Broadening the reach of antibiotic stewardship (AS) activities to include nurses has recently been recognized as important for the success of antibiotic stewardship programs (ASPs) by the American Nurses Association (ANA) and by the Centers for Disease Control and Prevention (CDC).1, 2 Concomitantly, accrediting and federal agencies have issued calls for interdisciplinary AS perspectives, indicating the need for nursing participation.3, 4 The growing number of position statements and endorsements for nurses as AS partners is encouraging, but practical guidance of how to best integrate nurses into AS is lacking. Here, we present specific examples of the potential role of bedside nurses in AS activities, and we provide a framework for integrating bedside nurses into AS activities.
Existing AS work by nurses
Work in long-term care settings focusing on the integration of nurses in initiatives to improve antibiotic use have proven successful; several such examples can be found in the literature. A behavioral intervention targeting frontline nurses and prescribers, consisting of education about appropriate indications for urine culture and algorithms for clinical decision support, resulted in sustained reductions in the inappropriate treatment of asymptomatic bacteriuria in long-term care residents.Reference Zabarsky, Sethi and Donskey5 Similarly, a clinical algorithm to assist nurses, as well as other providers, in deciding appropriate indications for urine cultures in patients with indwelling urinary catheters successfully reduced overtreatment of asymptomatic bacteriuria.Reference Naik, Skelton, Amspoker, Glasgow and Trautner6, Reference Trautner, Grigoryan and Petersen7 Additionally, a clinical pathway designed for nurses in managing nursing home residents with lower respiratory tract infections resulted in fewer hospitalizations and healthcare costs without affecting clinical outcomes.Reference Loeb, Carusone and Goeree8
Data are limited, however, regarding the role of the bedside nurse in AS activities in the acute-care setting. Recent studies have shed light on nursing perceptions of their potential contribution to AS efforts in hospitals, their perceived limitations to accomplish their role as stewards, and suggestions to overcome these limitations.Reference Monsees, Popejoy, Jackson, Lee and Goldman9–Reference Carter, Greendyke and Furuya11 When the role of bedside nurses in making AS interventions was examined, nurses reported a high degree of confidence with certain practices, such as assessing for antibiotic-associated adverse drug reactions, obtaining cultures prior to antibiotic initiation, and participating in patient and family education about appropriate antibiotic use.Reference Monsees, Popejoy, Jackson, Lee and Goldman9 Conversely, nurses were less confident or reluctant to initiate a 48-hour antibiotic time-outReference Monsees, Popejoy, Jackson, Lee and Goldman9 or to de-label patients with penicillin (PCN) allergies.Reference Carter, Greendyke and Furuya11
Leveraging nurses’ experience into AS strategies
As part of their daily work, nurses perform a number of activities that influence antibiotic prescribing decisions. A comprehensive list of such activities have been outlined in a White Paper developed by the ANA and the CDC.1 Through collaborative efforts with the Agency for Healthcare Research and Quality (AHRQ) Safety Program for Improving Antibiotic Use,12 which strives to improve the decision-making by frontline clinicians, including nurses, we consider the following practices, in which bedside nurses could play a key role in improving antibiotic prescribing practices, to have priority:
Ensuring appropriate Clostridioides difficile testing
Given their extensive direct patient contact, nurses are instrumental in identifying changes in the bowel habits of patients. Ensuring appropriate documentation of bowel movements (number and description) ensures accurate clinical interpretation both to make a clinical diagnosis of diarrhea and to evaluate clinical response. Accurate documentation also increases the likelihood that C. difficile testing is limited to patients with appropriate signs and symptoms of C. difficile infection (CDI). Studies have shown poor correlation between human sniffing ability and CDI.Reference Rao, Berland, Young, Walk and Newton13 Therefore, education may be necessary to ensure that testing is not based on the smell of bowel movements if the patient does not have a clinical picture consistent with CDI. Furthermore, nurses can alert prescribers when patients are receiving concomitant laxatives or tube feedings that can cause diarrhea when C. difficile testing is being considered. Preferably, reviewing the receipt of relevant medications and tube feeding should occur prior to testing, but these alternative causes for diarrhea should be reported to prescribers even if the C. difficile test is positive. Additionally, educating nurses regarding certain diagnostic test limitations may be beneficial to promote appropriate C. difficile testing. For example, some commonly used tests detect the presence of the gene that produces the C. difficile toxin(s) but not toxin production; hence, the distinction between carrier status and infection relies on clinical evaluation. Treating asymptomatic C. difficile carriers is not recommended because it may alter the patient’s protective intestinal flora or provoke C. difficile toxin production.
Ensuring appropriate indications prior to obtaining specimens for urine culture
Major factors driving unnecessary antibiotic use are inappropriate testing due to isolated changes in color or smell of urine (ie, “dark,” “cloudy,” or foul-smelling urine) or vague symptoms such as fatigue and the poor practice of testing urine from the catheter collection bag. Treatment of asymptomatic patients is a significant contributor to antibiotic misuse. Education focusing on the signs and symptoms of a urinary tract infection and the 2 primary indications for treatment of asymptomatic bacteriuria,Reference Nicolle14 which include pregnancy and urologic procedures expected to cause mucosal bleeding (eg, transrectal biopsies, transurethral prostatectomies), will enhance nurses’ confidence in identifying inappropriate indications for urine cultures and will facilitate communication with providers when urine cultures are not needed. By preventing unnecessary urine cultures, nurses can prevent exposing patients to unnecessary antibiotics and their associated adverse events (eg, CDI, antibiotic resistant bacteria, and antibiotic-associated adverse drug events).
Ensuring optimal antibiotic administration
Conversion of patients from intravenous (i.v.) to oral antibiotics can minimize the need for vascular lines (and the need for outpatient parenteral therapy) and reduce the length of stay, without compromising clinical care in many clinical situations. In several infectious diseases, oral step-down therapy is recommended when the patient improves, such as pneumonia and skin and soft-tissue infections. Nurses can aid in the transition of i.v. antibiotic therapies to the oral route by prompting providers when patients are tolerating oral feeding or other oral medications. When i.v. therapy is required, nurses are essential in ensuring the appropriate timing for therapeutic drug monitoring when monitoring is needed (eg, vancomycin, aminoglycosides). Nurses can also report observations and issues with vascular sites (eg, phlebitis) and difficulties encountered with vascular access (eg, sluggish or hard to flush lines) to trigger consideration of oral therapies if appropriate.
Obtaining and documenting accurate penicillin allergy histories
Allergic reactions to penicillin (PCN) are commonly reported; however, true PCN allergy is rare (only 1% of the general population is actually allergic to PCN).Reference Trubiano, Adkinson and Phillips15 In other words, most people who believe they have PCN allergies do not have IgE-mediated reactions to PCN.
Anaphylaxis, a severe type of allergic reaction, to PCN is extremely rare (<0.01% of the US population). Patients with a PCN allergy label have worse patient outcomesReference Blumenthal, Lu, Zhang, Li, Walensky and Choi16–Reference MacFadden, LaDelfa and Leen18 (eg, higher risk of treatment failure of bacterial infections and adverse events including CDI and surgical site infections) compared to patients without a PCN allergy label, likely because the former group receives therapy that deviates from recommended firstline options. When nurses obtain allergy histories, key elements such as specific reactions to antibiotics, timing of reactions, date of reactions, and severity of the reactions are critical for antibiotic decision-making and appropriate use.
Specific details on reactions that should be elicited include describing (1) the specific antibiotic instead of drug class wherever possible (eg, cephalexin rather than cephalosporins), (2) the specific reaction (eg, nonraised flat rash, not simply “rash”), (3) the severity of the reaction (eg, hospital admission, respiratory failure), (4) when the reaction occurred in relation to drug administration (eg, immediately after or several days later), and (5) the patient’s age at the time of the reaction. An allergy consultation is especially recommended, if available, for patients who report anaphylaxis or hives that occurred in the remote past because patients may overcome hypersensitivity to penicillin over time and thus become candidates for penicillin testing.
When there is discrepancy between a documented allergy history and the patient verbal report, nurses can notify providers or pharmacists for resolution and treatment considerations if nurses are uncomfortable making changes in the chart. After the nature of the allergy is clarified, nurses can update the medical record as appropriate. Many reported reactions represent side effects (eg, isolated fatigue, nausea, and headache) and should not prevent a patient from receiving the most appropriate antibiotic. Nurses are well-positioned to notify clinicians of allergy labels that have been placed in error, and as patient safety advocates, they can promote patient understanding by discussing the importance of PCN allergy clarification and the rationale for Allergy consult evaluations for questionable cases. Additional key points regarding reviewing PCN allergy histories are shown in Box 1.
Box 1: Additional Key Points Regarding Reviewing Penicillin Allergy Histories
80% of patients overcome an allergy after 10 years and may be able to safely receive penicillin (PCN). These patients should be further evaluated.
It is essential to distinguish hives (wheals with a pale center that typically appear within minutes to hours of antibiotic administration and represent a true allergic reaction) from nonhives rash.
The most common type of rash to PCN or cephalosporins is a non-allergic rash that appears after many days of antibiotic use, usually affects the trunk and extremities, does not affect eyes/mouth. The rash may feel rough to touch. This type of rash does not contraindicate future antibiotic use.
Because PCN cross reactivity (ie, the chance that the patient will have the same reaction if exposed to another antibiotic) with other antibiotics within the family is variable, a PCN allergy history does not contraindicate the use of all other cephalosporin or carbapenem antibiotics.
Nurses can refer to and adopt educational algorithms, clinical guides, and scriptsReference Sumner, Forsyth and Collette-Merrill19 to increase their participation in antibiotic optimization strategies. Box 2 provides an example on how to conduct an allergy history.
Box 2: Nurse-to-Patient Script on Clarifying Allergies to Penicillin
“What exactly happened when you took penicillin? How old were you when you experienced this reaction? What antibiotics have you taken after that? Have you seen an Allergy specialist?
I’m going to review your health information with the healthcare team. Sometimes your health care team may decide to give you an antibiotic even though you reported an allergy. This is because while many people report a history of being allergic to penicillin, most people who report an allergy to penicillin are not truly allergic. Also, a person with a true allergy may outgrow the allergy and can safely receive penicillin. It’s important to us that you receive the best therapy to treat your illness so we will work with you to address your concerns.” (Modified from Summer et al.Reference Sumner, Forsyth and Collette-Merrill19)
Prompting an antibiotic time out
Ensuring that appropriate durations of antibiotic therapy are being prescribed is a core component of AS. Antibiotic therapies may be prolonged beyond the recommended window for several reasons (eg, treatment end date is missing during patient transfer between units or teams, a system to address anticipated antibiotic duration and current day of therapy on a daily basis is lacking). Nurses can prevent patients from receiving unnecessarily prolonged antibiotic therapy by prompting the primary team to verbalize the planned duration of therapy. In Box 3, we present the “Four Moments of Antibiotic Decision-Making Adapted for Nursing.”Reference Tamma, Miller and Cosgrove20 Although the framework is focused on prescribers, it can be easily adopted by all individuals in the antibiotic decision-making process, such as those who administer or dispense antibiotics. This team-based approach ensures a critical review of an antibiotic prescription and improves antibiotic utilization.
Box 3: Four Moments of Antibiotic Decision MakingReference Tamma, Miller and Cosgrove20 Adapted for Nursing
(1) Does the patient have an infection that requires antibiotics?
(2) Have appropriate cultures been ordered before starting antibiotics? What empiric therapy should be initiated?
(3) A day or more has passed. Can antibiotics be stopped? Can therapy be narrowed? Can a change be made from IV to oral therapy?
(4) What duration of antibiotic therapy is needed for the patient’s diagnosis?
Key elements needed to integrate nurses into AS efforts
Education, communication strategies, and implementation models strategically embedded into work processes are primary components for formal nurse integration into AS activities. Leadership, including both physician and nursing leaders, supporting a workplace culture that fosters nurses’ participation and encourages them to play an active role in AS processes is key to the implementing and sustaining a number of AS interventions.Reference Manning, Pfeiffer and Larson21, Reference Manning and Giannuzzi22 In this section, we summarize the key elements required to integrate nurses into AS activities.
Enhancing education
Nurses have identified limited formal education on antibiotics and microbiology as a barrier to AS.Reference Monsees, Popejoy, Jackson, Lee and Goldman9–Reference Carter, Greendyke and Furuya11, Reference Monsees, Goldman and Popejoy23–Reference Manning and Pogorzelska-Maziarz25 Prescribers and pharmacists should make a concerted effort to discuss with bedside nurses why specific antibiotic treatment plans are being recommended for patients and when this does not happen, nurses should feel empowered to solicit these answers. Over time, this on-the-job learning will result in a broadened knowledge base about the spectrum of activity, potential drug interactions, and associated adverse drug events of specific antibiotics. In addition, these unstructured educational encounters may foster open communication and shared learning.
To enhance nurses’ contributions to AS, nurses should be offered the opportunity to learn more about microbiology reports and susceptibility testing, and to understand the difference between colonization and infection. The United Kingdom’s National Health Service (NHS) and the Scottish Antimicrobial Prescribing Group have developed an educational workbook targeting nurses and midwives that includes an overview of microbiology and antibiotic resistance.26 Computer-based learning modules for new hires or retraining purposes can be considered. A member of the ASP can collaborate with a nurse with an interest in AS and develop educational material for nurses.
Strengthening communication
Education should not be limited to clinical content but include other core components of AS, such as effective communication and teamwork. Barriers to including nurses in AS activities are often related to issues with unit culture, such as not being included in rounds, not having their input recognized or actively sought, and power differentials between disciplines.Reference Monsees, Popejoy, Jackson, Lee and Goldman9 In a recent survey querying healthcare system infection prevention and control administrators on nurse engagement, respondents frequently stated that nurses need confidence to question providers on antibiotic management.Reference Manning and Pogorzelska-Maziarz25 Strategies to enhance conversation between teams may neutralize potential communication challenges. The SBAR (situation, background, assessment and recommendation) tool provides a framework for organizing information in a clear and concise format. This communication style has been successfully used in healthcare to improve patient outcomes.Reference Carroll27–Reference Beckett and Kipnis29 We encourage bedside nurses to adopt this tool to strengthen communication with prescribers. Using the aforementioned practice of appropriate indications for C. difficile testing, we provide a clinical example of effective communication using SBAR in Box 4.
Box 4: Effective Communication Using the SBAR Tool
Situation: “Mrs. Flint is currently experiencing abdominal discomfort and watery stools.”
Background: “She is a 69 year-old woman with hypercholesterolemia and mild anemia who was admitted last night after a syncopal episode at her local grocery store. She was treated for a UTI 2 months ago with ciprofloxacin.”
Assessment: “Mrs. Flint reports taking laxatives at home because she is chronically on iron supplements. Her home bowel regimen has been continued in the hospital.”
Recommendation: “Even though she has a risk factor for C. difficile, I wanted to make sure you knew she is on laxatives. Should we stop the laxatives and reassess the need for C. difficile testing at a later time?”
The AHRQ Safety Program for Improving Antibiotic Use12 relies on both improvements in understanding the best practices in managing common inpatient infections and on improving teamwork, communication, and respect among healthcare providers. It encourages clinicians to recognize the opinions of the bedside nurse in formulating plans related to obtaining cultures and antibiotic treatment and also empowers nurses to feel comfortable voicing their concerns. The program consists of a series of webinars and other resources targeting both nursing and other clinicians to provide guidance on developing a collegial environment in which the common goal of optimizing patient care is a priority. These webinars and resources are expected to be publicly available in the summer of 2019.
Using an improvement model
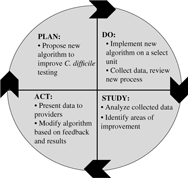
When sufficient resources are available, nurses can partner with other clinicians in identifying additional targeted interventions that may be necessary to improve diagnostic testing or antibiotic use. The Plan-Do-Study-Act (PDSA)Reference Deming30 framework for quality improvement can be considered a guide to implement such interventions (Fig. 1). For example, it can be used to implement an intervention to improve C. difficile testing:
Step 1. Plan: An algorithm with indications for appropriate C. difficile testing is developed, particularly with input from nurses who are primarily responsible for specimen collection. A plan to collect the data is established (eg, generate a list of all C. difficile tests in the electronic medical record during a specific time period).
Step 2. Do: The C. difficile algorithm is implemented on a select unit, ideally with high C. difficile ordering rates. A nurse champion in partnership with an AS leader provides support during the pilot process (eg, reviews cases with bedside nurses, seeks feedback on the algorithm, and identifies barriers to implement the algorithm).
Step 3. Study: A proportion or all C. difficile tests are reviewed for appropriateness. The number of C. difficile test orders is plotted on a run or statistical process control chart every week or month and is reviewed on a regular basis to evaluate impact of the intervention (algorithm). Depending on resources, the numerator can be the number of tests or the number of appropriate tests (standardized to the number of tests ordered).
Step 4. Act: Modifications to the algorithm are based on results and feedback. For example, if upon review of cases, receipt of laxatives is missing from the algorithm, the algorithm can be modified to include recent laxative use. Perform staff education to ensure optimal intervention implementation and compliance. Expand the intervention to additional units.
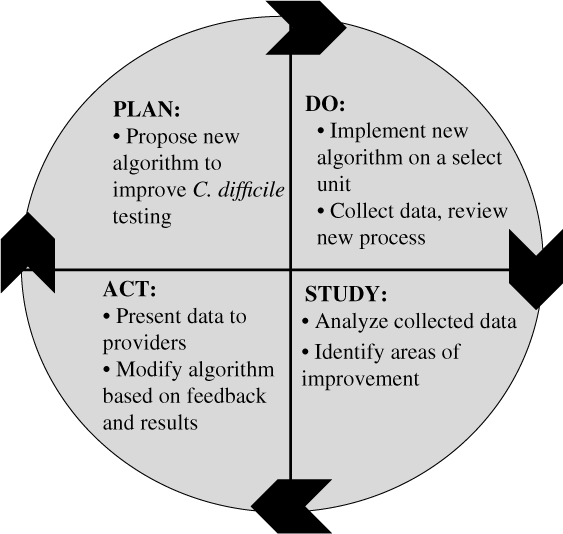
Fig. 1. The plan, do, study, act cycle.Reference Deming30
With such a framework, the user: (1) increases the belief that the change will result in improvement, (2) examines how the proposed changes will lead to the desired improvement and whether the proposed change will work in the environment of interest, (3) addresses pockets of skepticism and fear of clinical adverse events with proposed changes through the implementation of small graded steps, (4) enhances the monitoring process to prevent major setback and loss of confidence, and (5) minimizes resistance upon implementation. Elements to consider before implementing a nurse-driven AS intervention are summarized in Box 5.
Box 5: Elements to Consider Before Intervention
Assess organizational culture
Address organizational barriers
Tailor education based on observed practice deficits
Develop a usable intervention
Solicit feedback from nurses and identify a nurse champion to help develop education on proposed intervention
Consider train the trainer or other team-based modalities
Secure nursing and physician stakeholders support
Evaluating opportunities for workflow integration
After examining opportunities to boost AS education and communication techniques and adopting an implementation model, we suggest exploring workflow to determine the efficacy of new job aids. For example, nurses in an emergency department examined work processes and produced a group A Streptococcus pharyngitis algorithm that endorsed nurse evaluation prior to prescriber evaluation to minimize the broad testing of children with upper respiratory infections.Reference Durant31 Following several PDSA cycles, injudicious testing was reduced by 23% without impacting unit efficiency. As discussed earlier, several low-resource educational interventions have been published, including urine culture algorithms to reduce the treatment of asymptomatic bacteriuria.Reference Trautner, Grigoryan and Petersen7, Reference Norton, Lee and Harte32 Nurses should be part of the team that evaluates, advises, and collaborates on the development of potential AS job aids for them to be successful.
Barriers to integrating nursing into AS
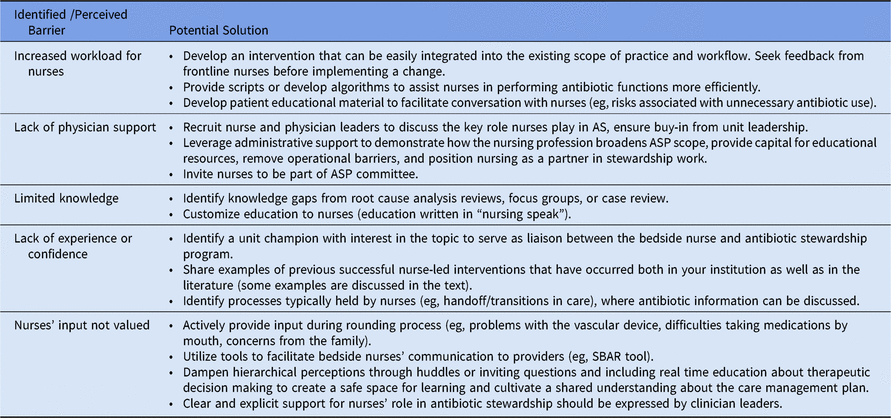
The perception of medical hierarchical tradition has contributed to limiting the role of nurses in performing tasks that may be perceived as “interfering” with medication prescribing. Overcoming this obstacle is an important step in integrating nurses in AS activities and can be achieved by strengthening communication between bedside nurses and prescribers and by increasing nurses’ confidence in antibiotic functions through education and decision support algorithms or guides. The potential barriers to integrating bedside nurses in AS and potential solutions are summarized in Table 1.
Table 1. Perceived Barriers to Antibiotic Stewardship (AS) by Acute-Care Nurses and Potential Solutions
Recent literature has revealed that nurses are receptive to strengthening their AS partnership and enthusiastically support their inclusion in programming efforts.Reference Monsees, Popejoy, Jackson, Lee and Goldman9–Reference Carter, Greendyke and Furuya11 Recommendations to expand ASPs with greater inclusion of bedside nurses is generating support at a national level.1, 2 Initial steps to integrate nurses into programming efforts include leveraging successful nurse leadership models and developing communication mechanisms to encourage nurses to speak up, participate actively in management discussions, and question practices, as appropriate.
Nurses view patient safety as an essential component of their work; however, antibiotic functions have not been formally integrated into their practice. Nurses generally interact with patients before the AS team, often before primary prescribers, and they are responsible for specimen collection, obtaining initial antibiotic allergy data, and antibiotic administration. Nurses spend more time with patients and families than most other clinicians; therefore, they are usually the first to observe, document, and report infectious symptoms. Also, they are often the first and most consistent point of contact for patients and families with the healthcare team. Nurses are therefore in a unique position to influence antibiotic decisions in a number of ways and to ensure safe use of antibiotics. Nurses should be included as valued partners in AS efforts.
Author ORCIDs
Elizabeth A. Monsees, 0000-0001-5277-7469
Acknowledgments
The findings and conclusions in this document are those of the authors, who are responsible for its content, and do not necessarily represent the views of AHRQ. No statement in this report should be construed as an official position of AHRQ or of the US Department of Health and Human Services.
Financial support
This work was supported by the Agency for Healthcare Research and Quality (AHRQ) (HHSP233201500020I/HHSP23337003T)
Conflicts of interest
All authors report no competing interests relevant to this article.