Implications
Selection for increased litter size in sows has also resulted in a disproportional increase in ovulation rate. The larger litter size has been associated with a lower birth weight and higher within litter variation in birth weight and consequently higher mortality rates during lactation, raising economic and welfare concerns. This paper shows that in multiparous sows, high ovulation rates results in an moderate increase in the number of viable embryos at day 35 of pregnancy, but compromise the placental development of the embryos surviving to day 35 and thus, may contribute to lower piglet birth weight.
Introduction
In pork production systems, an important aspect of profitability is a high number of piglets produced per sow per year. To accomplish this, pig breeding programs have been focusing on components such as litter size and pre-weaning mortality (Johnson et al., Reference Johnson, Nielsen and Casey1999). Selection for litter size has been shown to disproportionally increase ovulation rate (OR) and prenatal mortality (Johnson et al., Reference Johnson, Nielsen and Casey1999; van der Waaij et al., Reference Van der Waaij, Hazegeler, Soede, Laurenssen and Kemp2010; Vallet et al., Reference Vallet, McNeel, Miles and Freking2014) and OR of 25 to 30 are relatively common nowadays (Patterson et al., Reference Patterson, Wellen, Hahn, Pasternak, Lowe, De Haas, Kraus, Williams and Foxcroft2008; Wientjes et al., Reference Wientjes, Soede, Knol, van den Brand and Kemp2013). The increase in prenatal mortality with an increase in OR seems due to both an increase in pre-implantation and post-implantation mortality (van der Waaij et al., Reference Van der Waaij, Hazegeler, Soede, Laurenssen and Kemp2010). Pre-implantation mortality has been associated with embryonic heterogeneity within a litter; less developed embryos cannot develop further in a uterine environment that is advanced by the more developed embryos (reviewed by Pope et al., Reference Pope1990). As embryonic heterogeneity has been largely attributed to follicle heterogeneity (reviewed by Pope et al., Reference Pope1990), possibly sows with a high OR have a more heterogeneous follicle pool. Post-implantation mortality related with a high OR seems due to effects of intra-uterine crowding and associated competition for space and/or nutrients, both early post-implantation (Geisert and Schmitt, Reference Geisert and Schmitt2002) and throughout the remainder of pregnancy (Foxcroft et al., Reference Foxcroft, Bee, Dixon, Hahn, Harding, Patterson, Putman, Sarmento, Smit, Tse and Town2007). Several authors have found a relationship between placental weight and foetal weight at the end of gestation, indicating that foetal development is dependent on placental size (Van Rens, Reference Van Rens1989; Freking et al., Reference Freking, Leymaster, Vallet and Christenson2007) and Père et al. (Reference Père, Dourmand and Etienne1997) found that a high number of embryos at day 35 of pregnancy not only resulted in a higher foetal loss, but also resulted in a lower placental weight and foetal weight of the surviving embryos at 112 days of pregnancy. Thus, in a crowded uterus, placental growth is compromised, which subsequently limits foetal development. This intra-uterine crowding is apparent in high prolific sows as shown by the lower average piglet birth weight and increased variation in piglet birth weight in sows with a high litter size (Milligan et al., Reference Milligan, Fraser and Kramer2002; Quesnel et al., Reference Quesnel, Brossard, Valancogne and Quiniou2008). Therefore, if selection for litter size results in a substantial increase in OR, the associated high numbers of embryos in early gestation may negatively impact the growth of the surviving embryos and thereby result in a low piglet birth weight. As piglet birth weight and birth weight variation are important characteristics for piglet survival and further development (Milligan et al., Reference Milligan, Fraser and Kramer2002), a further insight in relationships between OR and foetal development and survival is warranted. Therefore, the aim of this experiment was to investigate the relationships between OR and embryonic mortality and embryonic and placental characteristics of multiparous sows at 35 days of pregnancy, to better understand mechanisms that lead to prenatal losses and reduced piglet birth weight in highly prolific sows.
Material and methods
Animals
Multiparous sows (parity 2 to 17, n=91), from one commercial farm and three different genetic backgrounds (sire line cross, n=46; purebred Landrace, n=17 and crossbred Yorkshire×Landrace, n=28; Topigs Norsvin, Vught, the Netherlands), were used.
Measurements
Sows were slaughtered at a local abattoir at day 35.0±0.1 (mean±SEM) of pregnancy and the uterus and ovaries of each sow were collected. OR was assessed by counting the number of corpora lutea on both ovaries. Both uterine horns were separated from the mesometrium and opened at the anti-mesometrial side. After opening the uterus, embryos were separated from their placentas and counted. Embryos were classified as vital or non-vital according to their visual appearance and were considered non-vital when there was a presence of haemolysed amniotic fluid, resorbed embryonic membranes, or both (Van der Waaij et al., Reference Van der Waaij, Hazegeler, Soede, Laurenssen and Kemp2010); and when there was evidence of implantation, combined with placental or embryonic remnants. The difference between OR and total number of embryos was considered as early embryonic mortality. The number of non-vital embryos was considered as late embryonic mortality.
The embryonic-placental units were separated from the uterine wall and all vital embryos and their placentas were individually weighed. In addition, placental length between the necrotic tips of the placenta was measured on a wet surface and also the length of the uterine horns was measured on a wet surface, from the utero-tubal junction to the uterine body. The length of each implantation site on the uterine wall was measured. Implantation sites were recognised by the reddening of the endometrium, compared to the whiter area (unoccupied space) in between. The middle of the implantation site was considered to be the embryonic position within the uterine horn. Embryonic spacing was determined as the distance between two embryonic positions. For the first embryo at the ovarian end, embryonic spacing was defined as the distance from the embryonic position to the utero-tubal junction. For the first embryo at the cervical end, embryonic spacing was defined as the length from embryonic position to the cervix. Furthermore, the length of the whiter, ‘unoccupied’, areas of the uterine wall on both sides of a embryonic-placental unit site were defined as the ‘empty space’ surrounding that embryo.
Statistical analyses
To analyse parity effects on several embryonic, placental and uterine characteristics, parity was divided into three categories: class 1 (parities 2 and 3, n=25), class 2 (parities 4 to 10, n=47) and class 3 (parities 11 to 17, n=19). Parity and genetic background were confounded as all second, third and fourth parity sows were from the same genetic background (sire line cross). Therefore, the effect of parity and genetic backgrounds could not be assessed simultaneously in the same model. To analyse effects of genetic background, only sows from parity class 2 (parities 4 to 10, n=47) were used. The fixed class effects of parity or genetic background on OR were each assessed using PROC GLM in SAS 9.3 (Proc. GLM; SAS Inst. Inc., Cary, NC, USA). Subsequently, to assess effects of parity or genetic background and OR on embryonic, placental and uterine characteristics, the fixed class effects of parity or genetic background and the fixed continuous effect of OR, and their interaction were assessed. The analysed characteristics were: embryo numbers (total, vital, non-vital, early embryonic mortality), average and standard deviation of vital embryonic and placental characteristics of the vital embryos (embryonic weight, embryonic spacing, implantation length, placental weight and length and empty spaces around each implantation site), and total uterine length. All characteristics were normally distributed, based on skewness and kurtosis analyses of variables and model residuals. Preliminary analyses demonstrated that the interaction between parity class and OR, and the interaction between genetic background and OR were never significant. Therefore, these interaction effects were excluded from the models. In addition, to check the linearity of the OR effects in the earlier models, OR was analysed as class variable (class 1 (range 17 to 21, n=20), class 2 (range 22 to 24, n=23), class 3 (range 25 to 28, n=24) and class 4 (range 29 to 38, n=24)), The interaction between OR classes and parity classes were never significant and were therefore excluded from the model.
To study if the effects on embryonic and placental characteristics were related to early or late embryonic mortality, early and late mortality were classified into four classes. For early embryonic mortality these classes were: class 1 (range −4 to 1, n=18), class 2 (range 2 to 3, n=21), class 3 (4 to 6, n=24) and class 4 (7 to 25, n=28) and for late embryonic mortality: class 1 (range 0 to 1, n=22), class 2 (range 2 to 3, n=27), class 3 (range 4 to 5, n=24) and class 4 (range 6 to 15, n=18). Early or late embryonic mortality class were included in a model together with the fixed class effect of parity and their interaction, and analysed in relation to embryonic spacing, implantation length, vital placental length and the empty spaces around embryos. Preliminary analyses demonstrated that the interaction between early embryonic mortality or late embryonic mortality classes with parity class were never significant. Therefore, the interactions were excluded from the models. Results are presented as LSMeans±SEM, and are considered significant at P⩽0.05.
Results
Descriptive statistics
Averages and standard deviations of the measured variables are presented in Table 1. Average OR was 25.5±5.0. The average total number of embryos was 20.1±5.2 and that of vital embryos was 16.4±3.9. The average number of non-vital embryos was 3.7±3.0. Average implantation length was 19.2±4.8 cm and the space between two embryos, or embryonic spacing, was 25.0±6.0 cm. The empty uterine space around each embryonic-placental unit was 15.4±7.1 cm and average placental length was 44.0±8.0 cm.
Table 1 Summary statistics for vital embryonic, placental and uterine variables from sows at 35 days of pregnancy

1 Number of corpora lutea that do not account for an embryo.
2 Distance between two embryos.
3 Total empty uterine space around each vital embryo-placental unit.
Parity and genetic background effects on ovulation rate
OR was lower in parity class 1 than in both other parity classes (P<0.001) and parity class 2 had a lower OR than parity class 3 (P=0.05, Table 2). OR was not affected by genetic background of the sows (P=0.93; Table 3).
Table 2 Effect of parity class and the ovulation rate on vital embryonic, placental and uterine characteristics in sows at 35 days of pregnancy
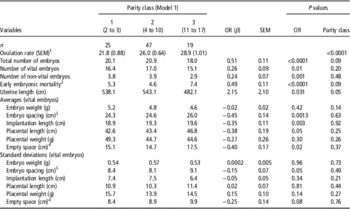
OR=ovulation rate.
1 Effect of parity classes on ovulation rate.
2 Number of corpora lutea that do not account for an embryo.
3 Distance between two embryos.
4 Total empty uterine space around each vital embryo-placental unit.
Table 3 Effect of genetic background class and the ovulation rate on embryonic, placental and uterine characteristics in sows at 35 days of pregnancy
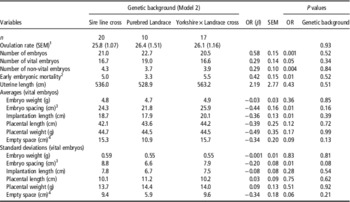
OR=ovulation rate.
1 Effect of genetic background classes on ovulation rate.
2 Number of corpora lutea that do not account for an embryo.
3 Distance between two vital embryos.
4 Total empty uterine space around each vital embryo-placental unit.
Effects of parity and ovulation rate on embryonic and placental characteristics
Parity class did not significantly affect any of the embryonic, placental or uterine characteristics when OR was included in the statistical model (see Table 2).
An increase in OR was significantly associated with an increase in number of vital embryos (β=0.26±0.09 n/ovulation, P=0.01), non-vital embryos (β=0.24±0.07 n/ovulation, P=0.001) and early embryonic mortality rate (β=0.49±0.11 n/ovulation, P<0.0001), as presented in Table 2. Figure 1 confirms the linearity of the relationships between OR class and number of vital embryos (Figure 1a), non-vital embryos or late embryonic mortality (Figure 1b) and early embryonic mortality (Figure 1c). The within-class relationships between OR and number of vital embryos were 1.24±0.5 n/ovulation (P=0.02), 0.10±0.4 n/ovulation (P=0.8), −0.15±0.3 n/ovulation (P=0.6) and 0.00±0.1 n/ovulation (P=0.9), for OR classes 1, 2, 3 and 4, respectively, indicating a relatively weak relationship between OR and number of vital embryos above an OR of 22.
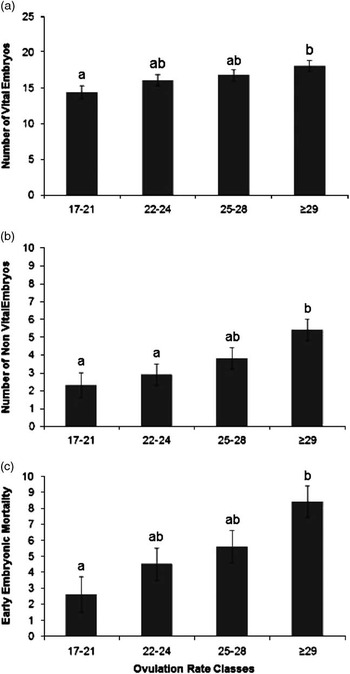
Figure 1 Estimated least square means for the effect of ovulation rate classes (number of corpora lutea (class 1, range 17 to 20, n=20; class 2, range 22 to 24, n=23; class 3, range 25 to 28, n=24 and class 4, range 29 to 38, n=24)), on number of vital embryos (a; P=0.05); number of non-vital embryos (b; P=0.01) and on early embryonic mortality incidence (c; P=0.01) at 35 days of pregnancy. Significant differences between classes are indicated by letters above the columns and the error bars indicated a single SE of the estimates.
An increase in OR was linearly associated with a decrease in vital embryonic spacing (β=−0.45±0.14 cm/ovulation; P=0.001), vital implantation length (β=−0.35±0.11 cm/ovulation; P=0.003), vital placental length (β=−0.38±0.19 cm/ovulation; P=0.05) and in the empty space around the implantation sites of the vital embryos (β=−0.40 cm/ovulation, P=0.02). However, there was no relationship between OR and average embryonic weight (P=0.42) or embryonic weight standard deviation (P=0.96) at day 35 of pregnancy. Figure 2 confirms the linearity of the OR relations with vital embryonic spacing, vital implantation length, vital placental length and empty uterine space around each vital embryo.
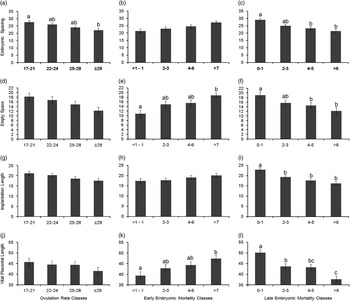
Figure 2 Estimated least square means for the effect of ovulation rate (OR) classes (number of corpora lutea; a, d, g and j), early embryonic mortality (EM) classes (corpora lutea that do not account for an embryo; b, e, h and k) and late embryonic mortality (LM) classes (evidence of implantation, combined with placental or embryonic remnants or both; c, f, I and l) on embryonic spacing (OR, P=0.003; EM, P=0.12; LM, P<0.0001) and empty space around embryonic-placental units (OR, P=0.06; EM, P=0.003; LM, P=0.02), on implantation length (OR, P=0.12; EM, P=0.08; LM, P<0.0001) and on vital placental length (OR, P=0.43; EM, P=0.01; LM, P<0.0001) at day 35 of pregnancy for the vital embryos. Significant differences between classes are indicated by letters above the columns and the error bars indicate a single SE of the estimates.
Effects of genetic background and ovulation rate on embryonic and placental characteristics
For sows with parity 4 to 10, genetic background did not significantly affect any of the measured embryonic, placental or uterine characteristics, when OR was included in the statistical analyses (Table 3). This limited dataset, therefore, shows similar relationships between OR and the number of vital embryos, non-vital embryos, total number of embryos and early embryonic mortality as in the model with parity class effect. In addition, a comparable negative relationship between the OR and average implantation length, average embryonic spacing and embryonic spacing standard deviation were found.
Relationships between early and late embryonic mortality with vital embryonic and placental characteristics
A higher incidence of early embryonic mortality tended to increase vital embryonic space (Figure 2b) and vital implantation length (Figure 2h) and increased vital placental length (Figure 2k) and empty uterine space around the vital embryos (Figure 2e). Figure 2 also shows that a higher incidence of late embryonic mortality was related with a decrease in these four parameters (Figure 2c, f, i and l).
Discussion
This study investigated relationships between OR and embryonic and placental characteristics at 35 days of pregnancy, aiming to clarify consequences at early pregnancy for litter characteristics at term. The results show that a higher OR in sows is related with a considerable increase in early and late embryonic mortality and only a moderate increase in number of vital embryos at day 35. Increased ORs also result in a compromised placental development in the vital embryos at day 35. The latter may cause reduced further growth and increased foetal mortality in later stages of pregnancy.
In the multiparous sows in this study, with ORs varying between 17 and 38, each extra ovulation represented an increase in the incidence of early embryonic mortality of 0.49. Many factors can explain this. First, one should consider that it might be related with fertilisation failure. Fertilisation rates are normally considered to be ~95% (Geisert and Schmitt, Reference Geisert and Schmitt2002), however, sows with a higher OR might have an earlier and higher rise in progesterone, which would affect sperm transport to the site of fertilisation and thereby reduce fertilisation rate, and/or would induce early embryo mortality (Day and Polge, Reference Day and Polge1968; Mao and Foxcroft, Reference Mao and Foxcroft1998; Soede et al., Reference Soede, Bouwman, van der Laan, Hazegeler, Jourquin, Langendijk and Kemp2012). The higher OR might also result in a higher early embryonic mortality related with a higher embryo diversity. This higher diversity might arise from an increase in follicular and oocyte diversity (reviewed by Pope et al., Reference Pope1990) or from a prolonged variation in ovulation time between oocytes, that might not only affect fertilisation rate, but also the time of fertilisation, contributing to embryo diversity (Soede and Kemp, Reference Soede and Kemp1993). Embryo diversity results in peri-implantation losses as the oestrogen production of the more developed embryos stimulate uterine secretions to their own benefit but creates an hostile environment for the less developed embryos (Pope, Reference Pope, Xie, Broerman and Nephew1992; Geisert and Schmitt, Reference Geisert and Schmitt2002), compromising their survival chances. Therefore, considering the relation between OR and early embryonic mortality, increased follicular heterogeneity and/or embryo diversity might account for part of the observed increase in the early embryonic mortality.
Besides an increase in early embryonic mortality, the current study also showed an increase in late embryonic mortality with an increase in OR of 0.24 non-vital embryos per extra ovulation at day 35 of pregnancy. The category of non-vital embryos included the sites in the uterus with evidence of implantation but without a vital embryo, so also sites with only placental remnants. It thereby gives an estimate of post-implantation mortality up to day 35 of gestation. This mortality presumably is related with competition for uterine space by the embryos. Around days 10 and 14 of pregnancy, embryos elongate and start to attach to the uterine wall. Further developed embryos will elongate quicker and get a larger implantation site (reviewed by Bazer et al. Reference Bazer, Spencer, Johnson, Burghardt and Wu2009), which will provide an increased placental surface area (Stroband and Van der Lende, Reference Stroband and Van der Lende1990). Foxcroft et al. (Reference Foxcroft, Wilson, Vonnahme, Town, Gourly, Wolf, Quirk-Thomas and Ford2000) showed that substantial embryonic losses occur between day 26 and 44 of pregnancy. Although placental attachment occurs around day 13 to 17, the placenta is not functionally complete until day 35 of pregnancy (reviewed by van der Lende et al., Reference Van der Lende, van Rens and Leenhouwers2000, Geisert and Schmitt, Reference Geisert and Schmitt2002). In the course of pregnancy, embryos become more dependent of their placenta for further growth, and placental functionality can therefore limit embryonic development and survival (reviewed by Vallet et al., Reference Vallet, McNeel, Miles and Freking2014). Thus, embryos that, due to the competition for space, have acquired a smaller implantation site and associated smaller placenta, might die during pregnancy in case of insufficient placental supply of nutrients. This process already takes place before day 35 of pregnancy, not only in animals with a very high OR (Van der Waaij et al., Reference Van der Waaij, Hazegeler, Soede, Laurenssen and Kemp2010; superovulation, 45 ovulations), but also in animals with a normal OR (Langendijk et al., Reference Langendijk, Chen, Athorn and Bouwman2012; 20 ovulations). In addition, Vonnahme et al. (Reference Vonnahme, Wilson and Ford2002) found a correlation between OR (average 26.6) and number of viable conceptuses at day 25 of pregnancy (r=0.50; P<0.0001), but not at day 36 of pregnancy (r=0.02; P=0.98) due to a further loss of embryos in sows with a high OR. Further, Van der Waaij et al. (Reference Van der Waaij, Hazegeler, Soede, Laurenssen and Kemp2010) also found a smaller implantation site and lighter and a shorter placenta in non-vital foetuses, which indeed suggests that limited uterine space was the cause of this mortality. In this study, the number of vital embryos at day 35 of pregnancy linearly increased by 0.26 with each extra ovulation, meaning that to achieve one more vital embryo at this stage at least four ovulations are needed. Further analyses – dividing sows in four classes of OR – showed hardly any increase in the number of vital embryos in sows in the OR classes above 21. Therefore, at this stage of pregnancy, the number of vital embryos seems to reach a plateau at about 17 embryos. At 40 days of pregnancy, Van der Waaij et al. (Reference Van der Waaij, Hazegeler, Soede, Laurenssen and Kemp2010) also found a plateau of about 17 vital foetuses in gilts with ORs varying between 20 and 50. Therefore, a further increase in OR does not result in higher embryo numbers at this early foetal stage of pregnancy. However, the increase in OR affected the development of the surviving embryonic-placental units at day 35 of pregnancy. The increase in OR was not related with a reduction in embryonic weight, but was related with a reduction in placental length and also with a reduction in embryonic spacing, in implantation length and in the empty spaces around each vital embryonic-placental unit. These results suggest that the development and survival of these vital embryos might become compromised in the remainder of pregnancy due to insufficient uterine space and reduced placental development (van der Lende et al., Reference Van der Lende, van Rens and Leenhouwers2000).
The observed vital embryonic and placental development at 35 days of pregnancy was found to be related to the incidence of early and of late embryonic mortality. We observed that sows with a high level of early embryonic mortality had a longer vital placental length and larger empty spaces around each embryonic-placental unit. This could indicate that sows that have a high OR followed by a high level of early embryonic mortality, provides the surviving embryos with more space and therefore a better opportunity to grow, as has also been shown by Van Rens (Reference Van Rens1989). We also observed that sows with a high level of late mortality had less foetal spacing and smaller empty spaces around each embryonic-placental unit, they had less implantation length and a shorter vital placenta length. This suggests that the increased late embryonic mortality is already a consequence of uterine crowding, in which the lack of space also compromises placental development of the – as yet – vital embryos. Such a decreased placental weight due to intra-uterine overcrowding was observed by Town et al. (Reference Town, Putman, Turchinsky, Dixon and Foxcroft2004) already at 30 days of pregnancy. Further, as the recently died embryos still occupy space that cannot or can hardly be used by the surviving embryos (Vallet et al., Reference Vallet, Freking and Miles2011), late embryonic mortality can also be seen as a cause, and not only a consequence, of the crowding conditions faced by the surviving embryos. Like in our study, Van der Waaij et al. (Reference Van der Waaij, Hazegeler, Soede, Laurenssen and Kemp2010) observed that embryonic-placental development and implantation length of vital embryos at day 40 of pregnancy was more related to late embryonic mortality than to early embryonic mortality. In contrast to our study, Van der Waaij et al. (Reference Van der Waaij, Hazegeler, Soede, Laurenssen and Kemp2010) also found a lower embryonic weight in gilts with a high level of late embryonic mortality. This difference may be related with the very high OR and associated levels of uterine crowding in the study of Van der Waaij et al. (Reference Van der Waaij, Hazegeler, Soede, Laurenssen and Kemp2010) (45 v. 25 CL in our study and 11.2 non-vital embryos v. 3.7 in our study). It could also be related with the somewhat later evaluation time (day 40 v. day 35 in our study).
The present study did not show relationships between OR and embryonic uniformity of the vital embryos at 35 days of pregnancy. However, as discussed before, a higher embryonic diversity in early pregnancy might be related with a higher mortality at earlier stages of pregnancy, reducing the impact of diversity on the surviving embryos. However, in sows with a high OR and low levels of early embryonic mortality, the compromised placental development in surviving embryos might increase the variation in foetal weight at a later stage of pregnancy, resulting in lower uniformity in large litters (Quesnel et al., Reference Quesnel, Brossard, Valancogne and Quiniou2008).
In conclusion, higher ORs especially increased early embryonic mortality and to a lesser extent late embryonic mortality. The resulting number of vital embryos at day 35 of pregnancy showed an increase with a higher OR, however, this increase seems to reach a plateau of ~17 embryos at an OR of 22 ovulations. This seems to confirm the findings of Johnson et al. (Reference Johnson, Nielsen and Casey1999), who found low correlated responses between an increase in OR and subsequent litter size. Further, at high ORs, vital embryos had a reduced uterine space and placental development, which might cause growth retardation and increased mortality in later stages of pregnancy and thereby affect piglet birth weight. Research is needed in later stages of pregnancy to confirm such relationships between OR and foetal development and survival. On the other hand, ovarian ultrasound during pregnancy may become a reliable technique to assess OR (Gonzalez-Añover et al., Reference Gonzalez-Añover, Encinas, Gomez-Izquierdo, Sanz, Sanchez-Sanchez and Gonzalez-Bulnes2009) and relate the findings to litter characteristics at term.
Acknowledgements
Capes – Coordenação de Aperfeiçoamento de Pessoal de Nível Superior, Brazil, for providing the scholarship and Topigs Norsvin® for providing the animals.







