mixture of c9, t11 and t8, c10 isomers when heated under specific conditions(Reference Destaillats, Japiot and Chouinard6). We recently showed that Syrian golden hamsters fed a semi-purified diet supplemented at 2 % (w/w) concentration with a commercially purchased t8, c10+c9, t11-CLA mixture did not result in significant changes in either body weight or body composition compared with LA control and c9, t11+t10, c12-CLA mixture-fed animals(Reference Bissonauth, Chouinard and Marin7). Rather, it was observed that animals fed the t8, c10+c9, t11-CLA mixture had increased levels of VLDL-TAG, VLDL-cholesterol and glycaemia(Reference Bissonauth, Chouinard and Marin7).
In the present study, the t8, c10+c9, t11-CLA-fed hamsters carried significantly lower body fat mass compared with the control animals. This difference in the present results may partly be explained by the differences in experimental conditions, including the type of control diet that was used (LA-control(Reference Bissonauth, Chouinard and Marin7)v. no LA-control), source of CLA (commercial(Reference Bissonauth, Chouinard and Marin7)v. laboratory-synthesised resulting in slightly different isomeric profiles), the level of dietary fat (10 %(Reference Bissonauth, Chouinard and Marin7)v. 5 %), and the use of lard and safflower-seed oil as the fat source, compared with lard only in the previous study(Reference Bissonauth, Chouinard and Marin7). In any case, it is to be noted that the present results may be attributable to the presence of all the fatty acids in the diet and not CLA alone. The present results on fat-mass reduction for the t8, c10+c9, t11-CLA group cannot be explained by differences in food intake, energy expenditure or digestibility of dietary fat. However, the results could be partly explained by the slight reduction of saturated fats in CLA diets compared with the control diet. It is also possible that t8, c10+c9, t11-CLA provoked a specific response on body fat. In this respect, in vivo measurements of hormones including leptin and adiponectin, and measurements at the adipocyte level, such as activity of lipoprotein lipase and enzymes involved in lipolysis, as well as PPAR-γ-targeted genes, would help to elucidate the mechanism by which the t8, c10+c9, t11-CLA mixture exerts its effects. We also observed that hamsters fed the t10, c12-CLA-supplemented diet had lower body fat mass compared with all the other experimental groups. This is in agreement with previous data showing that the t10, c12 isomer decreases fat accumulation in hamsters(Reference Navarro, Zabala and Macarulla20–Reference Tarling, Ryan and Bennett24). The t10, c12-CLA-supplemented animals also had higher lean body mass compared with the animals consuming the control and c9, t11-CLA diets. It has been reported that CLA has the ability to increase lean mass in rodent models, either in the form of a mixture(Reference Park, Albright and Liu25–Reference Park, Albright and Storkson27) or as pure isomers(Reference Halade, Rahman and Fernandes28, Reference Halade, Rahman and Fernandes29); the present results are congruent with these data. Mechanisms by which t10, c12-CLA may decrease body fat include inhibition of sterol regulatory element-binding protein-1 expression leading to decreased lipogenesis, induction of apoptosis of adipocytes, and influence on preadipocyte differentiation via reduced expression of PPAR target genes(Reference Silveira, Carraro and Monereo30). However, mechanisms involved in increasing lean body mass with CLA remain unclear(Reference Rainer and Heiss31).
In addition to exerting an influence on adipocyte metabolism, and adipokines such as leptin and adiponectin, CLA may elicit effects on body fat mass by increasing fatty acid β-oxidation and energy expenditure(Reference Park and Pariza16). In the present study we did not observe a difference in energy expenditure across the four experimental groups. Previous studies have shown that reduced whole-body TAG content and fat mass in rodents occurs independently of energy intake(Reference DeLany, Blohm and Truett26, Reference Bouthegourd, Even and Gripois32). In the present study, however, we observed a decrease in food intake with the t10, c12-CLA group compared with the control animals. CLA may also reduce body fat mass by decreasing fat digestibility leading to increased faecal fat output, but we observed no difference in faecal fat content or fat digestibility of the t10, c12-CLA and the t8, c10+c9, t11-CLA-fed animals compared with control. These results suggest that other mechanisms are responsible for the observed decrease in body fat mass, which need further exploration and verification.
CLA has been shown to affect cardiovascular risk markers such as circulating concentrations of TAG, and total and lipoprotein cholesterol, although the evidence in animals(Reference McLeod, LeBlanc and Langille33, Reference Mitchell and McLeod34) and human subjects(Reference Nestel35) remains varied and therefore inconclusive. In the present study we found that the t10, c12-CLA-fed animals had higher serum concentrations of non-HDL-cholesterol (sum of VLDL-, LDL- and IDL-cholesterol) compared with the c9, t11-CLA group. The present results are in contrast with those reported by previous studies showing that t10, c12-CLA supplementation in hamsters fed an atherogenic diet results in lower LDL-cholesterol levels when supplemented either in pure or mixture form at concentrations ranging from 0·5 % (w/w) to 1 % (w/w) of the diet(Reference Nicolosi, Rogers and Kritchevsky36, Reference Navarro, Macarulla and Fernández-Quintela37). However, the effect on non-HDL-cholesterol concentrations in the present study is in agreement with our previous data showing that the t10, c12 isomer supplemented at a level of 2 % (w/w) in the diet leads to higher levels of LDL-cholesterol(Reference Bissonauth, Chouinard and Marin38). Indeed, Liu et al. (Reference Liu, Wakefield and Joseph39) have recently shown in a dose–response experiment that a 2 % (w/w) t10, c12-CLA-supplemented diet resulted in significantly higher serum non-HDL-cholesterol concentrations in Syrian golden hamsters, an effect that was not observed in animals supplemented with the lower dose of 1 % (w/w) t10, c12-CLA. Our findings might therefore be explained by the higher concentration (2 %, w/w) of t10, c12-CLA used in the present study.
Our previous study with t8, c10+c9, t11-CLA showed that supplementation with this isomeric mixture induced higher blood glucose levels compared with LA-control and a c9, t11+t10, c12-CLA mixture in hamsters(Reference Bissonauth, Chouinard and Marin7). Results of the present study fail to support those data; no significant effect of the diets on blood glucose levels was observed across the four experimental groups. However, in the present study, circulating glucose levels remained within the normal range for hamsters(Reference Field and Sibold40). These differences may be explained by the differences in total fat content, as well as the fatty acid profile of the diets. Specifically, Bissonauth et al. (Reference Bissonauth, Chouinard and Marin7) used a lard-based (10 %) control diet which itself increased fasting blood glucose levels in the hamsters. However, the basal diets in the present study contained only 5 % total fat composed of an equal mixture of lard and safflower-seed oil.
The safety of CLA supplementation is constantly questioned. Some studies have demonstrated detrimental physiological effects including hepatic dystrophy in mice(Reference Poirier, Niot and Clement9, Reference Clement, Poirier and Niot10) subsequent to CLA consumption. In the present experiment, we observed that liver weights of animals fed t10, c12-CLA were higher compared with those of hamsters in the other groups. Studies in mice have established that t10, c12-CLA supplementation results in hepatomegaly due to severe steatosis(Reference Poirier, Niot and Clement9, Reference Clement, Poirier and Niot10, Reference DeLany, Blohm and Truett26, Reference Tsuboyama-Kasaoka, Takahashi and Tanemura41) mainly as a result of lipogenesis leading to increased TAG accumulation in the liver(Reference Ide42, Reference Takahashi, Kushiro and Shinohara43). However, not all animal species might respond in a similar manner. Hamsters fed the t10, c12 isomer are known to exhibit heavier livers resulting not necessarily from an increase in hepatic lipids, but from a greater number of hepatocytes(Reference de Deckere, van Amelsvoort and McNeill44–Reference Miranda, Fernández-Quintela and Churruca46). In keeping with these data, we found no significant difference in the amount of hepatic lipids (TAG and cholesterol) across different treatment groups. We also observed an increase in the concentration of serum alanine transaminase, a marker of liver necrosis, in hamsters fed the t10, c12 isomer. A previous study in Syrian golden hamsters, in which liver function tests were conducted, found no changes in the relevant hepatic enzymes due to CLA feeding(Reference Macarulla, Fernandez-Quintela and Zabala45). This difference in results might be explained by the three-fold lower concentration of CLA that was used (0·5 %, w/w) compared with the present study (2 %, w/w). However, these negative effects on the liver were not observed with the t8, c10+c9, t11-CLA mixture even when supplemented at 2 % (w/w) of the diet, and were similar to findings in the control and c9, t11-CLA-fed animals.
In conclusion, results suggest that feeding t8, c10+c9, t11-CLA under the current experimental conditions leads to lower fat mass in hamsters, without exerting the deleterious effects on blood lipids and liver observed with t10, c12-CLA supplementation. However, further experiments are needed, to not only elucidate the mechanistic aspects of t8, c10+c9, t11-CLA activity, but also to resolve safety issues, before undertaking human clinical trials.
Acknowledgements
The present study was supported by funds from the Advanced Foods and Materials Network, Canada.
The contributions of each author are as follows: S. V. J. was the doctoral student who contributed to the study design, conducted the animal trial, performed laboratory work, analysed data, and wrote the manuscript. X. L. was the master's student who conducted the animal trial and performed laboratory work. A. W. contributed to the study design and conducted the animal trial. P. Y. C. was a co-investigator and prepared the t8, c10+c9, t11-CLA mixture. P. J. H. J. and H. A. were grant holders and co-investigators, and major contributors to the study design. H. J. was the principal investigator and a grant holder, and played a major role in the design and execution of the study, interpretation of results, and writing of the manuscript. All the authors contributed to reviewing and editing of the manuscript.
The authors have no conflicts of interest to declare.