The increasing prevalence of overweight and obesity in children and adolescents is now a major public health concern in many countries. Excess body fatness has been shown to not only adversely affect children and adolescents by reducing their health-related quality of life(Reference Williams, Wake and Hesketh1, Reference Swallen, Reither and Haas2), but is also associated with several risk factors for later heart disease and other chronic diseases(Reference Berenson, Srinivasan and Bao3, Reference Mahoney, Burns and Stanford4). As established body fatness is difficult to treat successfully, investigation of modifiable lifestyle factors that influence body fatness in children and adolescents is a high public health priority.
Glycaemic index (GI) and glycaemic load (GL) have received increasing attention in this regard. GI is defined as the incremental area under the blood glucose response curve of carbohydrate in a food expressed as a percentage of the response to carbohydrate in a reference food (usually glucose), and thus represents the quality of carbohydrate(Reference Jenkins, Wolever and Taylor5). GL is the product of the GI and the carbohydrate content of the food, and thus represents both the quality and quantity of carbohydrate(Reference Salmeron, Ascherio and Rimm6). A diet with a low GI or GL, due to the slower blood glucose and insulin response following consumption, is hypothesised to stimulate increased satiety and reduce voluntary energy intake (EI)(Reference Ludwig7), reduce fat storage by regulating fuel partitioning(Reference Stevenson, Williams and Mash8), limit the decrease of resting energy expenditure in the fasting state(Reference Pereira, Swain and Goldfine9) and, in turn, prevent the accumulation of body fat(Reference Augustin, Franceschi and Jenkins10). However, epidemiological studies of dietary GI and GL in relation to the measures of body fatness in free-living children and adolescents are limited and have yielded inconsistent findings(Reference Nielsen, Bjornsbo and Tetens11–Reference Cheng, Karaolis-Danckert and Libuda19).
These heterogeneous results may be due in part to the differences in underlying dietary intake patterns. While evidence in children and adolescents is limited(Reference Murakami, Miyake and Sasaki12, Reference Barba, Sieri and Dello Russo13, Reference Cheng, Karaolis-Danckert and Libuda19–Reference Louie, Buyken and Heyer21), studies in adults have shown that the strength and direction of the associations of dietary GI and GL with particular food groups and nutrients vary considerably depending on the cultural food context(Reference Du, van der A and van Bakel22–Reference Lau, Toft and Tetens27). For example, while dietary GI was positively associated with carbohydrate and negatively with protein and fat in children in Italy(Reference Barba, Sieri and Dello Russo13), Australia(Reference Louie, Buyken and Heyer21) and Japan(Reference Murakami, Miyake and Sasaki12), a German study showed no associations with carbohydrate or fat with a negative association with protein(Reference Cheng, Karaolis-Danckert and Libuda19, Reference Buyken, Dettmann and Kersting20). Conversely, dietary GL was associated positively with carbohydrate and negatively with protein and fat in both German(Reference Cheng, Karaolis-Danckert and Libuda19, Reference Buyken, Dettmann and Kersting20) and Japanese(Reference Murakami, Miyake and Sasaki12) children, despite a large difference in food habits.
Additionally, associations between dietary GI and GL and measures of body fatness may be confounded by misreporting of dietary intake, a serious problem in all dietary surveys(Reference Livingstone and Black28–Reference Murakami, Sasaki and Takahashi32). For example, studies in adults have shown different associations between dietary GI and GL and BMI in a separate analysis of low-energy reporters and non-low-energy reporters(Reference Mendez, Covas and Marrugat23, Reference Lau, Toft and Tetens27). This issue has not been investigated in children and adolescents.
Therefore, the primary aim of the present study was to explore the associations of dietary GI and GL with food and nutrient intakes and indices of body fatness in British children and adolescents. The secondary aim was to examine the impact of misreporting of EI on these associations.
Subjects and methods
Survey design
The present cross-sectional study was based on the data from the National Diet and Nutrition Survey (NDNS): young people aged 4–18 years. Data from the NDNS were obtained from the UK Data Archive, University of Essex. Full details of the rationale, design and methods of the survey have been described elsewhere(Reference Gregory and Lowe33). Briefly, the sample was randomly selected from 132 randomly selected postal sectors within mainland Great Britain. Eligibility was defined as being aged 4–18 years. Only one eligible person per private household was selected at random. Data collection was conducted during a 12-month period (January–December 1997). The present study was conducted according to the guidelines laid down in the Declaration of Helsinki, and all procedures involving human subjects were approved by the National Health Service Local Research Ethics Committee covering each of the postal sectors. Verbal informed consent was obtained from all subjects and their parents/guardians. Verbal consent was witnessed and formally recorded.
Anthropometric measurements
All anthropometric measurements were performed in duplicate by trained fieldworkers, and the mean value of two measurements was used in the analysis. Height (to the nearest 0·1 cm) and weight (to the nearest 0·1 kg) were measured while subjects were barefoot and wearing light clothes only. BMI (kg/m2) was calculated as weight (kg) divided by height (m) squared and converted age- and sex-specific z-score according to British growth reference data(Reference Cole, Freeman and Preece34). Overweight (including obesity) was defined as BMI ≥ 85th percentile of the age- and sex-specific growth reference data(Reference Cole, Freeman and Preece34). For subjects aged ≥ 11 years, waist circumference was also measured at the midpoint between the iliac crest and the lower rib (to the nearest 0·1 cm). Waist:height ratio (WHtR) was calculated as waist circumference divided by height, and central obesity was defined as WHtR ≥ 0·5(Reference Ashwell, Gunn and Gibson35).
Dietary assessment and calculation of dietary glycaemic index and glycaemic load
Dietary data were collected by a 7 d weighed dietary record. A detailed description of the procedure has been published elsewhere(Reference Gregory and Lowe33). Briefly, the subject, the parent or both, depending on the age of the subject, were asked to keep a weighed record of all food and drinks consumed by the subject, both in and out of the home, over seven consecutive days. They were supplied with a set of digital food scales and recording diaries and given by trained interviewers both written and verbal instructions on how to weigh and record items in the diary, with an example of recording diary. When weighing was not possible (e.g. eating out), they were asked to record as much information as possible, including its brand name, the portion size consumed and details of any leftovers. Trained interviewers, who were responsible for coding the diaries, visited the household at least twice during the recording period and checked the completeness of food recording, and, if necessary, additional information was added. All the collected diaries were checked by trained nutritionists, who were responsible for converting descriptions of portion sizes to weights and all aspects of the diary, including coding, recorded weights and descriptions of items consumed. Estimates of daily intake for foods, energy and selected nutrients were calculated from the records of food consumption based on the Food Standards Agency nutrient databank(Reference Smithers36), which is based on McCance and Widdowson's composition of foods series(37) and manufactures' data where applicable.
To calculate dietary GI and GL, GI values were assigned to individual food items (n 4238) in the dietary record, according to the following strategy developed based on previous studies(Reference Louie, Flood and Turner38–Reference Flood, Subar and Hull40). GI values were obtained from the latest international table of GI(Reference Atkinson, Foster-Powell and Brand-Miller41).
Step 1. Determine whether the item has < 0·5 g of carbohydrate (sum of sugars and starch) per 100 g. If yes, assign a GI value of 0 (n 470; 11·1 %). If no;
Step 2. Determine whether there is a direct link to a food in the international GI table. If yes, assign that value (n 717; 16·9 %). If no;
Step 3. Determine whether there is a closely related food (based on macronutrient and fibre content) in the international GI table. If yes, assign that GI value (n 2511; 59·2 %). If no;
Step 4. Determine whether the median GI value of the food subgroup is available. If yes, assign the median GI value of the subgroup (n 95; 2·2 %). If no;
Step 5. Determine whether the item is categorised to one of the following: vegetables, dairy products, sauce, dressing, alcoholic beverages and flour. If yes, assign the following nominal GI value(Reference van Bakel, Slimani and Feskens39, Reference Atkinson, Foster-Powell and Brand-Miller41): 40 for vegetables; 30 for dairy products; 60 for sauce; 30 for dressing; 65 (GI of sucrose) for alcoholic beverages; and the GI value of bread made from the same flour for flour (n 380; 9·0 %). If no;
Step 6. Determine whether the item is categorised to one of the following: fats, egg, fish, meat, tea, coffee, spice and sugar-free foods or beverages. If yes, assign the nominal value of 0 (n 49; 1·2 %). If no;
Step 7. Assign a nominal GI value of 0 or 50, depending on carbohydrate content (n 16; 0·4 %).
Glucose was used as the reference (GI for glucose 100). Where possible, foods were given GI values that were derived from groups of eight or more healthy subjects with an appropriate methodology (i.e. Table A1 in the international GI table; n 4105; 96·9 %). If the only relevant value was available from studies in subjects with diabetes or impaired glucose metabolism, from studies using too few subjects or showing wide variability (i.e. Table A2 in the international GI table), this value was used. Where relevant (e.g. breakfast cereals and breads), only GI values from studies carried out in the UK were used. If more than one eligible GI value was available for a given food, we assigned the mean of the GI values.
Dietary GL was calculated by multiplying the GI value of each individual food item by the amount (g) of carbohydrate consumed from that food item, and then summing the products divided by 100. Dietary GI was calculated by dividing dietary GL by the total amount (g) of carbohydrate consumed, and then multiplying this value by 100.
Assessment of non-dietary variables
The socio-economic status of the head of the household (i.e. occupational social class) was reported and used as a proxy for children's social class. The following three categories were used: manual (i.e. skilled manual, partly skilled and unskilled occupations: social classes III manual, IV and V); non-manual (i.e. professional, managerial, technical and skilled non-manual occupations: social classes I, II and III non-manual); unclassified.
For subjects aged ≥ 7 years, a 7 d physical activity diary was carried out concurrently with the dietary record. A detailed description of the procedure has been published elsewhere(Reference Gregory and Lowe33). Briefly, the subject was asked to provide information on the time spent being active from a list of prompted moderate, vigorous and very vigorous activities. Information on activities that were not already listed and sleep was also provided. Trained interviewers checked the completeness of records at least twice during the recording period, and, if necessary, additional information was added. Subsequently, time spent daily in sleep, very light, light, moderate, vigorous and very vigorous-intensity activities was computed for each day of recording. Each type of activity was assigned a metabolic equivalent (MET) value from a published table: 1·0 for sleep; 1·5 for very light; 2·5 for light; 4·0 for moderate; 6·0 for vigorous; 10·0 for very vigorous-intensity activities(Reference Ainsworth, Haskell and Herrmann42). The number of hours spent per d on each activity was multiplied by the MET value of that activity, and all MET-h products were summed to produce a total MET-h score for the day. They were then divided by 24 h to give a physical activity level (PAL) value, and classified into four categories (sedentary (PAL ≥ 1·0 to < 1·4), low active (PAL ≥ 1·4 to < 1·6), active (PAL ≥ 1·6 to < 1·9) and very active (PAL ≥ 1·9 to < 2·5)) according to the US Dietary Reference Intakes(43). For subjects aged ≤ 6 years, for which activity diary was not collected, the ‘active’ level was assigned based on a result on total energy expenditure (TEE) measured by the doubly labelled water in the NDNS feasibility study(Reference Rennie, Jebb and Wright44).
Evaluation of energy intake reporting
We calculated each subject's estimated energy requirement (EER), based on information on age, weight, height and physical activity, with the use of equations published from the US Dietary Reference Intakes(43). The equations were developed from a meta-analysis of methodologically sound studies using doubly labelled water as the criterion measure of TEE(43). Sex-, age- and weight status-specific equations(43) were used.
Subjects were identified as acceptable reporters (AR), under-reporters (UR) or over-reporters of EI based on their ratio of EI:EER, according to whether the individual's ratio was within, below or above the 95 % confidence limits of the expected ratio of 1·0. The 95 % confidence limits ( ± 2 sd cut-offs) were calculated according to the following equation:
where CVEI is the within-person CV in reported EI; d is the number of days of dietary assessment; CVEER is the error in the EER equations; CVTEE is the day-to-day variation in TEE(Reference Huang, Roberts and Howarth45). The values used were 23·5–31·3 % for CVEI (calculated from the present NDNS data; values varying depending on the sex–age–BMI stratum), 7 for d, 3·0–6·5 % for CVEER (based on the US Dietary Reference Intakes data(43, Reference Huang, Roberts and Howarth45); values varying depending on the sex–age–BMI stratum) and 8·2 % for CVTEE (as previously reported from doubly labelled water studies(Reference Black and Cole46)). As the 95 % confidence limits were found to be similar across sex–age–BMI strata (26·6–30·0 %), an average of 28 % was used in the present analysis. Thus, AR were defined as having EI:EER in the range of 0·72–1·28, UR as EI:EER < 0·72 and over-reporters as EI:EER >1·28.
Analytic sample
Of the 2672 potentially eligible people identified for the study, 2127 (80 % of the eligible sample) participated in the survey. For the present analysis, we excluded a total of 443 subjects with missing information on the variables used (n 182 for anthropometric data; n 426 for dietary data; n 125 for physical activity data; some subjects had more than one missing value). We further excluded forty-eight underweight subjects (i.e. BMI ≤ 3rd percentile of the age- and sex-specific growth reference data(Reference Cole, Freeman and Preece34)). The final analysis sample comprised 1636 subjects aged 4–18 years (61 % of the eligible sample).
Statistical analysis
All statistical analyses were performed for children aged 4–10 years (n 818) and adolescents aged 11–18 years (n 818) separately, using SAS statistical software (version 9.2; SAS Institute, Inc.). Separate analyses for boys and girls showed similar patterns of the associations of dietary GI and GL with dietary intake and measures of body fatness, and tests for interaction with sex were not significant (data not shown). We therefore present the results for both sexes combined. Differences between AR and UR (but not over-reporters because there were only a few over-reporters) were tested by the independent sample t test (for continuous variables) and by the χ2 test (for categorical variables). Stepwise regression analyses were carried out to investigate the contribution of nineteen selected food groups to the inter-individual variation in dietary GI and GL. For those food groups contributing at least 1 % variation, multiple regression analyses were performed, with only those predictive food groups plus EI as explanatory variables and dietary GI or GL as the response variable. Associations of intakes of energy and selected nutrients with dietary GI and GL were investigated through Spearman's correlation analyses.
For the investigation of the associations between dietary GI and GL (independent variables) with measures of body fatness (dependent variables), dietary GI and GL were categorised at tertile points based on the distribution. With the use of the PROC GLM procedure, multivariate-adjusted mean (with their standard error) values for BMI z-score (and WHtR for adolescents) were calculated according to tertiles of dietary GI and GL. Trends of associations were assessed by a linear regression model that assigned consecutive integers (0–2) to the levels of the independent variables. Further, using the PROC LOGISTIC procedure, crude and multivariate-adjusted OR (and 95 % CI) for overweight (and central obesity for adolescents) for each tertile category of dietary GI and GL were calculated, with the lowest tertile category used as the reference category. Trends of associations were assessed by a multiple logistic regression model that assigned consecutive integers to the levels of the independent variables.
Potential confounding factors included in the multivariate models were age, sex, social class, physical activity, protein intake, dietary fibre intake and EI:EER, in addition to dietary fat intake for dietary GI. For the analysis on central obesity, BMI z-score was also included as a potential confounding factor. These potential confounding factors were selected based on a comprehensive literature review of epidemiological studies on dietary GI and GL and body fatness(Reference Nielsen, Bjornsbo and Tetens11–Reference Cheng, Karaolis-Danckert and Libuda19, Reference Du, van der A and van Bakel22, Reference Mendez, Covas and Marrugat23, Reference Du, van der A and van Bakel26, Reference Lau, Toft and Tetens27). The analyses were conducted not only for the entire population but also for AR only or UR only.
Values of nutrient intake were energy-adjusted using the density method (i.e. percentage of energy for energy-providing nutrients and amount per 10 MJ of energy for dietary fibre). We used crude values for dietary GI and energy-adjusted values by the density method (per 10 MJ) for dietary GL because dietary GI is, by definition, a measure of carbohydrate quality, not quantity, whereas dietary GL is a measure of the combination of carbohydrate quality and quantity(Reference Murakami, Miyake and Sasaki12). Use of energy-adjusted values (including dietary GI) by the residual method(Reference Willett, Howe and Kushi47) did not change the results materially (data not shown).
Data have not been weighted to take into account known sociodemographic differences between responders and non-responders, not only because the impact of this adjustment, applied as a weighting factor, for nutritional variables was extremely small and not significant(Reference Gregory and Lowe33), but also because we were only interested in the relationships between variables, rather than estimates of prevalence. All reported P values are two-tailed, and P< 0·05 was considered as statistically significant.
Results
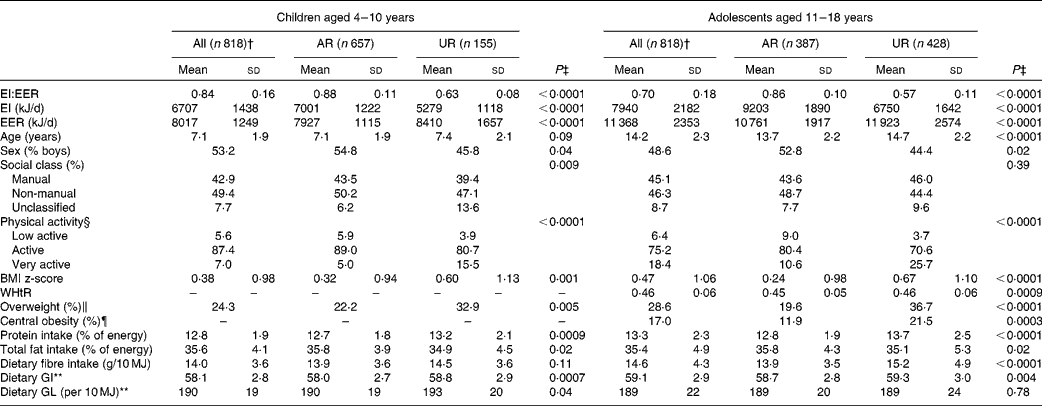
The mean value of EI:EER was 0·84 (sd 0·16) in children and 0·70 (sd 0·18) in adolescents (Table 1). The prevalence of overweight was 24 % in children and 29 % in adolescents. The prevalence of central obesity in adolescents was 17 %. The mean values of dietary GI and GL were 58 (sd 3) and 190 (sd 19) per 10 MJ in children and 59 (sd 3) and 189 (sd 22) per 10 MJ in adolescents, respectively.
Table 1 Characteristics of the subjects* (Mean values and standard deviations or percentages)

AR, acceptable reporters; UR, under-reporters; EI:EER, ratio of energy intake:estimated energy requirement; EI, energy intake; EER, estimated energy requirement; WHtR, waist:height ratio; GI, glycaemic index; GL, glycaemic load.
* AR were defined as subjects with EI:EER in the range of 0·72–1·28; UR defined as subjects with EI:EER < 0·72.
† Including over-reporters of energy intake (n 6 in children and n 3 in adolescents), defined as subjects with EI:EER > 1·28.
‡ P values for differences between AR and UR based on the independent-samples t test for continuous variables and the χ2 test for categorical variables.
§ There were no subjects classified into the ‘sedentary’ level.
∥ BMI ≥ 85th percentile of the age- and sex-specific British growth reference data.
¶ WHtR>0·5.
** Based on the GI of glucose (100).
The percentages of AR and UR were 80 and 19 % in children and 47 and 52 % in adolescents, respectively (only six children (0·7 %) and three adolescents (0·4 %) were classified as over-reporters). In both children and adolescents, compared with AR, UR had a lower mean value of EI:EER and EI and a higher mean value of EER and BMI z-score (and age and WHtR in adolescents), and were more likely to be girls, physically active and overweight, be in unclassified social class (children only) and centrally obese (adolescents only). In terms of dietary intake, UR had a higher mean intake of protein (% energy), but a lower mean intake of total fat (% energy). UR also had a higher mean GI, GL (children only) and fibre (g/10 MJ, adolescents only).
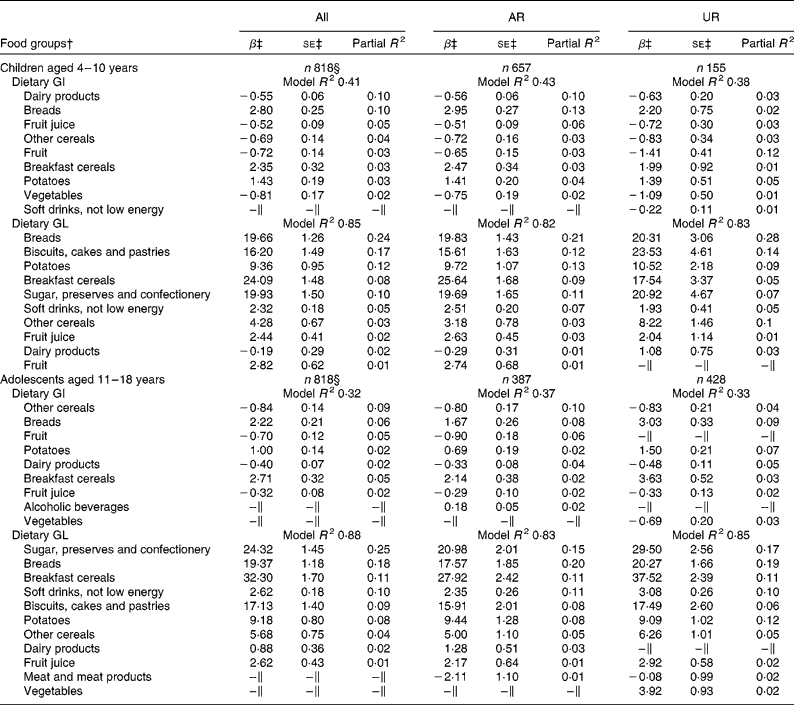
Irrespective of age, breads, breakfast cereals and potatoes were the positive predictive food groups for dietary GI, while dairy products, fruit juice, other cereals and fruit (and vegetables in children only) were the negative predictors (Table 2). In total, these food groups accounted for 41 % (children) and 34 % (adolescents) of the variation in dietary GI. For dietary GL, 85 % (children) and 88 % (adolescents) of the variation were explained by major carbohydrate-rich food groups, all of which showed positive associations (except for dairy products in children). Food groups identified as the predictors of dietary GI and GL were relatively similar in the analysis of only AR or only UR for both children and adolescents.
Table 2 Food groups contributing to the inter-individual variation in dietary glycaemic index (GI) and glycaemic load (GL)* (Regression coefficients with their standard errors and partial determination coefficients)

AR, acceptable reporters; UR, under-reporters.
* AR were defined as subjects with the ratio of energy intake:estimated energy requirement (EI:EER) of 0·72–1·28; UR defined as subjects with EI:EER < 0·72.
† Food groups listed are those contributing at least 1 % of the variation of dietary GI or GL based on stepwise regression analysis with nineteen food groups (i.e. breads; breakfast cereals; biscuits, cakes and pastries; other cereals; dairy products; egg and egg dishes; butter and spreads; meat and meat products; fish and fish dishes; vegetables; potatoes; fruit; sugar, preserves and confectionery; fruit juice; alcoholic beverages; tea, coffee and water; nuts and seeds; soft drinks, not low energy; soft drinks, low energy) as explanatory variables and dietary GI or GL as the response variable.
‡ Models with listed variables and energy intake as explanatory variables and dietary GI or GL as the response variable; regression coefficients mean the change in dietary GI or GL with a 100 g increase of each food group.
§ Including over-reporters of energy intake (n 6 in children and n 3 in adolescents), defined as subjects with EI:EER >1·28.
∥ Not contributing at least 1 % of the variation of dietary GI or GL.
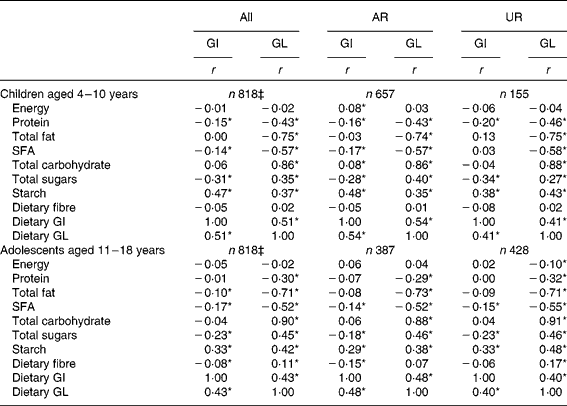
Both dietary GI and GL showed no association with EI (Table 3). Dietary GI was positively associated with starch and dietary GL and inversely associated with SFA and total sugars. Dietary GI was also negatively associated with protein in children and total fat and dietary fibre in adolescents. There was no association between dietary GI and total carbohydrate. Dietary GL was positively associated with total carbohydrate, total sugars and starch, and inversely with protein, total fat and SFA. Dietary GL was also positively associated with dietary fibre in adolescents. Relatively similar results were obtained when AR and UR were analysed separately. When crude (i.e. non-energy-adjusted) values, instead of energy-adjusted values, were used, the associations between dietary GI and nutrient intakes were unchanged while dietary GL was positively associated with all the nutrients examined (data not shown).
Table 3 Correlation of energy and nutrient intake with dietary glycaemic index (GI) and glycaemic load (GL)† (Spearman's correlation coefficients)

AR, acceptable reporters; UR, under-reporters.
* P< 0·05.
† Values are Spearman's correlation coefficients calculated using energy-adjusted values (i.e. percentage of energy for energy-providing nutrients and amount per 10 MJ of energy for dietary fibre and GL) except for energy and dietary GI (for which crude values were used). Dietary GI and GL were calculated based on the GI of glucose (100). AR were defined as subjects with the ratio of energy intake:estimated energy requirement (EI:EER) of 0·72–1·28; UR defined as subjects with EI:EER < 0·72.
‡ Including over-reporters of energy intake (n 6 in children and n 3 in adolescents), defined as subjects with EI:EER >1·28.
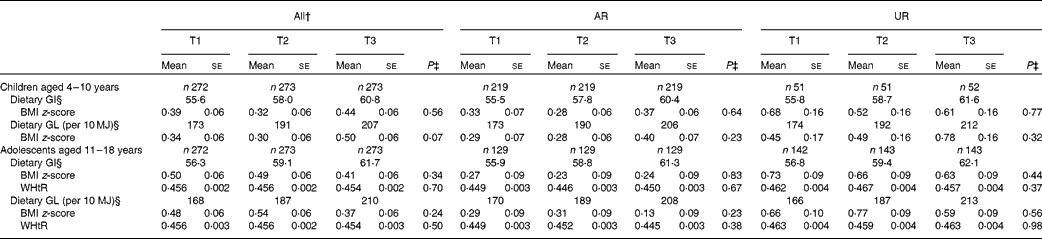
Both dietary GI and GL showed no association with BMI z-score in children and adolescents (Table 4). There was also no association between dietary GI and GL and WHtR in adolescents. The separate analysis of AR and UR did not show any associations.
Table 4 Measures of body fatness according to tertiles (T) of dietary glycaemic index (GI) or glycaemic load (GL)* (Mean values with their standard errors)

AR, acceptable reporters; UR, under-reporters; WHtR, waist:height ratio.
* Values are adjusted means (with their standard errors) unless otherwise indicated. Adjustment was made for age (years, continuous), sex (boys or girls), social class (manual, non-manual or unclassified), physical activity (low active, active or very active), protein intake (percentage of energy, continuous), dietary fibre intake (g/10 MJ, continuous), ratio of energy intake:estimated energy requirement (EI:EER, continuous) and total fat intake (percentage of energy, continuous, for dietary GI only). For WHtR, further adjusted for BMI z-score (continuous). AR were defined as subjects with EI:EER in the range of 0·72–1·28; UR defined as subjects with EI:EER < 0·72.
† Including over-reporters of energy intake (n 6 in children and n 3 in adolescents), defined as subjects with EI:EER >1·28.
‡ P values represent P for trend tested by linear regression.
§ Based on the GI of glucose (100). Values are medians.
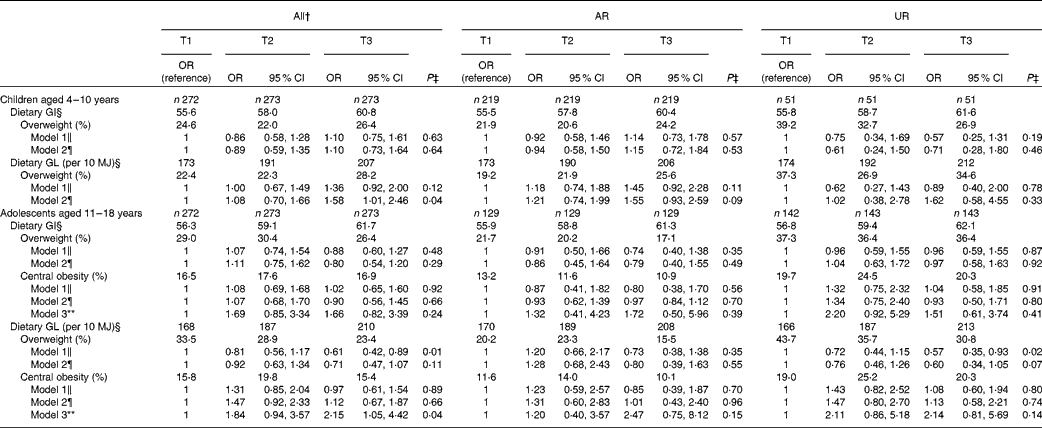
Table 5 shows OR (and 95 % CI) for overweight and central obesity according to tertiles of dietary GI and GL. In children, after adjustment for potential confounding factors (model 2), dietary GL was associated with a higher risk of overweight (P for trend = 0·04), while there was no association for dietary GI. In adolescents, dietary GI was not associated with the risk of overweight or central obesity. Additionally, there was no independent association between dietary GL and overweight. However, dietary GL was associated with a higher risk of central obesity (P for trend = 0·02) after adjustment for potential confounding factors including BMI z-score (model 3). In the analysis of AR or UR, the direction and strength of the independent associations (i.e. OR themselves) were similar, but none of the associations reached statistical significance. Excluding EI:EER from the models did not change the results materially (data not shown).
Table 5 OR for overweight and central obesity according to tertiles (T) of dietary glycaemic index (GI) or glycaemic load (GL)* (Odds ratios and 95 % confidence intervals)

AR, acceptable reporters; UR, under-reporters.
* OR (95 % CI) were calculated using logistic regression. Overweight was defined as BMI ≥ 85th percentile of the age- and sex-specific British growth reference data; central obesity was defined as waist:height ratio ≥ 0·5. AR were defined as subjects with the ratio of energy intake:estimated energy requirement (EI:EER) of 0·72–1·28; UR defined as subjects with EI:EER < 0·72.
† Including over-reporters of energy intake (n 6 in children and n 3 in adolescents), defined as subjects with EI:EER >1·28.
‡ P values represent P for trend tested by logistic regression.
§ Based on the GI of glucose (100). Values are medians.
∥ Crude model.
¶ Adjusted for age (years, continuous), sex (boys or girls), social class (manual, non-manual or unclassified), physical activity (low active, active or very active), protein intake (percentage of energy, continuous), dietary fibre intake (g/10 MJ, continuous), EI:EER (continuous) and total fat intake (percentage of energy, continuous, for dietary GI only).
** Adjusted for variables used in model 2 and BMI z-score (continuous).
Discussion
In the present cross-sectional study of British children and adolescents, breads, breakfast cereals and potatoes were the positive predictive food groups for dietary GI, while dairy products, fruit juice, other cereals and fruit were the negative predictors. Dietary GL was closely correlated with carbohydrate intake. Dietary GI was not associated with any measures of body fatness in both children and adolescents. However, dietary GL was independently positively associated with overweight in children. Dietary GL was also independently associated with a higher risk of central obesity (but not overweight) in adolescents. Nevertheless, none of the associations reached statistical significance in both AR and UR, although the direction and strength of the associations (i.e. OR themselves) were similar. Further, no association was seen when measures of body fatness were treated as continuous variables (i.e. BMI z-score and WHtR). Thus, the results must be interpreted with caution. To our knowledge, this is the first study to examine dietary GI and GL in relation to food and nutrient intakes and measures of body fatness in British children and adolescents, taking into account EI misreporting assessed by individualised measures of EER.
Information on dietary GI and GL in relation to food and nutrient intakes (in terms of dietary composition) in children and adolescents is limited, but, generally, dietary GI has shown to be positively associated with carbohydrate and starch and negatively with protein, fat, saturated fat, fibre and sugar(Reference Murakami, Miyake and Sasaki12, Reference Louie, Buyken and Heyer21). However, a positive association with fibre and sugar and no association with carbohydrate, fat and saturated fat have also been observed(Reference Barba, Sieri and Dello Russo13, Reference Cheng, Karaolis-Danckert and Libuda19, Reference Buyken, Dettmann and Kersting20). Dietary GL has shown to be positively associated with carbohydrate and negatively with protein, fat and fibre(Reference Murakami, Miyake and Sasaki12, Reference Buyken, Dettmann and Kersting20). The present findings provide further support for the diversity of these associations. In particular, we showed no association between GI and protein, fat, carbohydrate and fibre and a positive association between dietary GL and fibre, which has also been observed in adults(Reference Du, van der A and van Bakel22–Reference van Bakel, Kaaks and Feskens25). Studies in adults generally showed that dietary GL was associated with many carbohydrate-rich foods, while dietary GI was associated not only with a higher intake of major carbohydrate-rich foods (such as breads and potatoes) but also with a lower intake of other foods with lower carbohydrate content (such as fruit and dairy products)(Reference Du, van der A and van Bakel22–Reference van Bakel, Kaaks and Feskens25). However, the strength and direction of the associations varied considerably among studies(Reference Du, van der A and van Bakel22–Reference van Bakel, Kaaks and Feskens25), which is in line with the findings of the present study.
A limited number of observational studies have examined the association between dietary GI and GL and body fatness in children and adolescents. No association was observed in cross-sectional studies conducted in the USA(Reference Davis, Alexander and Ventura17), Hong Kong(Reference Hui and Nelson15) and Italy(Reference Scaglioni, Stival and Giovannini16). A series of prospective analyses in German children have also provided null findings(Reference Buyken, Cheng and Gunther18, Reference Cheng, Karaolis-Danckert and Libuda19). Conversely, a Danish cross-sectional study found that higher dietary GI and GL were related to a higher sum of skinfold thickness; however, this association was seen in 16-year-old boys only, and not in 10-year-old boys or 10- or 16-year-old girls(Reference Nielsen, Bjornsbo and Tetens11). Additionally, dietary GI, but not GL, was cross-sectionally associated with overweight and high waist circumference in Italian children aged 6–11 years(Reference Barba, Sieri and Dello Russo13). A cross-sectional study in Australian adolescents aged 13–15 years also showed a positive association between high waist circumference and dietary GL, but not GI(Reference O'Sullivan, Lyons-Wall and Bremner14). Further, dietary GL, but not GI, was associated with the risk of overweight in Japanese children aged 6–15 years (except for girls aged 12–15 years)(Reference Murakami, Miyake and Sasaki12). The findings on the present study (i.e. positive association of GL, but not GI, with overweight in children and central obesity in adolescents) are consistent with the associations observed in the Australian(Reference O'Sullivan, Lyons-Wall and Bremner14) and Japanese(Reference Murakami, Miyake and Sasaki12) studies, but at variance with those in other studies(Reference Nielsen, Bjornsbo and Tetens11, Reference Barba, Sieri and Dello Russo13, Reference Hui and Nelson15–Reference Cheng, Karaolis-Danckert and Libuda19). This variance may be, at least partly, explained by differences in the characteristics and lifestyles of the populations examined, dietary assessment methods used, measures of body fatness applied and potential confounding factors considered, in addition to differences in underlying dietary intake patterns in these studies. It is unclear why there was a positive association with overweight and central obesity for dietary GL only but not for GI in the present study. Both the quality and quantity of dietary carbohydrate rather than quality alone may be important, at least in the present population. Nonetheless, as dietary GL was strongly correlated with carbohydrate and dietary fat, which was also observed in several previous studies(Reference Murakami, Miyake and Sasaki12, Reference Du, van der A and van Bakel22, Reference Du, van der A and van Bakel26), any effects of dietary GL cannot be separated from those of macronutrient composition and overall diet quality in the present observational study. However, a repeated analysis using each of the other dietary variables (i.e. dietary fat, carbohydrate, protein or dietary fibre) as an independent variable instead of dietary GI or GL showed no association with overweight or central obesity (data not shown). Additionally, while dietary GL showed significant associations when body fatness measures were treated as categorical variables (i.e. overweight and central obesity), the trend of the associations was not very much clear. Furthermore, adjustment for BMI z-score in the model of central obesity may be an over-adjustment (given the wide CI). Nevertheless, we believe that this adjustment was worth conducting because of the following reasons. First, central obesity can develop independently of overweight through an accumulation of visceral fat. Second, central obesity may be more susceptible to dietary GL than overweight, given that visceral fat is more vulnerable to the influence of high insulin responses stimulated by high-GL diets compared with subcutaneous fat(Reference Du, van der A and van Bakel26). There was, however, no association when body fatness measures were treated as continuous variables. Thus, the results must be interpreted with caution.
Despite a high prevalence of UR and large differences in characteristics between UR and AR, which is quite common in dietary surveys(Reference Livingstone and Black28–Reference Murakami, Sasaki and Takahashi32), the associations of dietary GI and GL with food and nutrient intakes observed in UR were relatively similar to those observed in AR. It is reasonable to assume that the observed associations in UR are not artefacts produced by dietary under-reporting. Thus, exclusion of UR in the present analysis was not warranted. The exact reason for the similarity between AR and UR in terms of the associations between dietary GI and GL and food and nutrient intakes is unknown, and we are unaware of previous studies where the associations among dietary variables were compared according to categories of dietary misreporting status. However, it may be because the calculation of dietary GI and GL is based on carbohydrate intake only, independent of fat (which seems to be more prone to misreporting than other macronutrients)(Reference Subar, Kipnis and Troiano29–Reference Murakami, Sasaki and Takahashi32) as well as energy. In addition, the influence of dietary misreporting may be minimised by energy adjustment, as misreporting of any food and nutrient should be correlated with EI misreporting, at least to some extent(Reference Subar, Kipnis and Troiano29, Reference Murakami, Sasaki and Takahashi32).
In the present study, the positive associations between dietary GL and overweight in the total children population and central obesity in the total adolescent population were not observed in AR or UR; although the direction and strength of the associations (i.e. OR themselves) were similar, none of the associations reached statistical significance in both AR and UR. This may be due to insufficient statistical power in a separate analysis of AR and UR. Relatively similar, but non-significant, associations between dietary GI and GL and measures of body fatness observed in AR and UR are reasonable given that the associations between dietary GI and GL and food and nutrient intakes are relatively similar between AR and UR. Thus, there was no evidence that the association between dietary GI and GL and measures of body fatness was distorted by dietary misreporting (mainly under-reporting) in the present study. In line with this observation, previous studies showed that the associations of carbohydrate intake (expressed as percentage of energy) with BMI(Reference Huang, Roberts and Howarth45, Reference Mendez, Popkin and Buckland48) and fasting plasma insulin(Reference Rosell, Hellenius and De Faire30) were not affected by EI misreporting.
The advantages of the present study include the use of a 7 d weighed dietary record, a systematic assignment of GI values based on an updated and more representative GI table, measured anthropometric data and the use of individualised measure of EER to identify EI misreporters. However, there are also several limitations. First, the cross-sectional nature of the study does not permit the assessment of causality owing to the uncertain temporality of the association. Only a prospective study taking into account dietary misreporting would provide better understanding of the relationship between dietary GI and GL and measures of body fatness.
We used BMI and WHtR as proxy measures of body fatness. Since BMI reflects not only body fatness, but the relative length of the legs, body frame size and fat-free body mass as well(Reference Prentice and Jebb49), subjects with similar BMI (z-score) do not necessarily have the same amount of body fat, which may explain at least partly why overweight (defined by BMI z-score) was not associated with dietary GL while there was a positive association for central obesity (defined by WHtR) in adolescents. In any case, a more valid measure of body fat mass (e.g. dual-energy X-ray absorptiometry) may be needed for further investigation.
Another limitation of the present study is that only 61 % of the eligible sample was included in the present study, although the response rate was relatively high (80 %). The subjects included in the present analysis (n 1636) differed somewhat from those excluded from the analysis (n 491). The excluded subjects were more likely to be younger and be in social class classified as manual occupations (all P< 0·05). However, a previous analysis concluded that there was no evidence to suggest serious non-response bias in the NDNS(Reference Gregory and Lowe33). Additionally, although we adjusted for a variety of potential confounding variables, residual confounding could not be ruled out. In particular, we could not control for puberty status or parental weight status because of a lack of information.
Finally, we assessed misreporting of EI against calculated EER with the use of equations from the US Dietary Reference Intakes(43). In the absence of measured TEE, these equations with high R 2 values ( ≥ 0·95)(43) should serve as the best proxy. Nevertheless, the selection of physical activity category was based on self-report (i.e. 7 d physical activity diary) in subjects aged ≥ 7 years and fixed in subjects aged ≤ 6 years, which may be susceptible to systematic error. Additionally, we do not know the sensitivity and specificity of the procedure for identifying EI misreporters used. However, even though some misclassification of subjects according to EI reporting status did occur in the present study, we are confident of our conclusions, because the associations of dietary GI and GL with food and nutrient intakes and obesity measures observed here were not influenced by EI misreporting. Nonetheless, it should be stressed that the role of misreporting was mainly evaluated only in terms of under-reporting because over-reporting occurred in such a low number of cases that no conclusions could be drawn in this regard.
In conclusion, the present cross-sectional study in British children and adolescents showed that a high-GI diet, characterised by a high intake of breads, breakfast cereals and potatoes and a low intake of dairy products, fruit juice, other cereals and fruit, was not associated with overweight in both children and adolescents. However, dietary GL, which was closely correlated with carbohydrate intake, was independently associated with a higher risk of overweight in children and a higher risk of central obesity (but not overweight) in adolescents. Nevertheless, none of the associations reached statistical significance in both subjects with plausible EI and those with implausible EI, although the direction and strength of the associations (i.e. OR themselves) were similar. Further, dietary GI and GL were not associated with BMI z-score in children and adolescents or WHtR in adolescents. Thus, the results must be interpreted cautiously. Given the diverse observations in previous studies(Reference Nielsen, Bjornsbo and Tetens11–Reference Cheng, Karaolis-Danckert and Libuda19) and the present study, the association between dietary GI and GL and measures of body fatness may be a function of the food culture concerned. Further research with a prospective design in diverse populations is needed, preferably taking into account dietary misreporting as well as using more valid measures of body fatness so that any firm conclusions can be drawn with regard to the effect of dietary GI and GL on excess body fatness.
Acknowledgements
The present study was supported in part by the JSPS Postdoctoral Fellowships for Research Abroad, Japan Society for the Promotion of Science, Japan. K. M. designed the study, analysed and interpreted the data, and wrote the manuscript. T. A. M. and M. B. E. L. designed the study and helped in the writing of the manuscript. All authors read and approved the final manuscript. None of the authors had a conflict of interest.