INTRODUCTION
Salmonella is a major cause of gastrointestinal infection in England and Wales with 38 490 cases confirmed by the Health Protection Agency (HPA) Laboratory of Enteric Pathogens [now Laboratory of Gastrointestinal Pathogens (LGP)] from 2004 to 2006 [Reference Kafatos1]. During this time, 77·7% of infections were caused by the following: S. Enteritidis, 22 829 (59·3%); S. Typhimurium, 4479 (11·6%); S. Newport, 1072 (2·8%); S. Virchow, 1070 (2·8%); and S. Stanley, 474 (1·2%) [Reference Hutton2]. Laboratory reporting under-ascertains Salmonella infections within the community by at least a factor of 3 [Reference Wheeler3], the burden of illness caused by Salmonella is therefore considerable. In 2005, there were 11 983 cases of non-typhoidal Salmonella, 1200 hospitalizations and 100 deaths [4].
Salmonella enterica serovar Typhimurium (S. Typhimurium) is considered a broad host-range serovar, as it is associated with disease in numerous species, including humans, livestock, domestic pets and wildlife. It is also isolated frequently from foodstuffs and the environment [Reference Old, Thurston, Collier, Balows and Sussman5].
S. Typhimurium has previously been isolated from cattle, sheep, pigs and poultry in England and Wales [6] and this diversity of reservoirs is reflected by outbreaks associated with the consumption of raw milk in England [Reference Ashraf7], lamb kebabs and yoghurt relish in Wales [Reference Evans8], Roma tomatoes in Pennsylvania [Reference Sandt9], and a veterinary surgery clinic in New York state [Reference Cherry10]. Investigations worldwide have suggested that S. Typhimurium can contaminate a variety of fresh produce via a number of ways [Reference Islam11, Reference Islam12], and that it can cause illness in those consuming fresh produce, as evidenced in 1999 in Canada, where illness was associated with the consumption of clover sprout seeds [Reference Brooks13], and an outbreak in England in 2000 attributed to consumption of lettuce [Reference Horby14]; as well as pork and pork products in Switzerland [Reference Schmid15], and pork products in Denmark [Reference Ethelberg16]. Similarly, contaminated peanut butter and peanut butter-containing products have been associated with an outbreak involving 666 case-patients across 45 states in the USA [17].
Between 21 February and 19 August 2008 the HPA's LGP confirmed 171 primary indigenous case-patients of S. Typhimurium phage type (PT) U320 infection in England, Wales and Northern Ireland. The laboratory described this newly defined phage type as fully antimicrobial-sensitive and plasmid free. We investigated the outbreak of the new strain to identify its source, so that the outbreak could be controlled.
METHODS
Case definition and identification
A case was defined as a resident in England, Wales or Northern Ireland infected with S. Typhimurium PT U320 as confirmed by the LGP.
Case-patients were excluded if they had underlying gastrointestinal conditions, if they had travelled in the week prior to onset of illness, or if a household member or someone with whom they had close contact had diarrhoea in the week prior to their illness.
Notification of a patient from LGP prompted an initial follow-up call to the referring General Practitioner to acquire the onset date, travel history in the week prior to illness, hospitalization status, post code and contact telephone number.
Hypothesis generation
Between 12 and 14 March 2008, 11 newly identified case-patients were interviewed by telephone with a standardized 7-day retrospective hypothesis-generating trawling questionnaire to identify possible foodborne and environmental risk factors for further investigation. These case-patients were excluded from recruitment into the case-control study. Food items mentioned by two thirds of the case-patients were included in the standardized case-control questionnaire.
Epidemiological investigation
A national case-control study was initiated on 18 March 2008 to test the hypothesis that S. Typhimurium PT U320 infection was associated with the food groups listed below. Case-patients were defined as primary indigenous symptomatic residents of the UK, infected with a plasmid-free strain of S. Typhimurium PT U320, whose isolate had been received and confirmed by the LGP or the Scottish Salmonella, Shigella and Clostridium difficile Reference Laboratory (SSSCDRL) after 1 March 2008, with no history of foreign travel or close contact (including household) with other individuals with diarrhoea in the 7 days before onset. Case-patients were selected chronologically, after the decision had been made to conduct the case-control study, and up to 10 attempts were made to contact them by telephone before the next case-patient was recruited. Case-patients were identified through enhanced notification, such that a request had been circulated to all Health Protection Units to ensure that all Salmonella isolates were referred to LGP.
A control was defined as a resident of the UK who had not had diarrhoea, not travelled outside the UK, nor had close contact with other individuals with diarrhoea in the 7 days prior to interview. Two controls were recruited for each case, utilizing progressive digit-dialling based on the case-patient's telephone number by adding ‘3’ to the telephone numbers attempted; To minimize selection bias, we requested an interview with the person in the household with the next birthday in the house. If the next birthday was that of a child aged <16 years, the parent or guardian was asked to respond on their behalf.
For each food item consumed (Table 1), the product name and provenance was sought. We enquired about shopping habits in the 2 weeks prior to interview. Identical questions were posed to controls for the 3-day period or 2 weeks (shopping habits) prior to interview. Epidemiologists from the Department of Gastrointestinal, Emerging and Zoonotic Infections (GEZI) interviewed all case-patients and controls, and responses were double-entered onto a bespoke MS Access outbreak database.
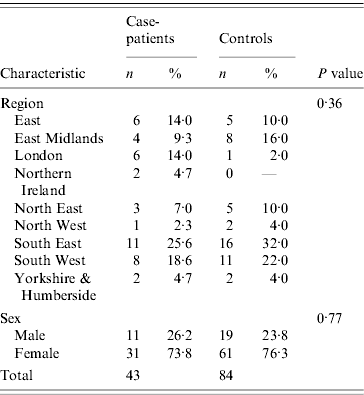
Table 1. Characteristics of case-patients and controls recruited to the Salmonella Typhimurium PTU320 study, England, March 2008
Sample size calculations, conducted prior to the start of the case-control study, suggested that 60 case-patients and 120 controls were required to detect a statistically significant (i.e. 95%) odds ratio of 3·0 with 80% power, assuming 16% exposure in controls. After the start of the study, exposure data from a previous national outbreak of S. Bareilly [18], specific to the hypotheses under investigation in this outbreak, was made available to the investigators. This suggested that exposure in controls was more likely to be around 3%, and repeated sample size calculations revealed that only 12 case-patients and 24 controls would need to be recruited successfully to attain sufficient statistical power.
Statistical analysis
The two datasets were compared using EpiCompare™ and discrepancies checked and corrected in a master dataset. Statistical analysis was conducted using Stata version 10.0 (StataCorp., USA). Age was calculated from the dates of birth given at interview. Demographic details for case-patients and controls were compared using Student's t test and χ2 test. Crude odds ratios (OR), with 95% confidence intervals (CI) were calculated for all exposures and risk factors. While the proportion of males and females did not differ between case-patients and controls, controls tended to be older than case-patients (t test P⩽0·01), therefore ORs were calculated adjusted for age only, where models adjusting for age included only one food item at a time.
A review of the data demonstrated that both case-patients and controls used the variables ‘bagged lettuce’, ‘mixed leaves with herbs’, and ‘other mixed leaves’ interchangeably, i.e. the same item was reported in both categories by different interviewees. Therefore, a new variable was created (‘bagged salad’) to represent exposure to any of these variables.
Exposures positively associated with being a case-patient with a P value of <0·25 from univariate analysis were selected for further investigation [Reference Hosmer and Lemeshow19]. Logistic regression was applied and controlled for age and the a priori confounding effect of gender. All variables were included in the model initially, with backwards stepwise exclusion of variables using the likelihood ratio test with a cut-off of P=0·05 until only significant variables remained. Interactions between retail shopping habits and food exposures significant at the univariate level but not included in the final logistic regression model were examined.
Environmental investigation
A meeting was held with retail chain A and the Food Standards Agency, and they reviewed their records surrounding the period of production for the egg sandwiches following our discussions. As a vehicle of transmission had not been proven with microbiological testing, no actions could be taken beyond this review.
RESULTS
Between 21 February and 19 August 2008, 171 case-patients infected by S. Typhimurium PT U320 infection were identified in England, Wales and Northern Ireland. This was a newly defined strain, previously designated ‘reacted but did not conform’ (RDNC). These strains are isolated sporadically by the LGP, and without testing every single RDNC retrospectively against the new pattern, it is impossible to determine whether there were historical averages with which to compare this increase in incidence. However, there had been no previous increase of RDNC strains during the same time in previous years. Three case-patients were known to be secondary case-patients, and two had travelled outside the UK in the week prior to illness so were excluded from further analysis. Four asymptomatic case-patients and 12 with underlying conditions were also excluded on the basis of unobtainable onset dates; 150 case-patients were included.
Of the 140 case-patients reported in England, 105 (70%) were reported in the South East, London and East of England and South West regions. Five were identified from Wales, and one from Northern Ireland. Ages of case-patients ranged from 5 months to 92 years (median=46) and 1·7 times more women were affected than men (93 women, 55 men, two unknown). This differs from the gender ratios for all S. Enteritidis at the same time (0·98; t test=3·19, P⩽0·01). Onset dates were available for 116 case-patients, and ranged from 1 January to 9 May 2008. The shape of the epidemic curve (Fig. 1) showed a sudden increase in cases-patients, with 88 (77%) case-patients experiencing onset of symptoms between 16 February and 1 March, suggesting a point-source outbreak.
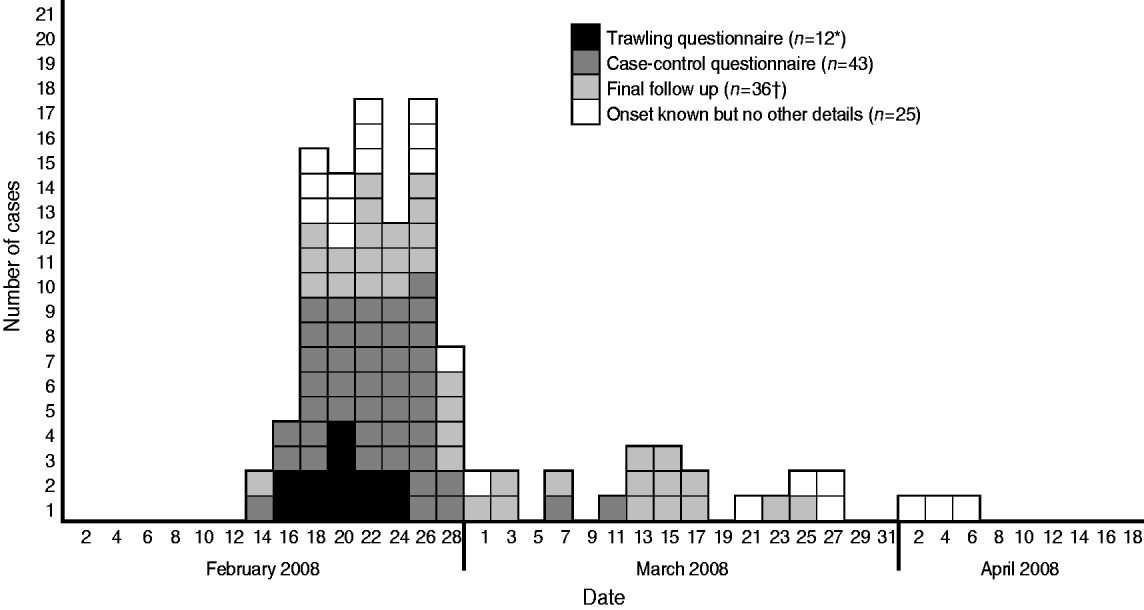
Fig. 1. Epidemic curve showing the onset dates for 115 case-patients with disease caused by S. Typhimurium PTU320 in England, February–May 2008 (one case reported onset of 1 January and is not shown). * Data from one trawling questionnaire had not been entered at the time of the decision to study and the results were not altered by its late inclusion. † One case involved in the final follow-up had previously been interviewed as a case.
Hypothesis generation
Food exposures reported by at least two thirds of the 11 interviewed case-patients were: dairy products other than milk or cheese (n=10), fruit (n=9), hot poultry dishes (n=9), minced beef dishes (n=9), salad vegetables (n=9), pre-packaged sandwiches (n=7), habitually shopping at retail chain A (n=8) or retail chain C (n=7). Eggs were not eaten by at least two thirds of the 11 case-patients. These food items and shopping habits were more frequently selected by case-patients than expected when compared to food consumption and shopping habit data from a previous S. Bareilly case-control study [Reference Cowden20].
Case-control study
Forty-three case-patients and 84 controls were recruited to the case-control study, which was conducted from 18 March to 7 April 2008. Two case-patients were ineligible as their onset of symptoms was prior to 1 February 2008, the earliest date selected to limit recall bias. One control aged <16 years was recruited and the parent/guardian answered questions on their behalf. Telephone numbers were recorded for 65 (77%) controls, allowing the calculation that a median of 11 telephone calls (range 1–183, mean 21, interquartile range 4–25) were attempted before a control was successfully interviewed. In four instances, 100 telephone calls were made prior to successfully conducting a control interview. For 39 interview sets, where dates of interview were available for the case and at least one control (dates of interview for both controls were available for 36 sets), an average of 29 days elapsed between the onset of disease for the case and the interview date for their corresponding controls (median 28 days).
Case-patients' onset dates ranged from 6 February to 13 March 2008 (Fig. 1). Diarrhoea (100%), abdominal pain (84%), nausea (77%) and fever (77%) were the most commonly reported symptoms. Half of the case-patients reported vomiting (53%) and a third of them (35%) reported blood in stools. The median duration of illness was 9 days (range 0–42 days) and seven case-patients were admitted to hospital (16%). The mean age of case-patients and controls was 40 and 54 years, respectively (P=0·004; median 47 and 55). In terms of gender, 25·6% case-patients were men (95% CI 14·3–40·1) and 22·6% of controls were men (95% CI 14·6–32·5) (Table 1). The mean number of days between onset of symptoms and case interview was 27·8 days.
Univariate analysis
Case-patients were more likely to have consumed bagged salad and pre-packaged egg sandwiches during the 3-day exposure period compared to controls (Table 2). More case-patients than controls reported the consumption of minced beef, but the difference was not significant.
Table 2. Association between food items, risk factors and Salmonella Typhimurium PTU320 infection: case-control study, England, March 2008 (n=number of people reporting exposure)
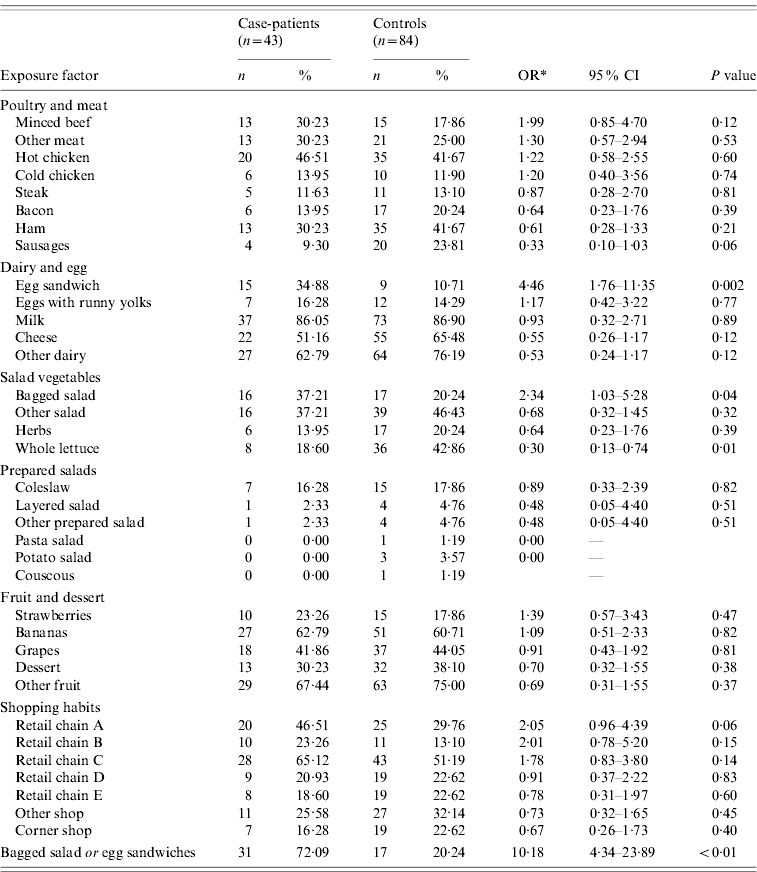
* Odds ratio adjusted for age.
Thirty-seven per cent of case-patients consumed bagged salad, 35% consumed pre-packaged egg sandwiches. Of the 16 case-patients who reported consuming bagged salad, 44% also reported consumption of egg sandwiches, while of the 15 case-patients that ate egg sandwiches, 47% also ate bagged salad. Thirty-one case-patients (72%) reported either exposure while seven case-patients (16%) reported both exposures.
Case-patients were less likely to have consumed whole lettuce than controls and the difference in exposure to sausages between case-patients and controls approached significance (OR 0·33, 95% CI 0·10–1·03, P=0·06). In terms of shopping habits, case-patients were more likely to have purchased food from retailer A in the 2 weeks prior to interview than controls.
Multivariate analysis
The effects of consumption of bagged salad, pre-packaged egg sandwiches and minced beef on being a case were investigated further using logistic regression, while controlling for age and gender. Consumption of pre-packaged egg sandwiches (adjusted OR 3·29, 95% CI 1·19–9·09) was significantly associated with illness. The exposures to egg sandwiches and bagged salad were not strongly correlated (P=0·15).
Source investigations
Of the case-patients and controls who reported exposure to pre-packaged egg sandwiches, eight out of 15 case-patients purchased their egg sandwich from retail chain A (53·3%) whereas none of the eight controls who could recall where they purchased their sandwiches reported consuming sandwiches from this retailer (χ2=7·20, P⩽0·01). Due to the null exposure in controls it was not possible to include these data in the final logistic regression model as a fitted effect. However, the lower limit for the odds ratio for the above was estimated as 2·22.
It was not possible to perform similar calculations for bagged salad due to the variety of goods available and the quality of the descriptions of the provenance and brand of the products reported by case-patients and controls.
The processing records of retail chain A and those of their suppliers did not indicate a problem during the processing of the egg sandwiches, although none of the food was available for microbiological testing. There were no identified anomalies, and the producers that they sourced raw materials from had not changed. Retail chain A is a speciality grocery store rather than a bulk shopping venue, and egg sandwiches from this store were anecdotally considered to be a ‘treat’.
Subsequent incidence
The last case investigated as part of this outbreak was reported on 23 April 2008. Since 19 August 2008, 11 case-patients have been reported, with the last case-patient identified on 22 September 2009. This gives a total of 182 case-patients in England, Wales and Northern Ireland reported since this outbreak was identified [21].
DISCUSSION
This investigation of an outbreak of a newly defined phage type of S. Typhimurium designated PT U320 identified a strong epidemiological association between the consumption of egg sandwiches and cases of disease. Not all of the cases of illness during this outbreak could be explained by consumption of egg sandwiches (35%), but consumption of either egg sandwiches or bagged salad was reported by 72% of case-patients, therefore it is hypothesized that the outbreak was caused by a salad ingredient that went into multiple, complex, ready-to-eat foods (Fig. 2), including the sandwiches. The absence of a significant association with consumption of bagged salad in the final logistic model can be explained by the likely wide dissemination of an individual ingredient across many ready-to-eat food products on the market. This variability in the products, and the amount of the individual ingredient spread across such a wide market decreased the likelihood of finding an association unless sample-size calculations included this assumption to create a study with sufficient power. Eggs were not implicated as the vehicle of this outbreak, despite being a common cause of Salmonella outbreaks.
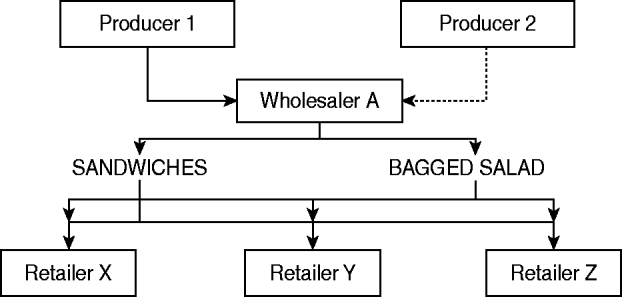
Fig. 2. Possible route of ingredients found at retailers of complex ready-to-eat foods.
Between 2003 and 2006, there were four national and nine local outbreaks of salmonellosis in England associated with the consumption of ready-to-eat fresh produce [21]. Items of food associated with these outbreaks include: pre-packaged egg and cress sandwiches [22], lettuce [Reference Irvine23] and sandwiches from one retailer [21]. Similarly, an outbreak of E. coli O157 infection was associated with chicken wraps based on epidemiological results rather than microbiological findings [Reference Whittaker24].
Although several outbreaks have been linked to raw fruit and vegetables, the original source of these outbreaks are seldom identified. A large school dinner-related outbreak of E. coli O92:H– infection in Denmark in 2006 was associated with freshly made pesto, where imported basil was believed to have been the source, although as none was left over for testing, this could not be confirmed [Reference Pakalniksiene25]. In Northern Ireland, investigations of an outbreak of S. Newport found epidemiological evidence of an association with lettuce but, again, there was no microbiological proof [Reference Irvine23].
This apparent increase in foodborne outbreaks relating to salad vegetables and fruit is not limited to the UK – similar events have been reported from Australia [Reference Dalton26], the USA [Reference Sivapalasingam27] and Japan [Reference Michino28]. In this outbreak, as with others, most of the case-patients were middle-aged women (⩾40 years) [29–31]. The open market across the EU means that concurrent illnesses have occurred in other European countries during outbreaks attributed to lettuce contaminated at source [Reference Long32]. This is despite food hygiene practices reflecting that Salmonella spp. should be absent in all ready-to eat salad vegetables, fresh herbs and fruit [33].
During the case-control study, previously unavailable information on sandwich consumption in the population was used to re-estimate the required sample size. Indeed, consumption of raw vegetables and salad is common and increasing within the population [34], requiring a higher level of power, or more case-patients and controls to be interviewed, to detect a difference in exposure between them at a multivariate level.
Selection bias was minimized using the next-birthday selection method, to randomly choose a person from the household for interviewing without increasing the likelihood of refusal and to reflect the case age distribution [Reference Beebe35, Reference Battaglia36]. However, the controls were older than the case-patients, perhaps attesting to the inadequacy of the application of the next-birthday selection method. Some controls refused to provide dates of birth, thereby the data were lost. This emphasizes the importance of collecting some marker of age during the interview, possibly asking the respondent to indicate an age range if birth date is unacceptable.
Retrospective case-control studies of laboratory-confirmed case-patients depend on their recall, the accuracy of which is affected over time. The median delay from illness onset to case interview was 39 days (range 17–67 days) due to the time taken to access a medical doctor or hospital, and delays in specimen submission, laboratory identification, reference typing and reporting. This means that case-patients were likely to have forgotten exactly what they had eaten in the days leading up to their illness. In most outbreaks, associations will tend to be underestimated as a proportion of case-patients will have forgotten that they had eaten the vehicle of infection. To optimize recall by controls, food exposure was restricted to the 3 days prior to the interview.
During analysis, bagged lettuce and mixed leaves were recoded, based on a review of the details that were provided by the interviewees. As this was conducted at the analysis stage, it had no bearing of how the case-patients or controls reported these exposures. However, it does raise the issue of salad nomenclature in regards to consumer perception of ‘bagged’ vs. ‘loose’ salad, e.g. ‘leaves’ vs. ‘whole’ lettuce and the affect of this to risk identification, management and communication. Recent guidance on the fresh produce terminology used by the food industry would assist in the investigations of future outbreak investigations of this kind, and facilitate risk communication of hazards which have arisen.
Vehicles in foodborne outbreaks in England are either identified via microbiological evidence (e.g. the bacteria is found in a sample of the food) or epidemiological evidence (e.g. a case-control or cohort study identifies an association between illness and a food vehicle). The vehicle is more frequently identified by analytical studies than microbiological evidence in prepared salad outbreaks (60%) than in outbreaks caused by dissemination of other food types (e.g. eggs) [17]. Identifying a fresh produce item incorporated into a variety of ready-to-eat foods as the vehicle of transmission requires an epidemiological investigation of substantial size; and unless there have been a large number of cases of illness, they are impossible to undertake. A number of factors militate against securing microbiological evidence of crop contamination in situ, these include: the short shelf-life of salad vegetables and herbs; complete harvesting (no samples for testing); the rapid production/distribution timescales; complex international distribution chains; heterogeneity of contamination levels (both in produce and the environment); the processing and inclusion in a diverse range of products (e.g. bagged salads, prepared salads, sandwiches and other ready-to-eat foods); and their distribution through a range of retailers and caterers. This means that contamination of a particular crop might lead to a number of different products becoming contaminated and these might be distributed over increasingly wide geographical areas (Fig. 2). Under these circumstances it is likely that different case-patients will have become infected through the consumption of different foods and that no single product is likely to have been eaten by all of the case-patients. These factors need to be taken into consideration when designing and analysing epidemiological studies which will, for the reasons outlined above, constitute the main body of evidence available to enforcement authorities. Identifying the specific ingredient that was contaminated is often extremely difficult.
The increasing requirement to make timely expert use of epidemiological evidence drawn from outbreak investigations to inform risk management poses a serious challenge to enforcement authorities. Recent outbreaks from around the world show that the outbreaks linked to contaminated salad vegetables, herbs and fruit can have a major public health impact [Reference Pezzoli37–Reference Emberland39]. Therefore seeking what is often elusive microbiological evidence of product contamination to support already established epidemiological evidence risks delaying the introduction of preventive measures which would protect people from becoming infected. Given the volumes of trade in these products there is an imperative to respond as quickly as possible to prevent large-scale avoidable morbidity and mortality. Furthermore, there is a clear need to ensure that the largely unpublished body of epidemiological evidence that has been growing over recent years is made accessible so that the public health impact posed by contaminated salad vegetables, herbs and fruit can be objectively assessed. Without taking due consideration of this body of evidence, health lobbyists and legislators may not be able to appropriately put into place procedures and policies designed to protect the health of the public.
ACKNOWLEDGEMENTS
This work was conducted within the remit of the provision of service of the Health Protection Agency. The authors thank K. Harker, C. Lane, S. LeBaigue for their contribution to data collection and T. Peters for conducting PFGE typing on isolates of S. Typhimurium PT U320 that were confirmed throughout this outbreak and the resulting investigation.
DECLARATION OF INTEREST
None.






